Published online Jul 14, 2007. doi: 10.3748/wjg.v13.i26.3638
Revised: February 2, 2007
Accepted: March 8, 2007
Published online: July 14, 2007
To alert clinicians to a potential novel adverse drug effect of interferonβ 1a, we herein report a patient with relapsing-remitting multiple sclerosis who developed ulcerative colitis following treatment with interferonβ 1a. Ulcerative colitis persisted despite discontinuation of interferonβ 1a treatment and switching the patient to glatiramer acetate. Tacrolimus (FK506), 6-mercaptopurine, and prednisolone were required to induce remission. Both ulcerative colitis and multiple sclerosis were eventually well controlled using this regimen. Our report underscores that caution should be exercised when prescribing immunostimulatory agents in patients with inflammatory bowel disease (IBD) and challenges current efforts to stimulate innate immunity as a novel therapeutic concept for IBD.
- Citation: Schott E, Paul F, Wuerfel JT, Zipp F, Rudolph B, Wiedenmann B, Baumgart DC. Development of ulcerative colitis in a patient with multiple sclerosis following treatment with interferonβ 1a. World J Gastroenterol 2007; 13(26): 3638-3640
- URL: https://www.wjgnet.com/1007-9327/full/v13/i26/3638.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i26.3638
Interferonα has been demonstrated to potently suppress synthesis of both IL-5 and IL-13 in human leucocytes, making it an attractive agent for the treatment of ulcerative colitis (UC). Interferon-α therapy showed no benefit in patients with Crohn's disease[1]. Few cases of aggravation or de novo development of UC with interferons have been reported[2,3]. We herein report a patient with relapsing-remitting multiple sclerosis who developed ulcerative colitis following treatment with interferon ®1a, aiming to alert clinicians to a potential novel adverse drug effect of interferon ®1a.
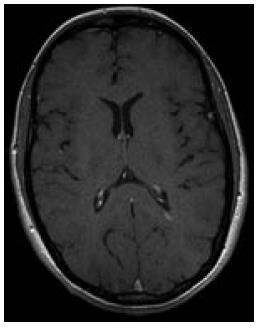
A 29-year-old Caucasian female noticed visual blurring that persisted for one week and then resolved spontaneously. Thirteen months later, paresthesia and weakness of the left arm developed. Diagnosis of relapsing-remitting multiple sclerosis (MS) was established using standard diagnostic criteria (Figure 1)[4].
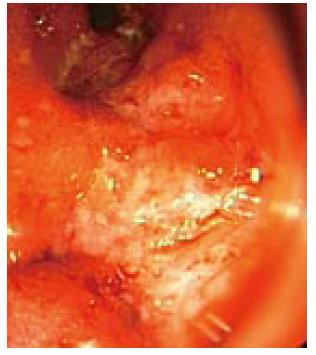
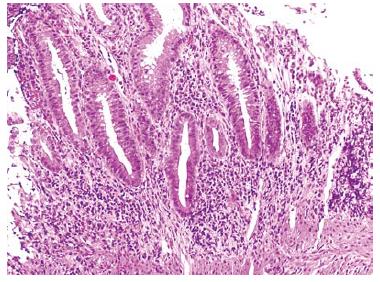
Therapy with interferon®1a (Avonex®) was initiated at a dose of 30 μg (6 million international units, im qw). Twelve months into treatment with interferon®1a, the patient noticed bloody diarrhea, cramping and abdominal pain. She was referred for colonoscopy (Figures 2 and 3) and subsequently diagnosed with ulcerative colitis (pancolitis). She experienced a short-term improvement on 5-amino-salicyclic acid (5-ASA) 1000 mg po tid, but later required intravenous steroids for another flare-up.
Interferon®1a was discontinued, and she was switched to glatiramer acetate (20 mg sq qd). When the steroids were tapered, her symptoms worsened again and she was referred to our Inflammatory Bowel Disease Center. Since her symptoms did not improve on intravenous prednisolone, her medication regimen was supplemented with low-dose oral tacrolimus at a dose of 0.1 mg/kg body weight aiming for serum trough levels of 4 to 8 ng/mL and 6-mercaptopurine at a dose of 75 mg po qd, which she tolerated well. Treatment with glatiramer acetate was paused after tacrolimus was started.
Her symptoms gradually improved. The steroids were successfully tapered. To date, both her MS and ulcerative colitis are well controlled on 6-mercaptopurine and tacrolimus. Tacrolimus is currently being tapered.
Patients with inflammatory bowel disease (IBD) have an increased risk of demyelinating diseases, but in our patient multiple sclerosis (MS) preceded the development of ulcerative colitis (UC) by approximately two years[5,6]. TypeIinterferons are immunostimulatory agents and development of autoimmune diseases during treatment with interferons are common.
Few cases of aggravation or de novo development of UC with interferons have been reported[2,3]. All but one, where a renal cancer patient received alpha and gamma interferon, occurred in patients treated with pegylated interferons for chronic HCV infection. In our patient, no signs of IBD were present before the initiation of interferon®1a treatment. A treatment course of 10 mo prior to the occurrence of the first symptoms of UC suggests de novo development of IBD as a consequence of long-term immunostimulatory treatment with interferonβ1a.
Importantly, colitis activity did not decrease after discontinuation of interferon®1a therapy, suggesting triggering of a persistent specific autoimmune response by interferon β1a as the underlying mechanism.
After discontinuation of interferon®1a, therapy with glatiramer acetate was initiated. Glatiramer acetate is a synthetic copolymer mimicking a portion of myelin basic protein, one of several putative autoantigens in MS; however, mechanism of its action is not fully understood. In a mouse model of experimental colitis, it ameliorated colitis symptoms and suppressed the production of TNF-α, a key mediator of inflammation in both MS and IBD[7,8]. However, it failed to demonstrate any additional benefit for the induction of remission in our patient.
Recent data from our group and others suggest that tacrolimus is an effective treatment option for refractory UC[6,9,10]. Circumstantial and experimental evidence suggests that patients with MS may benefit from tacrolimus[11]. In a murine model of MS, experimental autoimmune encephalomyelitis (EAE), tacrolimus significantly decreased the amount of damage and ameliorated the disease course[12].
The failure of the innate immune system to maintain mucosal integrity has recently received great attention as a possible cause of IBD[13]. Proponents of this theory argue that T-cell responses in patients with IBD are secondary, rather than primary events in the pathogenesis of IBD. Therefore, some investigators suggested that the enhancement rather than the suppression of the innate immune system would result in an alleviation of symptoms of IBD. Pilot studies with filgrastim (granulocyte colony-stimulating factor) and sargramostim (granulocyte-macrophage colony-stimulating factor) in patients with Crohn's disease demonstrated clinical benefit[14]. However, several placebo-controlled follow-up trials of sargramostim in patients with active Crohn's disease failed to achieve their primary endpoint[15,16]. Likewise, controlled studies with pegylated interferon α2a and interferon ®1a in patients with active UC failed to demonstrate clinical benefit[17,18].
In addition to the lack of efficacy in patients with IBD, immunostimulatory regimens may be potentially harmful and therefore put patients at risk to aggravate or induce other autoimmune processes.
S- Editor Liu Y L- Editor Kumar M E- Editor Wang HF
| 1. | Gasché C, Reinisch W, Vogelsang H, Pötzi R, Markis E, Micksche M, Wirth HP, Gangl A, Lochs H. Prospective evaluation of interferon-alpha in treatment of chronic active Crohn's disease. Dig Dis Sci. 1995;40:800-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Watanabe T, Inoue M, Harada K, Homma N, Uchida M, Ogata N, Funada R, Hasegawa K, Soga K, Shibasaki K. A case of exacerbation of ulcerative colitis induced by combination therapy with PEG-interferon alpha-2b and ribavirin. Gut. 2006;55:1682-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Sprenger R, Sagmeister M, Offner F. Acute ulcerative colitis during successful interferon/ribavirin treatment for chronic hepatitis. Gut. 2005;54:438-439; author reply 439. [PubMed] |
| 4. | McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, McFarland HF, Paty DW, Polman CH, Reingold SC. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4792] [Cited by in RCA: 4776] [Article Influence: 199.0] [Reference Citation Analysis (0)] |
| 5. | Gupta G, Gelfand JM, Lewis JD. Increased risk for demyelinating diseases in patients with inflammatory bowel disease. Gastroenterology. 2005;129:819-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 199] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 6. | Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1247] [Cited by in RCA: 1357] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 7. | Aharoni R, Eilam R, Domev H, Labunskay G, Sela M, Arnon R. The immunomodulator glatiramer acetate augments the expression of neurotrophic factors in brains of experimental autoimmune encephalomyelitis mice. Proc Natl Acad Sci USA. 2005;102:19045-19050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 167] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1299] [Cited by in RCA: 1509] [Article Influence: 83.8] [Reference Citation Analysis (2)] |
| 9. | Baumgart DC, Pintoffl JP, Sturm A, Wiedenmann B, Dignass AU. Tacrolimus is safe and effective in patients with severe steroid-refractory or steroid-dependent inflammatory bowel disease--a long-term follow-up. Am J Gastroenterol. 2006;101:1048-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Ogata H, Matsui T, Nakamura M, Iida M, Takazoe M, Suzuki Y, Hibi T. A randomised dose finding study of oral tacrolimus (FK506) therapy in refractory ulcerative colitis. Gut. 2006;55:1255-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 329] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 11. | Yoshida EM, Devonshire VA, Prout AJ. Remission of multiple sclerosis post-liver transplantation. Can J Neurol Sci. 2004;31:539-541. [PubMed] |
| 12. | Gold BG, Voda J, Yu X, McKeon G, Bourdette DN. FK506 and a nonimmunosuppressant derivative reduce axonal and myelin damage in experimental autoimmune encephalomyelitis: neuroimmunophilin ligand-mediated neuroprotection in a model of multiple sclerosis. J Neurosci Res. 2004;77:367-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Marks DJ, Harbord MW, MacAllister R, Rahman FZ, Young J, Al-Lazikani B, Lees W, Novelli M, Bloom S, Segal AW. Defective acute inflammation in Crohn's disease: a clinical investigation. Lancet. 2006;367:668-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 287] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 14. | Korzenik JR, Dieckgraefe BK. An open-labelled study of granulocyte colony-stimulating factor in the treatment of active Crohn's disease. Aliment Pharmacol Ther. 2005;21:391-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Korzenik JR, Dieckgraefe BK, Valentine JF, Hausman DF, Gilbert MJ. Sargramostim for active Crohn's disease. N Engl J Med. 2005;352:2193-2201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 247] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 16. | Schering AG. Germany announces results of two clincial trials with sargramostim in Crohn's disease patients. Schering AG, Berlin, Germany. July 31st 2006. Available from: http: //www.schering.de/html/en/50_media/download/_files/2006/pi/060731_Leukine_en.pdf.. |
| 17. | Musch E, Andus T, Kruis W, Raedler A, Spehlmann M, Schreiber S, Krakamp B, Malek M, Malchow H, Zavada F. Interferon-beta-1a for the treatment of steroid-refractory ulcerative colitis: a randomized, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2005;3:581-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Tilg H, Vogelsang H, Ludwiczek O, Lochs H, Kaser A, Colombel JF, Ulmer H, Rutgeerts P, Krüger S, Cortot A. A randomised placebo controlled trial of pegylated interferon alpha in active ulcerative colitis. Gut. 2003;52:1728-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |