Published online Jul 21, 2007. doi: 10.3748/wjg.v13.i27.3692
Revised: January 20, 2007
Accepted: January 25, 2007
Published online: July 21, 2007
AIM: To compare different preconditioning strategies to protect the liver from ischemia/reperfusion injury focusing on the expression of pro- and anti-apoptotic proteins. Interventions comprised different modes of ischemic preconditioning (IP) as well as pharmacologic pretreatment by α-lipoic acid (LA).
METHODS: Several groups of rats were compared: sham operated animals, non-pretreated animals (nt), animals receiving IP (10 min of ischemia by clamping of the portal triad and 10 min of reperfusion) prior to sustained ischemia, animals receiving selective ischemic preconditioning (IPsel, 10 min of ischemia by selective clamping of the ischemic lobe and 10 min of reperfusion) prior to sustained ichemia, and animals receiving 500 μmol α-LA injected i.v. 15 min prior to the induction of 90 min of selective ischemia.
RESULTS: Cellular damage was decreased only in the LA group. TUNEL-positive hepatocytes as well as necrotic hepatocyte injury were also decreased only by LA (19 ± 2 vs 10 ± 1, P < 0.05 and 29 ± 5 vs 12 ± 1, P < 0.05). Whereas caspase 3- activities in liver tissue were unchanged, caspase 9- activity in liver tissue was decreased only by LA pretreatment (3.1 ± 0.3 vs 1.8 ± 0.2, P < 0.05). Survival rate as the endpoint of liver function was increased after IP and LA pretreatment but not after IPsel. Levels of lipid peroxidation (LPO) in liver tissue were decreased in the IP as well as in the LA group compared to the nt group. Determination of pro- and anti-apoptotic proteins showed a shift towards anti-apoptotic proteins by LA. In contrast, both our IP strategies failed to influence apototic cell death.
CONCLUSION: IP, consisting of 10 min of ischemia and 10 min of reperfusion, protects only partly against ischemia/reperfusion injury of the liver prior to 90 min of selective ischemia. IPsel did not influence ischemic tolerance of the liver. LA improved tolerance to ischemia, possibly by downregulation of pro-apoptotic Bax.
- Citation: Duenschede F, Erbes K, Riegler N, Ewald P, Kircher A, Westermann S, Schad A, Miesmer I, Albrecht-Schöck S, Gockel I, Kiemer AK, Junginger T. Protective effects of ischemic preconditioning and application of lipoic acid prior to 90 min of hepatic ischemia in a rat model. World J Gastroenterol 2007; 13(27): 3692-3698
- URL: https://www.wjgnet.com/1007-9327/full/v13/i27/3692.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i27.3692
Ischemic preconditioning (IP), first described by Murry et al[1], has been reported to induce tolerance to ischemia/reperfusion injury (IRI) in many organs, such as brain, heart, and skeletal muscle[2]. Especially in IRI of the liver, there are numerous studies that describe the protective effects of IP[3].
With both in vivo and in vitro models, the cycle of preconditioning seems to be critical for the induction of ischemic tolerance. Hypoxic periods shorter than 5 min duration or exceeding 15 min failed to induce protection. Similarly, periods of reperfusion of shorter than 5 min or exceeding 15 min also had no protective effect[4]. Ischemic tolerance of the rat liver was demonstrated by IP consisting of 5 min of ischemia and 30 min of reperfusion[5]. IP has shown contradictory effects in numerous experimental studies using different animal species[6-8]. In humans, IP has been carried out as a protective strategy against hepatic IRI[9,10].
Apart from the beneficial effects on hepatic IRI, the mechanism by which IP confers protection remains to be elucidated. Blocking the generation of reactive oxygen species (ROS) is considered one of the most likely mechanisms of action of IP[5,11]. IP has been shown to protect the mouse liver by inhibition of apoptosis through a caspase-dependent pathway[4].
Pro- and anti-apoptotic proteins, such as Bax and Bcl-2, play a key role in mediating apoptotic cell death in hepatic IRI. Bax was described to directly induce the release of cytochrome c from isolated mitochondria, whereas overexpression of Bcl-2 strongly attenuated the hepatic IRI by inhibition of cytochrome c release[12].
Previous studies of our group showed that LA is a fast, easy, and safe method to attenuate IRI[13]. As lipoamide, LA is a cofactor in multiple enzyme complexes that catalyze the oxidative decarboxylation of γ-keto acids, such as pyruvate, γ-ketogluterate and branched-chain γ-keto acids. It was found to be synthesized by animals and humans. Recently, it has been found that LA exerts powerful antioxidant effects. These effects have led to studies of the use of LA in a number of oxidative stress conditions[14].
Although IP is presently the best accepted strategy to attenuate IRI, IP has only rarely been compared to other interventions. A comparison of a “traditional” IP strategy (i.e. 10 min of ischemia and 10 min of reperfusion) with both a novel selective, partial clamping method and a promising pharmacologic intervention (LA) may provide insights into the mechanisms of action of IRI. Selective portal triad clamping (IPsel) has been described to minimize IRI of the liver during ablative treatment of colorectal metastases. However, studies on the influence of IPsel on IRI of the liver are rare[15].
The aim of the present study was to compare both the extent and potential mechanism of action of three different preconditioning strategies.
Male Brown Norway rats weighing 175-200 g (Harlan, Paderborn, Germany) were used. All animals had access to water and rat chow ad libitum (Global Diet Harlan). The animals were maintained in accordance with the ”Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86-23, revised 1985). The study was approved by our Institutional Animal Care and Use Committee.
IP was carried out by placing a microvascular clamp on the portal triad. IPsel was carried out by placing a microvascular clamp on the corresponding branch of the portal vein, hepatic artery, and bile duct of the left (acute experiment) or the median (animal survival) liver lobe proximal to its origin. As a model for acute experiments, 90 min ischemia of the left liver lobe was performed by clamping the corresponding branch of the portal vein, hepatic artery, and bile duct proximal to its origin. Reperfusion was initiated by removal of the clamp. Thereafter, the abdominal cavity was closed with running sutures, and animals were allowed to awaken without resuscitation of additional fluid. The animals had free access to water and rat chow ad libitum. After 4 h of reperfusion, anesthetized animals were sacrificed, and liver tissue and plasma were stored for further examination.
Several experimental groups were studied: (1) sham-operated animals (n = 3); (2) 90 min ischemia without any pretreatment (n = 5), (3) 90 min ischemia with IP (10 min of ischemia by clamping of the portal and 10 min of reperfusion) prior to sustained ischemia (n = 5), (4) 90 min ischemia with IPsel of the ischemic lobe (10 min of ischemia by selective clamping of the left lobe and 10 min of reperfusion) prior to sustained ichemia (n = 5), and (5) 500 μmol LA (120 μg/mL saline) via the abdominal vena cava 15 min prior to sustained ischemia (n = 5). Body temperature (37 ± 1°C) was monitored and maintained by a heating lamp. The sham, untreated, IP, and IPsel groups received vehicle (1 mL).
Animal survival was investigated by resecting non-ischemic liver tissue at the time of reperfusion. After induction of anesthesia with inhaled ether, the abdominal cavity was accessed through a midline incision. Inflow occlusion of the median lobe was performed by placing a microvascular clamp on the median branch of the portal vein, hepatic artery, and bile duct. Ischemia was maintained for 90 min, immediately after which the non-ischemic liver tissue, i.e. the right, the caudate, and the left liver lobes, was resected (70% liver tissue). Thereafter, the abdominal cavity was closed with running sutures, and animals were allowed to awake without resuscitation of additional fluid.
Several experimental groups were studied: (1) control animals only treated by liver resection (n = 3); (2) 90 min of ischemia of the median liver lobe followed by liver resection (n = 5), (3) 90 min of ischemia of the median liver lobe with IP (10 min of ischemia by clamping of the portal triad and 10 min of reperfusion) prior to sustained ischemia and liver resection (n = 8), (4) 90 min ischemia of the median liver lobe with IPsel (10 min of ischemia by selective clamping of the median liver lobe and 10 min of reperfusion) prior to sustained ichemia and liver resection (n = 5), and (5) 90 min ischemia of the median liver lobe with 500 μmol LA (120 μg per 1 mL saline) administered via the abdominal vena cava 15 min prior to sustained ischemia and liver resection (n = 8). Body temperature was monitored and maintained at 37 ± 1.0°C by a heating lamp. The control, untreated, IP, and IPsel groups received vehicle (1 mL).
Liver tissue was weighed prior to homogenization, and then diluted 1:5 with 0.01 mol/L PBS (phosphate buffered saline). Homogenization was carried out with a Potter tissue homogenizer. A homogeneous mixture was achieved by vortexing at 800 r/min. Next, the mixture was centrifuged at 1000 ×g for 10 min and the supernatant pipetted into an Eppendorf tube and centrifuged at 10 000 ×g for 20 min. The clear supernatant was pipetted into a fresh Eppendorf tube and stored frozen at -20°C until further measurement.
α-GST in plasma 4 h after the onset of reperfusion was measured by absorption using a microplate reader (Bio Rad). The ELISA kit was run in accordance with the manufacturer’s instructions (Biotrin, Sinsheim-Reihen, Germany).
LPO was determined with a standardized test (Cayman Chemical, Grünberg, Germany). Absorption measurements were done at 500 nm, using a microplate reader. The test measures the lipid hydroperoxides based on the calibration curve, utilizing the redox reactions with ferrous ions. The resulting ferric ions are detected, using thiocyanate ion as the chromogen. This procedure eliminates any interference caused by hydrogen peroxide or endogenous ferric ions in the sample, and provides a sensitive and reliable assay for lipid peroxidation.
Caspase 3- and 9- activity was measured using a colorimetric reaction (Caspase Colorimetric Assay, R&D Systems Germany, Wiesbaden) at a wavelength of 405 nm. All samples were prepared in pairs; one pair was measured with and one without substrate according to the manufacturer’s instructions. The results are expressed as fold increase in caspase activity in post-ischemic versus non-ischemic sham liver tissue[16].
The protein concentration was determined by spectrophotometry with a standardized test (Protein Assay ESL, Roche Diagnostics).
Formalin-fixed hepatic tissue samples were embedded in paraffin, and 5 μm sections were cut. Replicate sections were either stained with hematoxylin and eosin (H&E) for the evaluation of morphologic features of necrosis or stained with the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay for the evaluation of apoptosis.
The liver sections were fixed in freshly prepared neutral buffered formalin (PBS). Frozen sections (5 μm) of the fixed tissue were prepared and stained with the TUNEL method, using a commercial kit (Chemicon International, ApoTag peroxidase in situ apoptosis detection kit, Hampshire, UK). The percentage number of TUNEL-positve hepatocytes was estimated by evaluating the number of hepatocytes per high power field (hpf) compared with number of TUNEL-positive hepatocytes (× 400).
Morphological criteria of necrosis, such as shrinkage, loss of architecture, eosinophilia and karyolysis, were evaluated in serial sections stained with H&E. The percentage of necrosis was estimated by evaluating the number of microscopic fields with oncosis compared with the entire histologic section. All histologic evaluations were done in a blinded fashion.
The expression of Bax and Bcl-2 was assessed by RT-PCR. The expression of 18 S ribosome was used as the housekeeping gene. Total RNA was isolated using RNeasy Mini-Kits (Qiagen). After photometric determination of the amount of RNA, a first strand cDNA was synthesized from 4 μg RNA from each liver, using the following procedure: the transcription mix contained 5 μL oligo(dT) (Gibco-BRL), 3.6 μL dNTP-Mix (10 mmol/L) (Gibco-BRL), 8 μL first strain buffer, 4 μL DTT (0.1 mol/L), 1.5 μL reverse transcriptase (MLV-T, Roche Diagnostics, Mannheim, Germany) and 1.5 μL RNA free water (Qiagen). Controls were performed without the addition of reverse transcriptase. Cycle conditions were chosen as follows: 90 min at 37°C, 10 min at 94°C, and 30 s at 4°C. The cDNA was checked by amplification of 18 S robosome. Primers were designed specifically for rats with Primer 3 Software (Whitehead Institute, Boston, USA) and synthesized by MWG Biotech, Germany.
The following primers were used: Bcl-2 neu forward: 5'-TGC-AGA-GAT-GTC-CAG-TCA-GC-3' and Bcl-2 neu reverse: 5'-CAT-CCA-CAG-AGC-GAT-GTT-GT-3' (expected product 200 bp), Bax forward: 5'-ACA-GAT-CAT-GAA-GAC-AGG-GG-3' and reverse 5'-CAA-AGT-AGA-AGA-GGG-CAA-CC-3' (expected product 203 bp).
All sequences were compared with the complete rat Gene Bank library to ensure that each primer was unique for its intended target and that areas of sequence polymorphisms were avoided.
We additionally performed quantitative real-time PCR analysis of transcripts for Bax and GAPDH with predesigned and optimized TaqMan® Gene Expression Assays (Applied Biosystems) on an Applied Biosystems 7500 Real-Time PCR System, according to the manufacturer's instructions as described previously[17]. Relative quantitation was carried out using the delta-delta-CT method.
All data are expressed as mean ± SD. Statistical differences between experimental groups were calculated by GraphPad Prism Software 1994-1999 using a one-way analysis of variance (ANOVA) with subsequent post hoc Tukey-tests. Survival curves were calculated using the Kaplan and Meier method and compared by a log-rank test. Proportions were calculated using Fisher's exact test. Differences were considered statistically significant at Ρ < 0.05.
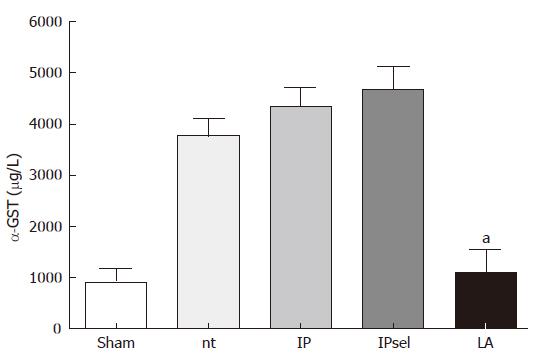
After 90 min of ischemia and 4 h of reperfusion, α-GST release increased markedly in the untreated, IP, and IPsel groups in comparison to the sham-operated group, whereas α-GST increased only slightly after 4 h reperfusion in the LA-group. α-GST was used as a sensitive marker of hepatocellular injury for several reasons: α-GST exhibits a specific but ubiquitious hepatic distribution, has a short serum in vivo half life time (< 1 h) and is released rapidly and substantially into the circulation in large amounts after acute hepatocellular damage[18] (Figure 1).
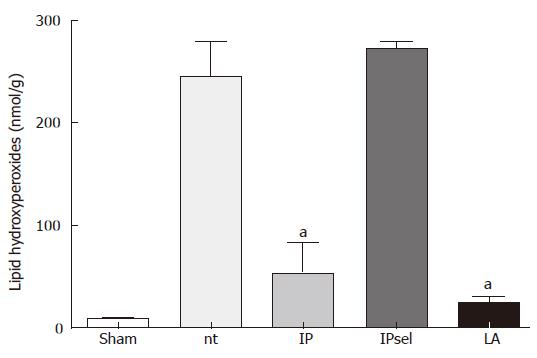
LPO values obtained 4 h after reperfusion showed a substantial increase in the untreated and IPsel livers compared to the sham-operated group (Figure 2). In the LA and IP groups, LPO values were decreased by about 90%.
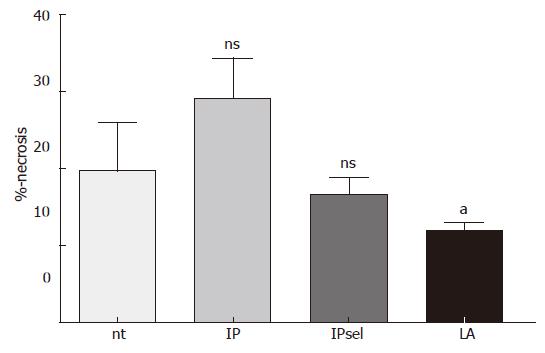
After 90 min of ischemia and 4 h of reperfusion there was a marked increase in oncotic hepatocytes in the untreated, IP, and IPsel groups compared to sham-operated animals (not detectable). In the LA-pretreated group, we observed a significant decrease of necrotic hepatocytes after 4 h of reperfusion (Figure 3).
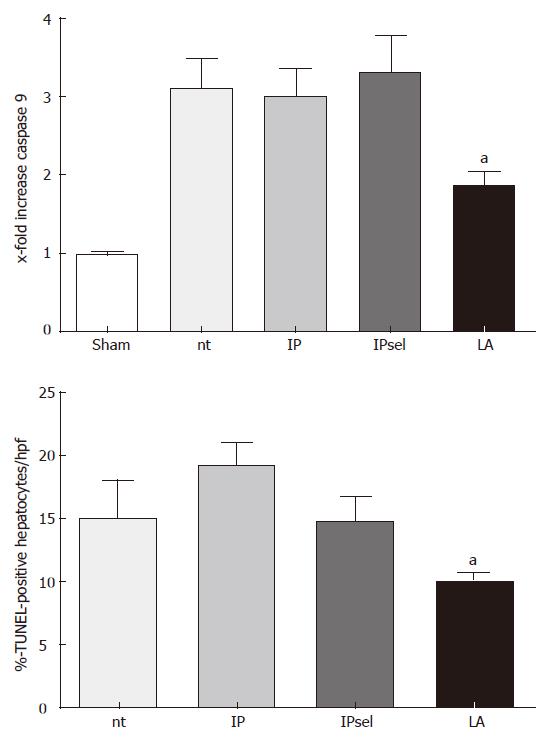
No TUNEL-staining was observed in the livers of sham-operated animals (data not shown). In the untreated, IP, and IPsel groups, the number of TUNEL-positive cells was increased after 90 min of ischemia and 4 h of reperfusion. The LA-treated group showed a lower incidence of TUNEL-positive hepatocytes at the same time point (P < 0.05, Figure 4).
After 4 h of reperfusion following 90 min of ischemia, we found no measureable caspase 3- activity in the untreated group in comparison to the sham operated group (data not shown). Caspase 9- activity was increased in the non-treated group in comparison to the sham-operated group. In contrast, caspase 9- activity was decreased by LA after 4 h of reperfusion in comparison to the untreated group. IP and IPsel did not alter caspase 9- activity compared with the untreated animals.
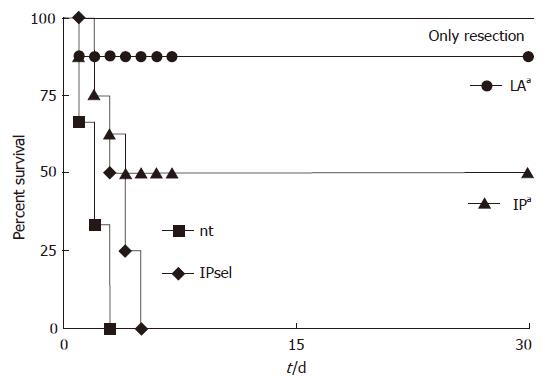
We compared the survival rates of animals from the two IP-treated groups with the non-treated group and the group undergoing only liver resection (Figure 3). All animals with liver resection and without ischemia survived the 30-d monitoring period, whereas all animals of the control group died within the first three days, as did all animals of the IPsel group. In contrast, 4 of 8 animals survived in the IP group (P < 0.05 vs control group). In the LA group, 7 of 8 animals survived (P < 0.05 vs IP group; Figure 5).
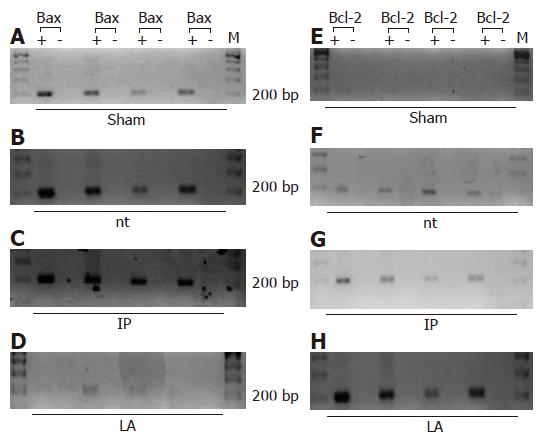
Using RT-PCR, we saw no expression of Bcl-2 mRNA in liver of sham-operated animals, while mRNA of Bax was detectable. In the untreated group, there was expression of Bax and Bcl-2 mRNA after 90 min of ischemia and 4 h of reperfusion (Figure 6). In the IP and IPsel group (data not shown), there was expression of Bax and only weak expression of Bcl-2. After LA-pretreatment, Bcl-2 was strongly detectable at the same time point but mRNA of Bax was absent.
Data for Bax mRNA were confirmed by quantitative real time-PCR analysis showing upregulation of Bax mRNA in the untreated and IP group in comparison to the sham operated animals (2.5 ± 0.1 and 7.6 ± 2.8 vs 1.0 fold increase, P < 0.05). Bax expression was not different in the LA group in comparison to the sham operated animals (1.3 ± 0.1 vs 1.0 fold increase, not significant, n = 4 each group).
Results of the current study demonstrate that IP consisting of 10 min of ischemia and 10 min of reperfusion only partly protects the rat liver against IRI in a model of 90 min of selective ischemia. In contrast, selective IP consisting of 10 min of ischemia and reperfusion each does not attenuate liver cell injury in the same experimental setting. Pretratment with LA, however, protects strongly against IRI of the rat liver.
In general, the effects of IP segregated into early and late phases[3]. The early phase follows ischemia immediately and lasts for 2-4 h. The late phase begins 12-24 h after ischemia and lasts for about 2 d. In our current study, we investigated the early phase of IP with a short term experiment, and we compared animal survival of the groups as pivotal endpoints to study late effects.
In acordance with our previous studies, we observed necrosis and apoptosis-related cell death after 90 min of ischemia and 4 h of reperfusion. Our study suggests different protective mechanisms for IP and LA. The reduction in hepatic injury by IP is associated with attenuation of LPO. In contrast, the protective effect of LA is associated with a reduction in apoptotic and oncotic-related cell death. These latter effects are related to a shift in expression of pro- and anti-apoptic gene products towards the anti-apoptic Bcl-2[19].
The functional roles of Bax and Bcl-2 are believed to involve the mitochondrial membrane. Whereas Bax causes cytochrome c release and apoptotic cell death via activation of caspase 9-, Bcl-2 prevents these changes in mitochondria[20]. We demonstrated a reduction of caspase 9-activity after pretreatment with LA and downregulation of Bax, but neither caspase 9-activity nor Bax or Bcl-2 expression were altered with IP. The results of TUNEL-staining in liver tissue further underlined the attenuation of apoptotic cell death by LA.
The reduction in LPO in liver tissue under pretreatment by IP contrasts with the results of others[21], and we were unable to demonstrate a reduction of necrosis-related cell death, measured by α-GST and the percentage of necrosis in liver tissue, in our model. These differences might be explained by the different durations of reperfusion. In our view, these results demonstrate the protective effects of IP in the early phase of IRI. ROS, mediating LPO, are generated in the early phase of reperfusion[22] and so it seems obvious that IP attenuates generation of ROS. On the other hand, IP increases animal survival in our long term study. In our view, this result may suggest the late phase protection of IP. Based on our results, the question of how IP exerts its late phase protection remains speculative. In long term experiments, it has been demonstrated that hepatoprotective effects of IP are associated with activation of nuclear factor kappa B (NF-κB), p38 MAPK and with the entry of hepatocytes into the cell cycle[23]. We speculate that these phenomena contribute to the observed late phase protection of IP.
We recently demonstrated reduction of hepatocellular injury by LA after 90 min of ischemia and 1 h of reperfusion accompained by downregulation of Bax-mRNA in our RT-PCR[19]. Anti-apoptotic action of LA in hepatocytes is mediated by inactivation of pro-apoptotic Bad[24]. Our current data add important additional information about this concept of LA as a regulator of pro and anti-apoptotic proteins by shifting the expression level towards anti-apoptotic Bcl-2.
In summary, IP consisting of 10 min of ischemia and reperfusion each decreases LPO in liver tissue accompanied by increased animal survival in a model of 90 min of selective hepatic ischemia. IPsel does not improve ischemic tolerance of the liver in our experimental setting. LA is an effective strategy against IRI of the liver and seems to fulfill its protective properties by influencing pro- and anti-apoptotic proteins towards the anti-apoptotic Bcl-2.
Aim of the study was to compare a fairly new method for prevention of ischemia/reperfusion inury (IRI) of the liver (lipoic acid pretreatment, LA) with the established method of ischemic preconditioning (IP).
The article gives important and new information about mechanism of action of hepatic IRI and protective strategies.
We demonstrate advances of lipoic acid in comparison with IP in attenuation of IRI of the liver. Moreover, LA is not time consuming, easy to administer and without serious side effects.
The article supports LA as a new method for prevention hepatic IRI.
The manuscript compared different preconditioning strategies to protect the liver from ischemia/reperfusion injury. It is a clearly written manuscript, with clear goals, sound experiments, and a well-balanced discussion of the discrepancies of the present results with results published previously.
S- Editor Wang J L- Editor Lutze M E- Editor Lu W
| 1. | Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5406] [Cited by in RCA: 5538] [Article Influence: 142.0] [Reference Citation Analysis (0)] |
| 2. | Tsai BM, Wang M, March KL, Turrentine MW, Brown JW, Meldrum DR. Preconditioning: evolution of basic mechanisms to potential therapeutic strategies. Shock. 2004;21:195-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Carini R, Albano E. Recent insights on the mechanisms of liver preconditioning. Gastroenterology. 2003;125:1480-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 135] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Yadav SS, Sindram D, Perry DK, Clavien PA. Ischemic preconditioning protects the mouse liver by inhibition of apoptosis through a caspase-dependent pathway. Hepatology. 1999;30:1223-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 199] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 5. | Glanemann M, Vollmar B, Nussler AK, Schaefer T, Neuhaus P, Menger MD. Ischemic preconditioning protects from hepatic ischemia/reperfusion-injury by preservation of microcirculation and mitochondrial redox-state. J Hepatol. 2003;38:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Schulz R, Walz MK, Behrends M, Neumann T, Gerken G, Heusch G. Minimal protection of the liver by ischemic preconditioning in pigs. Am J Physiol Heart Circ Physiol. 2001;280:H198-H207. [PubMed] |
| 7. | Carini R, De Cesaris MG, Splendore R, Bagnati M, Albano E. Ischemic preconditioning reduces Na(+) accumulation and cell killing in isolated rat hepatocytes exposed to hypoxia. Hepatology. 2000;31:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Rüdiger HA, Graf R, Clavien PA. Sub-lethal oxidative stress triggers the protective effects of ischemic preconditioning in the mouse liver. J Hepatol. 2003;39:972-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Clavien PA, Selzner M, Rüdiger HA, Graf R, Kadry Z, Rousson V, Jochum W. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg. 2003;238:843-850; discussion 851-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 368] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 10. | Clavien PA, Yadav S, Sindram D, Bentley RC. Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg. 2000;232:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 346] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 11. | Lee WY, Lee SM. Ischemic preconditioning protects post-ischemic oxidative damage to mitochondria in rat liver. Shock. 2005;24:370-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Reed JC. Bcl-2 family proteins. Oncogene. 1998;17:3225-3236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 742] [Cited by in RCA: 745] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 13. | Müller C, Dünschede F, Koch E, Vollmar AM, Kiemer AK. Alpha-lipoic acid preconditioning reduces ischemia-reperfusion injury of the rat liver via the PI3-kinase/Akt pathway. Am J Physiol Gastrointest Liver Physiol. 2003;285:G769-G778. [PubMed] |
| 14. | Packer L. alpha-Lipoic acid: a metabolic antioxidant which regulates NF-kappa B signal transduction and protects against oxidative injury. Drug Metab Rev. 1998;30:245-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 159] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | van der Bilt JD, Kranenburg O, Verheem A, van Hillegersberg R, Borel Rinkes IH. Selective portal clamping to minimize hepatic ischaemia-reperfusion damage and avoid accelerated outgrowth of experimental colorectal liver metastases. Br J Surg. 2006;93:1015-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Gurtu V, Kain SR, Zhang G. Fluorometric and colorimetric detection of caspase activity associated with apoptosis. Anal Biochem. 1997;251:98-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 259] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Duenschede F, Westermann S, Riegler N, Miesner I, Erbes K, Ewald P, Kircher A, Schaefer H, Schneider J, Schad A. Different protection mechanisms after pretreatment with glycine or alpha-lipoic acid in a rat model of warm hepatic ischemia. Eur Surg Res. 2006;38:503-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Knapen MF, Peters WH, Mulder TP, Steegers EA. A marker for hepatocellular damage. Lancet. 2000;355:1463-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Duenschede F, Erbes K, Kircher A, Westermann S, Schad A, Riegler N, Ewald P, Dutkowski P, Kiemer AK, Kempski O. Protection from hepatic ischemia/reperfusion injury and improvement of liver regeneration by alpha-lipoic acid. Shock. 2007;27:644-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Zhao Y, Li S, Childs EE, Kuharsky DK, Yin XM. Activation of pro-death Bcl-2 family proteins and mitochondria apoptosis pathway in tumor necrosis factor-alpha-induced liver injury. J Biol Chem. 2001;276:27432-27440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Peralta C, Bulbena O, Xaus C, Prats N, Cutrin JC, Poli G, Gelpi E, Roselló-Catafau J. Ischemic preconditioning: a defense mechanism against the reactive oxygen species generated after hepatic ischemia reperfusion. Transplantation. 2002;73:1203-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:G355-G362. [PubMed] |
| 23. | Teoh N, Dela Pena A, Farrell G. Hepatic ischemic preconditioning in mice is associated with activation of NF-kappaB, p38 kinase, and cell cycle entry. Hepatology. 2002;36:94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Diesel B, Kulhanek-Heinze S, Höltje M, Brandt B, Höltje HD, Vollmar AM, Kiemer AK. Alpha-lipoic acid as a directly binding activator of the insulin receptor: protection from hepatocyte apoptosis. Biochemistry. 2007;46:2146-2155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |