Published online Jul 28, 2007. doi: 10.3748/wjg.v13.i28.3824
Revised: April 13, 2007
Accepted: April 18, 2007
Published online: July 28, 2007
AIM: To investigate the effect of 5, 7-dihydroxy-8-nitrochrysin (NOChR) on apoptosis of human gastric carcinoma SGC-7901 cell line.
METHODS: SGC-7901 cells were cultured in vitro and the inhibitory effect of NOChR on proliferation of SGC-7901 cells was measured by using an MTT assay. NOChR-induced apoptosis rate of SGC-7901 cells was detected using flow cytometry (FCM) with PI staining. DNA ladder bands were observed by DNA agarose gel electrophoresis. The influence of NOChR on the proxisome proliferator-activated receptor-γ (PPARγ), Bcl-2 and Bax protein expression of SGC-7901 cells was analyzed by Western blot.
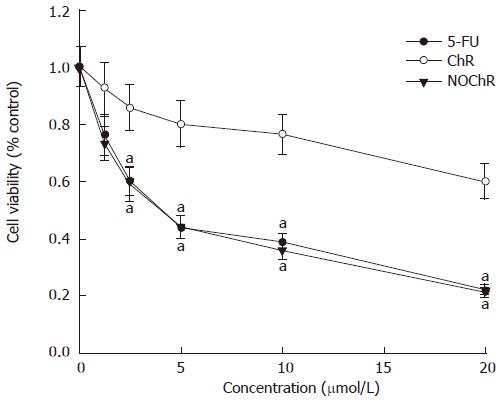
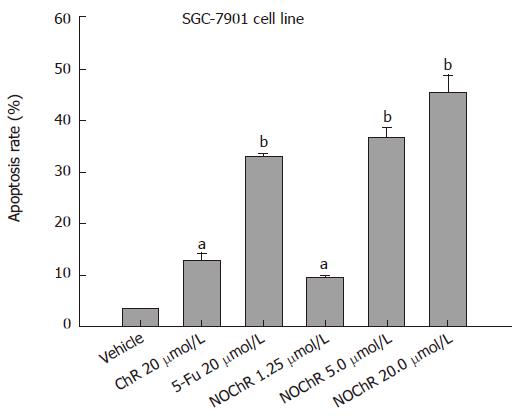
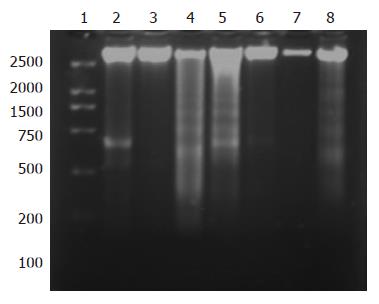
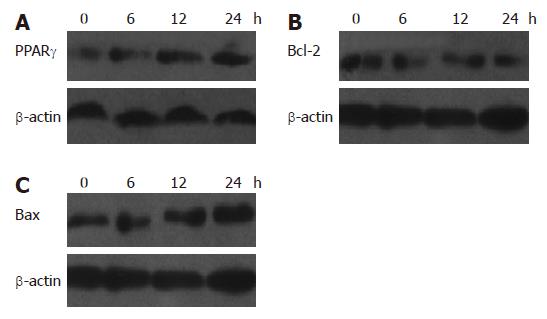
RESULTS: MTT assay showed that NOChR markedly inhibited proliferation of SGC-7901 cells in a dose-dependent manner, and when IC50 was 4.14 μmol/L, the potency of NOChR was 10 times than that of lead compound, chrysin (ChR, IC50 was 40.56 μmol/L), and was similar to 5-fluorouracil (5-FU, IC50 was 4.51 μmol/L). FCM with propidium iodide (PI) staining demonstrated that the apoptosis rates of SGC-7901 cells treated with 1.25, 5.00 and 20.00 μmol/L NOChR for 48 h were 9.8% ± 0.2%, 36.8% ± 1.9% and 45.5% ± 3.5%, respectively, and were significantly higher when treated with 5.00 and 20.00 μmol/L NOChR than that with 20.00 μmol/L ChR (12.9% ± 1.5%). DNA agarose gel electrophoresis showed that treatment of SGC-7901 cells with 20.00 μmol/L NOChR for 48 h resulted in typical DNA ladder bands of DNA of SGC-7901 cells, which could be eliminated by treating with 10.00 μmol/L GW9662, a blocker of PPARγ. Western blot analysis revealed that after 24 h of treatment with 20.00 μmol/L NOChR, PPARgamma and Bax protein expression of SGC-7901 cells increased but Bcl-2 expression decreased; however, pre-incubation with 10.00 μmol/L GW9662 could efficiently antagonize and weaken the regulatory effect of 20.00 μmol/L NOChR on Bax and Bcl-2 protein expression of SGC-7901 cells.
CONCLUSION: NOChR induces apoptosis of SGC-7901 cell lines by activating PPARγ and decreasing ratio of Bcl-2 to Bax.
-
Citation: Ai XH, Zheng X, Tang XQ, Sun L, Zhang YQ, Qin Y, Liu HQ, Xia H, Cao JG. Induction of apoptosis of human gastric carcinoma SGC-7901 cell line by 5, 7-dihydroxy-8-nitrochrysin
in vitro . World J Gastroenterol 2007; 13(28): 3824-3828 - URL: https://www.wjgnet.com/1007-9327/full/v13/i28/3824.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i28.3824
Gastric cancer is one of the most common malignancy in mankind and its incidence and mortality rank first in China[1]. Recent data indicate the mortality of gastric cancer in China is tending to increase and it severely threatens health and life of people[2]. At present, the managements of gastric cancer mainly include surgery and chemotherapy, but the curative effect of the existing chemotherapeutic drugs is not good enough and they have numerous side effects, therefore, it has been a focus to search the drugs capable of preventing and treating gastric cancer and other malignancy.
Proxisome proliferator-activated receptor-γ (PPARγ) is a member of the nuclear hormone receptor superfamily; a ligand-dependent transcription factor that plays an important role in lipid and glucose metabolism[3,4]. In recent years, over-expression of PPARγ has been found in a variety of tumor cells and PPARγ agonists can induce apoptosis[5,6]. It has been reported that chrysin (ChR) and its derivatives activate PPARγ to inhibit COX-2 and iNOS activity through various pathways with thiazolidones[7].
It has been demonstrated that ChR can markedly inhibit the growth of human thyroid cancer cell line[8], and has an effect on the inhibition of proliferation and induction of apoptosis in human myeloid leukemia cells as well[9,10]. Comte et al[11] reported that, through alkylation, the hydrophobic degree of ChR is increased, its KD value is decreased, and its binding affinity towards P-glucoprotein (P-gp) in cells is enhanced. Our previous study showed that the effect of 5, 7-dihydroxy-8-nitrochrysin (NOChR) on the inhibition of proliferation of the colon cancer cell line HT-29 and the gastric cancer cell line SGC-7901 was stronger than the effect of ChR[12]. We also confirmed that a series of B-ring trifluoromethylated derivatives of ChR markedly inhibited the growth of HT-29 and SGC-7901 cell lines[13] and NOChR had an inhibitory effect on primary Lewis lung carcinoma transplanted subcutaneously into mouse and spontaneous metastasis of mouse Lewis lung carcinoma in a dose-dependent manner[14].
In this study, we aimed to investigate whether NOChR induces apoptosis of SGC-7901 cell line by activation of PPARγ and whether Bcl-2 and Bax take part in this mechanism, thereby providing a new idea for research about pharmaceutical prevention and cure of human gastric cancer.
Human gastric cancer SGC-7901 cell lines were purchased from the China Center for Type Culture Collection (CCTCC), Wuhan, China and were cultured with RPMI 1640 medium supplemented with 100 mL/L calf serum at 37°C in a humidified atmosphere containing 50 mL/L CO2 in air.
NOChR was synthesized in the Institute of Pharmacology, Nanhua University as previously described[12], with a molecular weight of 299 ku, characteristic yellow crystals and purity of 99.0%. NOChR was dissolved in dimethyl sulfoxide (DMSO), diluted with phosphate buffer solution (PBS), and finally prepared as 2 mmol/L mother liquor by filtration sterilization. RPMI 1640, MTT and DMSO were purchased from Huanei Biotechnology Company. 5-fluorouracil (5-FU) was from Jinghua pharmaceutical Corporation Ltd, Nantong. The apoptotic DNA Ladder Apoptotic DNA Ladder Detection Kit was purchased from Bodataike Company, Beijing. Mouse anti-human Bcl-2 monoclonal antibody, mouse anti-human Bax monoclonal antibody and rabbit anti-human PPARγ polyclonal antibody were purchased from Santa Cruz Company.
SGC-7901 cells were seeded in a 96-well plate at a density of 2.0 × 104 cells/well as previously described[15]. Different concentrations of drugs were added to each well and cultured for 48 h, followed by incubation with 5 mg/L MTT for 4 h. The supernatant was removed after centrifugation. Finally, 100 μL of DMSO was added and adsorbance at 570 nm wavelength (A570) was measured by means of Enzyme-labeling instrument (EX-800 type). Relative cell proliferation inhibition rate (IR) = (1 - average A570 of the experimental group/average A570 of the control group) × 100%.
As previously described[16], SGC-7901 cells were treated with serum-free medium for 24 h, followed by treatment with media containing different concentrations of drugs for 48 h, respectively. Cells were collected and prepared as a single cell suspension by mechanical blowing with PBS, washed with cold PBS twice, fixed with 700 mL/L alcohol at 4°C for 24 h, stained with propidium iodide (PI) and cell apoptosis was detected using FCM (American BD Company, FACS420).
As previously described[17], cells were cultured with 20 μmol/L NOChR and 20 μmol/L NOChR plus 10 μmol/L GW9662, a PPAR-γ antagonist, for 0, 24, 48 and 72 h, respectively. Cells were washed twice with PBS and DNA was extracted with an Apoptotic DNA Ladder Detection Kit according to the manufacturer’s instructions. The extracted DNA was kept at 4°C overnight. Then 8.5 μL of DNA sample was mixed with 1.5 μL of 6 × Buffer solution, electrophoresed on 20 g/L agarose gel containing ethidium bromide at 40 V, and observed through DBT-08 gel image analysis system.
As previously described[18], cells were treated with 20 μmol/L NOChR for 0, 6, 12 and 24 h, respectively. Cells were collected, washed three times with PBS, lysed in cell lysate containing 0.1 mol/L NaCl, 0.01 mol/L Tris-Cl (pH 7.6), 0.001 mol/L EDTA (pH 8.0), 1 μg/mL Aprotinin, 100 μg/mL PMSF, and then centrifuged at 13 000 ×g for 10 min at 4 °C. The extracted protein sample (25 μg total protein/lane) was added in the same volume of sample buffer solution and subjected to denaturation at 100°C for 10 min, then electrophoresed on 100 g/L or 60 g/L SDS-PAGE at 100 mA for 3 h, and finally transferred onto PVDF membrane. The PVDF membrane was treated with TBST containing 50 g/L skimmed milk at room temperature for 2 h, followed by incubation with the first antibodies PPARγ, Bcl-2 and Bax (1:500 dilution), respectively, at 37°C for 2 h or at 4°C overnight. After being washed with TBST for 30 min, the corresponding secondary antibody was added and incubated at room temperature for 1 h. The membrane was then washed three times for 15 min each with TBST. Fluorescence was produced from solution A and B containing a chemiluminescence-generating chemical. The results were analyzed with Image analyzer and the product of area and optical density was expressed as integral absorbance (IA).
Experimental data in each group were expressed as mean ± SD. Analysis of variance was performed with SPSS software for windows 11.5 by using one way ANOVA and pairwise comparison with Student’s t test. P < 0.05 was considered statistically significantly.
The MTT assay demonstrated that NOChR obviously inhibited proliferation of SGC-7901 cells in a dose-dependent manner (Figure 1); and when IC50 was 4.14 μmol/L, the potency of NOChR was 10 times the potency of lead compound, ChR (IC50 was 40.56 μmol/L), and was similar to that of 5-FU ( IC50 was 4.51 μmol/L).
FCM with PI staining showed that the apoptosis rates of SGC-7901 cell line treated with 1.25, 5.00, 20.00 μmol/L NOChR for 48 h were 9.8% ± 0.2%, 36.8% ± 1.9% and 45.5% ± 3.5%, respectively, and were significantly higher when treated with 5.00 and 20.00 μmol/L NOChR than that with 20.00 μmol/L ChR (12.9% ± 1.5%) (Figure 2).
DNA gel electrophoresis showed that treatment of SGC-7901 cells with 20.00 μmol/L NOChR for 48 and 72 h resulted in typical DNA ladder bands, which could be eliminated or attenuated by treating with 20 μmol/L NOChR plus 10.0 μmol/L GW9662 for 48 h and partly eliminated by treating with 20 μmol/L NOChR plus GW9662 10.0 μmol/L for 72 h (Figure 3).
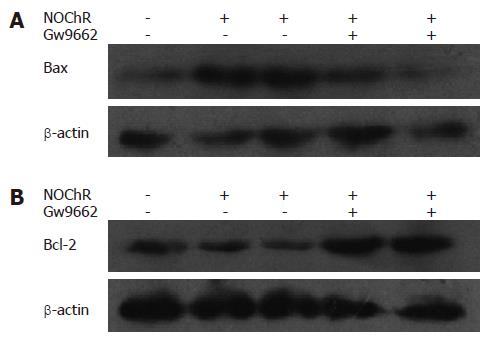
Western blot analysis showed that the relative densities of PPARγ, Bcl-2 and Bax protein bands of SGC-7901 cells treated with 20 μmol/L NOChR for 12 and 24 h were 154% and 165%, 62% and 49%, and 128% and 160% of the SGC-7901 cells not treated with NOChR, respectively (P < 0.05) (Figure 4). This indicates that NOChR can increase the PPARγ and Bax protein expression and decrease Bcl-2 protein expression.
Western blot analysis demonstrated that when SGC-7901 cells were pre-incubated with 10 μmol/L GW9662, a blocker of PPARγ, for 30 min, the effects of NOChR on up-regulation of Bax protein expression and down-regulation of Bcl-2 protein expression were antagonized or weakened (Figure 5), thereby suggesting that the effects of NOChR on up-regulation of Bax protein expression and down-regulation of Bcl-2 protein expression were associated with the activation of PPARγ.
Tumorigenesis and tumor progression are strongly associated with abnormal apoptosis. A number of anti-tumor drugs exert their therapeutic effects by inducing or promoting apoptosis. Based on the previous reports[12,14], our experiment was to investigate the apoptosis of human gastric carcinoma SGC-7901 cell line induced by NOChR and to provide theoretical and experimental evidences for its application as an anti-tumor drug.
Apoptosis usually possesses the typical morphological and biochemical characteristics, including condensed chromatin in cells, appearance of apoptotic bodies, presence of hypodiploid peak in FCM analysis and DNA ladder bands on agarose electrophoresis[19,20]. In this study, treatment of SGC-7901 cells with NOChR resulted in formation of DNA ladder bands and appearance of marked hypodiploid peak. Thus, this experiment suggested that NOChR can induce apoptosis of human gastric carcinoma SGC-7901 cell lines in vitro.
Apoptosis is a complex process involving several genes, such as Bcl-2, Bax, and a great attention has been given to Bcl-2 family. Bcl-2 family members have double functions: Bcl-2 and Bcl-Xl can inhibit apoptosis and promote cell survival, but Bax, Bak, Bad and Bcl-Xs can promote apoptosis. Bax inhibits the activity of Bcl-2 or Bcl-Xl to promote apoptosis either by forming a homodimer (with itself) or a heterodimer with either Bcl-2 or Bcl-XI[21]. The ratio of Bcl-Xl or Bcl-2 to Bax determines whether the cells undergo apoptosis or not. Possible mechanisms of action of members in Bcl-2 family are as follows: (1) Bax can destroy mitochondrial membrane to release cytochrome c, thereby activates cyt c/Apaf-1/caspase-9 signaling pathway to cause apoptosis[22], while the mechanism of Bcl-2 is quite opposite[23]; (2) anti-apoptotic protein, such as Bcl-2 and Bcl-Xl, can prevent Bax or Bak from conformational change, oligomerization or inserting in mitochondrial outer membrane to inhibit apoptosis[24,25]; (3) high level of Bcl-2 can inhibit transactivating effect of p53 on its target genes (such as p21/WAF1, Bax), thereby exerts its potential inhibitory effect on p53 to regulate apoptosis[26]. The results in this study confirmed that Bcl-2 expression in non-treated SGC-7901 cells was higher than those that treated with 20 μmol/L NOChR for 48 h; in contrast, Bax expression was lower. Thus, the ratio of Bcl-2 to Bax in SGC-7901 cells treated with NOChR was lower than that of non-treated SGC-7901 cells, which indicated that NOChR-induced SGC-7901 cells apoptosis was associated with down-regulation of Bcl-2 expression, up-regulation of Bax expression and reduction of the ratio of Bcl-2 to Bax.
PPARγ is a kind of ligand-activated nuclear transcri-ption factor belonging to a member of nuclear receptor superfamily. PPARγ ligands include natural ligands such as 15d-PGJ2 and linoleic acid, and synthetic ligands such as antidiabetic medicine thiazolidinediones, including troglitazone, rosiglitazone and pioglitazone and non-steroidal anti-inflammatory drugs (indomethacin, sulindac and ibuprofen)[27,28]. Chen et al[29] confirmed that PPARγ ligands may markedly inhibit NF-KB expression and reduce Bcl-2 expression to inhibit cell growth and induce apoptosis of colonic cancer HT-29 cell line by activation of PPARγ. Chen et al[26] reported that PPARγ ligands can inhibit cell growth and induce apoptosis of human gastric cancer cell line. It has been reported that chrysin-induced apoptosis is mediated via caspase activation and p-Akt inactivation in U937 leukemia cell and cervical carcinoma Hela cell[10,30]. Liang et al[7] have recently shown that ChR is activated by different ways with thiazolidones, and PPARγ inhibits activation of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase. In order to prove whether NOChR decreases Bcl-2 protein expression to induce apoptosis of SGC-7901 cells by activation of PPARγ, we pre-incubated SGC-7901 cell with GW9662, a selective antagonist of PPARγ, and observed the effect of NOChR on apoptosis and PPARγ and Bcl-2 protein expression of SGC-7901 cell lines. Our results showed that pre-incubation of GW9662 could effectively antagonize NOChR-induced apoptosis of SGC-7901 cell lines and down-regulation of Bcl-2 protein expression, thereby suggested that apoptosis of SGC-7901 cells induced by NOChR is dependent on activation of PPARγ.
In summary, NOChR possesses stronger anti-gastric cancer effect in vitro than lead compound ChR, and it exerts its apoptotic effect by activation of PPARγ, down-regulation of Bcl-2 protein expression, up-regulation of Bax protein expression, and reduction of the ratio of Bcl-2 to Bax. NOChR might be a promising candidate for treatment of gastric cancer.
S- Editor Zhu LH L- Editor Kumar M E- Editor Ma WH
| 1. | Sun X, Mu R, Zhou Y, Dai X, Qiao Y, Zhang S, Huangfu X, Sun J, Li L, Lu F. 1990-1992 mortality of stomach cancer in China. Zhonghua ZhongLiu ZaZhi. 2002;24:4-8. [PubMed] |
| 2. | Sun XD, Mu R, Zhou YS, Dai XD, Zhang SW, Huangfu XM, Sun J, Li LD, Lu FZ, Qiao YL. Analysis of mortality rate of stomach cancer and its trend in twenty years in China. Zhonghua ZhongLiu ZaZhi. 2004;26:4-9. [PubMed] |
| 3. | Hamm JK, el Jack AK, Pilch PF, Farmer SR. Role of PPAR gamma in regulating adipocyte differentiation and insulin-responsive glucose uptake. Ann N Y Acad Sci. 1999;892:134-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Rahimian R, Masih-Khan E, Lo M, van Breemen C, McManus BM, Dubé GP. Hepatic over-expression of peroxisome proliferator activated receptor gamma2 in the ob/ob mouse model of non-insulin dependent diabetes mellitus. Mol Cell Biochem. 2001;224:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Leung WK, Bai AH, Chan VY, Yu J, Chan MW, To KF, Wu JR, Chan KK, Fu YG, Chan FK. Effect of peroxisome proliferator activated receptor gamma ligands on growth and gene expression profiles of gastric cancer cells. Gut. 2004;53:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Li M, Lee TW, Mok TS, Warner TD, Yim AP, Chen GG. Activation of peroxisome proliferator-activated receptor-gamma by troglitazone (TGZ) inhibits human lung cell growth. J Cell Biochem. 2005;96:760-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Liang YC, Tsai SH, Tsai DC, Lin-Shiau SY, Lin JK. Suppression of inducible cyclooxygenase and nitric oxide synthase through activation of peroxisome proliferator-activated receptor-gamma by flavonoids in mouse macrophages. FEBS Lett. 2001;496:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 167] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Yin F, Giuliano AE, Van Herle AJ. Growth inhibitory effects of flavonoids in human thyroid cancer cell lines. Thyroid. 1999;9:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Ko WG, Kang TH, Lee SJ, Kim YC, Lee BH. Effects of luteolin on the inhibition of proliferation and induction of apoptosis in human myeloid leukaemia cells. Phytother Res. 2002;16:295-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Woo KJ, Jeong YJ, Park JW, Kwon TK. Chrysin-induced apoptosis is mediated through caspase activation and Akt inactivation in U937 leukemia cells. Biochem Biophys Res Commun. 2004;325:1215-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Comte G, Daskiewicz JB, Bayet C, Conseil G, Viornery-Vanier A, Dumontet C, Di Pietro A, Barron D. C-Isoprenylation of flavonoids enhances binding affinity toward P-glycoprotein and modulation of cancer cell chemoresistance. J Med Chem. 2001;44:763-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Zheng X, Meng WD, Xu YY, Cao JG, Qing FL. Synthesis and anticancer effect of chrysin derivatives. Bioorg Med Chem Lett. 2003;13:881-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 111] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Zheng X, Cao JG, Meng WD, Qing FL. Synthesis and anticancer effect of B-ring trifluoromethylated flavonoids. Bioorg Med Chem Lett. 2003;13:3423-3427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Xu YY, Zheng X, Zhu BY, Cao JG. Synthesis and Antitumor effect of 5,7-dihydroxyl-8-nitrio chrysin. Nanhua Daxue Xuebao (Medical Edition). 2004;32:283-289. |
| 15. | Cao JG, Tang XQ, Shi SH. Multidrug resistance reversal in human gastric carcinoma cells by neferine. World J Gastroenterol. 2004;10:3062-3064. [PubMed] |
| 16. | Tang XQ, Bi H, Feng JQ, Cao JG. Effect of curcumin on multidrug resistance in resistant human gastric carcinoma cell line SGC7901/VCR. Acta Pharmacol Sin. 2005;26:1009-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Yang FG, Zhang ZW, Xin DQ, Shi CJ, Wu JP, Guo YL, Guan YF. Peroxisome proliferator-activated receptor gamma ligands induce cell cycle arrest and apoptosis in human renal carcinoma cell lines. Acta Pharmacol Sin. 2005;26:753-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Liu H, Zang C, Fenner MH, Liu D, Possinger K, Koeffler HP, Elstner E. Growth inhibition and apoptosis in human Philadelphia chromosome-positive lymphoblastic leukemia cell lines by treatment with the dual PPARalpha/gamma ligand TZD18. Blood. 2006;107:3683-3692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Chen YC, Shen SC, Lee WR, Hsu FL, Lin HY, Ko CH, Tseng SW. Emodin induces apoptosis in human promyeloleukemic HL-60 cells accompanied by activation of caspase 3 cascade but independent of reactive oxygen species production. Biochem Pharmacol. 2002;64:1713-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 165] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Darzynkiewicz Z, Bedner E, Smolewski P. Flow cytometry in analysis of cell cycle and apoptosis. Semin Hematol. 2001;38:179-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 187] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Yang E, Korsmeyer SJ. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 1996;88:386-401. [PubMed] |
| 22. | Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1120] [Cited by in RCA: 1126] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 23. | Martinou JC, Green DR. Breaking the mitochondrial barrier. Nat Rev Mol Cell Biol. 2001;2:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 686] [Cited by in RCA: 721] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 24. | Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1428] [Cited by in RCA: 1466] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 25. | Perez D, White E. TNF-alpha signals apoptosis through a bid-dependent conformational change in Bax that is inhibited by E1B 19K. Mol Cell. 2000;6:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Miyashita T, Harigai M, Hanada M, Reed JC. Identification of a p53-dependent negative response element in the bcl-2 gene. Cancer Res. 1994;54:3131-3135. [PubMed] |
| 27. | Kliewer SA, Umesono K, Noonan DJ, Heyman RA, Evans RM. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992;358:771-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1312] [Cited by in RCA: 1330] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 28. | Marcus SL, Capone JP, Rachubinski RA. Identification of COUP-TFII as a peroxisome proliferator response element binding factor using genetic selection in yeast: COUP-TFII activates transcription in yeast but antagonizes PPAR signaling in mammalian cells. Mol Cell Endocrinol. 1996;120:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Chen GG, Lee JF, Wang SH, Chan UP, Ip PC, Lau WY. Apoptosis induced by activation of peroxisome-proliferator activated receptor-gamma is associated with Bcl-2 and NF-kappaB in human colon cancer. Life Sci. 2002;70:2631-2646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 105] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Zhang T, Chen X, Qu L, Wu J, Cui R, Zhao Y. Chrysin and its phosphate ester inhibit cell proliferation and induce apoptosis in Hela cells. Bioorg Med Chem. 2004;12:6097-6105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |