Published online Jul 14, 2007. doi: 10.3748/wjg.v13.i26.3625
Revised: March 29, 2007
Accepted: April 16, 2007
Published online: July 14, 2007
AIM: To explore the mechanism of intra-uterine transmission, the HBV infection status of placental tissue and in vitro cultured placental trophoblastic cells was tested through in vivo and in vitro experiments.
METHODS: A variety of methods, such as ELISA, RT-PCR, IHC staining and immunofluorescent staining were employed to test the HBV marker positive pregnant women's placenta and in vitro cultured placental trophoblastic cells.
RESULTS: The HBV DNA levels in pregnant women's serum and fetal cord blood were correlated. For those cord blood samples positive for HBV DNA, their maternal blood levels of HBV DNA were at a high level. The HBsAg IHC staining positive cells could be seen in the placental tissues and the presence of HBV DNA detected. After co-incubating the trophoblastic cells and HBV DNA positive serum in vitro, the expressions of both HBsAg and HBV DNA could be detected.
CONCLUSION: The mechanism of HBV intra-uterine infection may be due to that HBV breaches the placental barrier and infects the fetus.
- Citation: Bai H, Zhang L, Ma L, Dou XG, Feng GH, Zhao GZ. Relationship of hepatitis B virus infection of placental barrier and hepatitis B virus intra-uterine transmission mechanism. World J Gastroenterol 2007; 13(26): 3625-3630
- URL: https://www.wjgnet.com/1007-9327/full/v13/i26/3625.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i26.3625
The maternal-fetal transmission of hepatitis B virus (HBV) remains one of the important causes of chronic HBV infection in China[1,2]. At present, the combined immunity of hepatitis B vaccine and high-titer hepatitis B immunoglobulin (HBIG) has already achieved relatively excellent efficacies in blocking the maternal-fetal transmission of HBV[3-6]. However, still among around 10% of the mothers with positive HBV serum marker, the immunological blockade failed in the born neonates who later became chronically infected with HBV[7,8]. It is currently thought that the major cause of unsuccessful immunological blockade is the intra-uterine infection of HBV. The mechanism of HBV intra-uterine infection remains unclear at present. But, during the process of intra-uterine transmission, the placental tissue plays an important role. The HBV DNA in maternal body needs to trespass the placental barrier to infect the fetus. During pregnancy, the direct contact of trophoblast cells with the maternal blood is the first step of HBV passing through the placental barrier[9-11]. Therefore the studies of HBV infections of placental tissue and placental trophoblast cells are of great significance for elucidating the mechanism of HBV intra-uterine transmission.
A total of 20 cases of HBV-infected pregnant women during normal delivery for the period of October 2002 to December 2004 on the ward of Department of OB & GYN at the Shengjing Hospital Affiliated to CMU were selected. The age range of pregnant women was 23-38 years old. 20 cases of neonate were delivered normally. After birth, the fetuses (9 males and 11 females) were all healthy. They received HB vaccine and anti-hepatitis B immunoglobulin at birth and 1 mo after birth, and HB vaccine 6 mo after birth. Follow-up visit of HBV serum markers in the neonates were at 6 mo after birth. None of 20 pregnant women received anti-viral treatment. At admission, the LFT examination was normal with no symptom of hepatitis. The serologic HBV testing of their spouses was all negative. The peripheral venous blood and fetal cord blood were retained to isolate the serum and stored in a -20°C freezer. The appearance of their placenta was normal, with intact fetal membranes and no evidence of abruption. The surgical scalpel was used to slice the placental tissue into the blocks of size around 1.0 cm3. Formalin fixation was performed. 5 pregnant women with normal delivery were taken as the negative control. Patient permission was sought to retain the samples of placental tissue.
The ELISA method was used to perform the serological testing of HBV marker. The experimental methods followed those specified within the reagent kit (Shanghai Kehua Biotech Co., Ltd) package insert.
Real-time fluorescence quantitative PCR was used to test the HBV DNA level in serum samples: The handling procedures were performed in strict accordance with the reagent kit (Shenzhen PG Biotech Co., Ltd.) package insert. The primer was provided in the kit, the reaction volume was 40 μL, and the reaction condition was 37°C for 5 min, 94°C for 1 min then 40 cycles as 95°C for 5 s and 60°C for 30 s. The test was repeated twice to confirm the results.
The routine ABC method was adopted for staining. The handling methods specified within the reagent kit (ZYMED Co., Ltd. USA) package insert were followed and the experimental results considered to properly adjusting the incubation time. Visualization was achieved through DAB. The pregnant women's placenta with negative HBV serum marker was taken as the negative control while the tissue samples from liver puncture of chronic hepatitis B patients as the positive control.
The digestive lysis method of proteinase K was used to extract the tissue DNA[12] and the method of real-time fluorescence quantitative PCR(reagent kit was manufactured by Shenzhen PG Biotech Co., Ltd.)for testing. The test was repeated twice to confirm the results.
The product of tissue section and HE staining were produced by department of pathology of shengjing hospital affiliated to china medical university.
Six months post-birth the newborns were examined for HBV markers in the serum by ELISA.
Primary generation culture of human early-pregnancy villous membrane trophoblast cells[9]: early-pregnancy placental villus was digested by the combined trypsin/DNAase method to harvest the single cells. The trypsin was produced by Sigma Co., Ltd. and DNAase by Worthington Co., Ltd. USA. The trophoblast cells were separated and purified by Percoll (Pharmacia Co., Ltd.) density gradient centrifugation, and rat tail collagen (Sigma Co., Ltd.) was employed to promote the cellular wall-adsorption. The primary generation culture was performed. The in vitro culture model of trophoblast cells was established.
Propagation culture of trophoblast cell line JEG-3: The trophoblast cell line JEG-3 (generous gift of Prof. Wang Yanling, Institute of Zoology, CAS)was propagated in DMEM/F12 medium (Hyclone Co., Ltd.) with 10% FCS ( Hyclone Co., Ltd.) until the cells stayed within the logarithmic growth period for experiments. Prior to inoculation, the slide covers were pre-placed into the 6-hole culture plate (Costar Co., Ltd.).
The serum samples from HBV carriers (normal LFT) with a high level of HBV DNA (> 1.0 × 108 copies/mL) and those from the healthy volunteers with a negative serum HBV marker were collected. A 0.22 μm filtration device (Costar Co., Ltd.) was used to filter away the bacteria. At 56°C for 30 min, the complement was inactivated and stored aseptically.
DMEM/F12 (1:1) serum-free cell culture medium (Hyclone Co., Ltd.) containing 20% HBV positive serum was added into the primary cultured cells and JEG-3 cells. For the control group, the HBV negative control serum was added. The cells were taken out after co-incubating with HBV positive serum for 8, 24 and 48 h in the cell incubator at 37°C with 5% CO2. After washing and fixing, the samples were frozen and stored for later testing.
The frozen cell slides were taken out and blocked with 10% bovine serum albumin (BSA) (Sigma Co., Ltd.). After addition of 1:50 HBsAg monoclonal antibody (Beijing Zhongshan Co., Ltd.) diluted with 1% BSA, the samples were stored at 4°C overnight. The FITC-tagged secondary antibody (Beijing Zhongshan Co., Ltd.) was added and the staining results observed under the fluorescent microscope (Nikon, Japan).
The routine ABC method was adopted for staining. The handling methods specified within the reagent kit (Beijing Zhongshan Co., Ltd.) package insert were followed. HBcAg monoclonal antibody (Beijing Zhongshan Co., Ltd.) was used as first antibody. Visualization was achieved through DAB.
The testing method of HBV DNA in trophoblast cells was identical to that for HBV DNA in tissue.
The experiment data were expressed as mean ± SD. And the software of SPSS for Windows 10.0 was employed to perform statistic analysis of the data. The t-test, Spearman's rank correlation test and Wilcoxon's rank sum test for 2-sample geometric mean comparison were used for statistic analysis. The LSD method was used for inter-group paired comparison and the ANOVA method for single factor analysis. P < 0.05 was limit of significant difference.
The serum HBV DNA in 20 mothers were all positive. The HBV DNA was positive in 6 samples of fetal cord blood while the HBV DNA level of fetal maternal blood in these 6 cases was all above 1.0 × 107 copy/mL. The positive rate of HBV DNA in cord blood was 30%. And the HBV DNA level in fetal cord blood was markedly lower than that in maternal blood.
The authors compared the relationship of maternal blood HBV DNA level and cord blood HBV DNA level. The results are shown in Table 1.
| n | Maternal serum HBV DNA level | ||
| Xln | sln | ||
| Cord blood HBV DNA positive | 6 | 19.2556a | 1.5614 |
| Cord blood HBV DNA negative | 14 | 16.0952 | 2.8454 |
The results have indicated that the cord blood HBV DNA level was closely correlated with the maternal blood HBV DNA level. For the fetus with positive cord blood HBV DNA, the maternal serum HBV DNA was at a higher level. As compared with those with negative cord blood HBV DNA, there was significant difference.
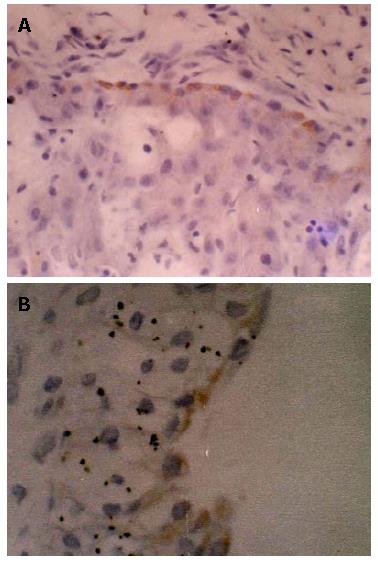
IHC staining of HBsAg and HBcAg in placental tissue of 20 pregnant women was carried out. The results showed that there were 6 cases of positive HBsAg IHC staining in placental tissue of pregnant women. No positive HBcAg IHC staining in placental tissue was found. Among the pregnant women with positive staining of placental tissue, 5 cases of cord blood were tested positive for HBV DNA. Among them, 1 case of cord blood was tested negative for HBV DNA while another 1 case positive for cord blood HBV DNA and no HBsAg positive cell was observed in placental IHC staining. The HBsAg positive staining cell was most commonly seen at the villous surface of trophoblast cells in placental tissue and also among the villous interstitial cells. Within one case, HBsAg positive staining was seen within the endothelial cells of villous capillaries in pregnant women's placental villus. For control group, there was no positive staining observed (Figure 1).
Five cases of placental tissue in 6 fetuses with positive cord blood were tested positive for HBV DNA. The level of HBV DNA was as low as 5.0 × 102-3.0 × 103 copy/mL.
After 6 mo, 19 neonates received follow-ups. Among which, 2 cases tested positive for serum HBsAg, HBeAg and anti-HBc. Both these two cases were positive for HBV DNA in cord blood and placental tissue. The remaining 17 cases were HBsAg negative. Ten cases were anti-HBs positive.
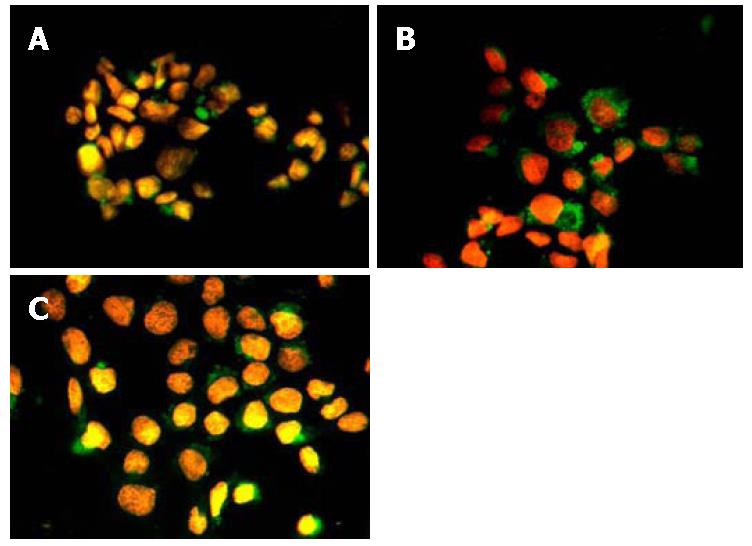
After co-incubating with HBV-infected serum, the trophoblast cells might show positive signal of HBsAg by immunofluorescence staining. At 8 h of co-incubating, the positive signal was weaker and the number of infected cells was fewer. While at 24 h of co-incubating, the positive signal became stronger and the number of infected cells showed a rising trend. Afterward when at 48 h of co-incubating, the number of HBsAg positive cells in trophoblast cells had no marked increase (Figure 2). Through numeration, the number of HBsAg positive cells for every 100 cells at each time point was recorded (500 cells counted for each glass slide). The number of HBsAg positive trophoblast cells at different time points was compared[10] (n = 5). The results are summarized in Table 2. IHC staining also showed that the positive expression of HBcAg could be seen within the nucleus of trophoblast cells at 24 h and 48 h of co-incubating with HBV-infected serum. In the negative control group, no HBsAg or HBcAg positive cell was seen.
| Co-incubation time | n | Numbers of HBsAg positive cells (per 100 cells) |
| 8 h | 5 | 18.93 ± 0.83 |
| 24 h | 5 | 30.87 ± 0.83a |
| 48 h | 5 | 25.80 ± 1.11 |
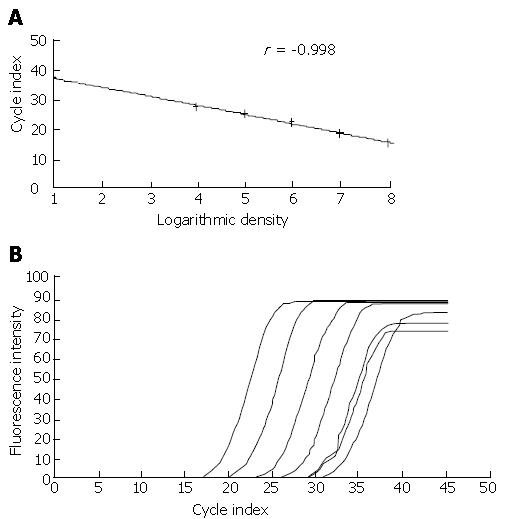
The fluorescence quantitative PCR test results showed that, after co-incubating with HBV-infected serum for 8 h, 24 h and 48 h, the presence of HBV DNA could be detected in primary generation cultured trophoblast cells and trophoblast cell line JEG-3 cell (Figure 3).
In clinical practice, the tests of HBsAg and HBV DNA in fetal cord blood or neonatal peripheral blood are often performed to diagnose intra-uterine HBV infection[15]. The authors carried out the test of HBV DNA in cord blood and placental tissue of fetuses born of 20 pregnant women. As a result, among 6 cases of fetal cord blood positive for HBV DNA, 5 cases of placental tissue were also positive for HBV DNA. At 6 mo post-birth, the follow-up results indicated that, among 6 neonate cases of positive cord blood, only 2 cases were still positive for serum HBsAg. The remaining 4 neonate cases were all negative for HBsAg and 3 cases produced anti-HBs. In mothers with cord blood and placental tissue positive for HBV DNA, the serum HBV DNA was all at a high level. It illustrates that the high level of serum HBV DNA in pregnant women is one of the high-risk factors for occurrence of HBV intra-uterine infection[16,17]. But the positive cord blood of HBV DNA does not indicate that HBV intra-uterine infection occurs in fetus. After fetal birth, despite the combined blockage of hepatitis B vaccine and HBIG, over one half of neonates can still acquire successful immunity. Since the positive rate of HBV DNA in cord blood is obviously correlated with the HBV DNA level of maternal blood and cord blood HBV DNA is always positive in cases of intra-uterine infection, it illustrates that the positive HBV DNA of cord blood is one of the high-risk factors of occurrence of intra-uterine infection in fetus[18]. For the relative ease of collecting the cord blood in clinical setting, so the test of HBV DNA in cord blood may be used as one of the predicative parameters for occurrence of intra-uterine infection.
To further explore the mechanism of HBV intra-uterine infection, the authors proved through two parts of clinical trial and in vitro experiment that HBV enters the fetal body through infecting the placental barrier. Within the clinical trial part, the IHC method was employed to test the placental tissue of HBsAg positive pregnant women. The results showed that the HBsAg positive cell could be seen in all the cell layers of the placental tissue. The expression of HBsAg was at a low level in the placental tissue, and HBV DNA was detected in the placenta of the pregnant women that were positive for HBV DNA in cord blood. It illustrates that HBV could infect the placental cell[19]. The HBV infection of placenta was markedly correlated with the HBV DNA in cord blood. Among 6 cases of pregnant women with placental HBV infection, 5 cases were tested positive for HBV DNA in cord blood. Another case was tested positive for HBV DNA in cord blood while the IHC staining of the placental tissue was negative. At follow-up 6 mo post-birth, the neonates didn't have occurrence of HBV infection, and anti-HBs were positive. It did not rule out the possibility of the cord blood being contaminated by the maternal blood. And for the cases of positive staining of the placental tissue and negative HBV DNA in cord blood, there was no occurrence of HBV infection in neonates even at the post-birth follow-up. It illustrates that the HBV DNA in cord blood may be acquired through the placental infection[20].
To further prove the hypothesis that HBV intra-uterine infection occurs through the infection of placental barrier, the authors carried out the experiment of HBV infecting the in vitro cultured trophoblast cells of human villous membrane. Based upon the literature reports[21-23] and our studies, both the primary cultured cell and human trophoblast cell line JEG-3 could be infected with HBV. Therefore the authors introduced the primary cultured cell and trophoblast cell line JEG-3 into the experiment and established it as the study model of placental barrier. The HBV DNA positive serum was added into the cell culture medium to simulate the in vivo situation of the trophoblast cells being immersed in the maternal blood. Both cell IHC staining and cellular immunofluorescent staining showed that, after co-incubating with HBV-infected serum for 8 h, 24 h and 48 h, the expression of HBsAg could be consistently detected in the primary generation cultured trophoblast cells and JEG-3 cell. The HBsAg positive cell was distributed diffusely in the trophoblast cells. At 24 h and 48 h post-infection, the expression of HBcAg was also detected in the trophoblast cells. When the test of HBV DNA was performed upon the trophoblast cells co-incubated with the HBV positive serum, the results showed that HBV DNA could be detected in the trophoblast cells. This further proved that HBV could infect the trophoblast cells. However, as found by the authors in the experiment, with the elapsing of time post-infection, the number of infected cells showed no obvious increase. It illustrates that other potential mechanisms may influence the HBV infection of the trophoblast cells[24-26]. Further studies will be needed.
The HE staining of the placental tissue slides revealed no marked cellular lesion and damage of intercellular conjunction. As observed under the inverted microscope, after the addition into the HBV-infected serum, there was no marked change of growth speed of trophoblast cells, cellular structure and intercellular tight junction. It illustrates that the intra-uterine transmission of HBV may be not related with the breached integrity of placental barrier or its increased permeability[27].
It was reported that HBV uptake by trophoblasts is increased if the HBsAg is complexed with anti-HBs[28,29]. The serologic profile of the mothers was detected before delivery. We didn't found the conditions that both HBsAg and anti-HBs were positive in serum of the mothers. So whether the 6 infected newborns in this study were associated with HBsAg and anti-HBs complex couldn't be proved in this study.
As concluded from the above, through the clinical cases and in vitro infection experiment of trophoblast cells, the authors prove that the fetal mechanism of HBV intra-uterine infection occurs when the placental barrier becomes infected.
S- Editor Liu Y L- Editor Alpini GD E- Editor Wang HF
| 1. | Bai H, Zhao GZ. Progresses in the study on the mechanism of HBV transmission from mother to infant. Guowai Yixue Liuxingbingxue Chuanranbingxue Fence. 2005;32:99-102. |
| 2. | Michielsen PP, Van Damme P. Viral hepatitis and pregnancy. Acta Gastroenterol Belg. 1999;62:21-29. [PubMed] |
| 3. | van der Sande MA, Waight P, Mendy M, Rayco-Solon P, Hutt P, Fulford T, Doherty C, McConkey SJ, Jeffries D, Hall AJ. Long-term protection against carriage of hepatitis B virus after infant vaccination. J Infect Dis. 2006;193:1528-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Kripke C. Hepatitis B vaccine for infants of HBsAg-positive mothers. Am Fam Physician. 2007;75:49-50. [PubMed] |
| 5. | Xiao XM, Li AZ, Chen X, Zhu YK, Miao J. Prevention of vertical hepatitis B transmission by hepatitis B immunoglobulin in the third trimester of pregnancy. Int J Gynaecol Obstet. 2007;96:167-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Yue Y, Yang X, Zhang S. Prevention of intrauterine infection by hepatitis B virus with hepatitis B immune globulin: efficacy and mechanism. Chin Med J (Engl). 1999;112:37-39. [PubMed] |
| 7. | Wang JS, Chen H, Zhu QR. Transformation of hepatitis B serologic markers in babies born to hepatitis B surface antigen positive mothers. World J Gastroenterol. 2005;11:3582-3585. [PubMed] |
| 8. | Wang Z, Zhang J, Yang H, Li X, Wen S, Guo Y, Sun J, Hou J. Quantitative analysis of HBV DNA level and HBeAg titer in hepatitis B surface antigen positive mothers and their babies: HBeAg passage through the placenta and the rate of decay in babies. J Med Virol. 2003;71:360-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Bhat P, Anderson DA. Hepatitis B virus translocates across a trophoblastic barrier. J Virol. 2007;81:7200-7207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Wang XP, Li FJ, Xu DZ, Yan YP, Men K, Zhang JX. Uptake of hepatitis B virus into choriocarcinoma cells in the presence of proinflammatory cytokine tumor necrosis factor-alpha. Am J Obstet Gynecol. 2004;191:1971-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Xu DZ, Yan YP, Zou S, Choi BC, Wang S, Liu P, Bai G, Wang X, Shi M, Wang X. Role of placental tissues in the intrauterine transmission of hepatitis B virus. Am J Obstet Gynecol. 2001;185:981-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Ausubel F. Short protocols in molecular biology. 4th ed. New York: John Wiley & Sons, Inc. Publishers 2000; 687-693. |
| 13. | Bai h, He LX, Ma L, Li Y, Zhao GZ. Establishment of the model in vitro culture of trophoblastic cells of human placenta of first-trimester. Zhongguo Zuzhi Huaxue yu Xibao Huaxue Zazhi. 2006;15:319-322. |
| 14. | Fujino T, Iwamoto I, Otsuka H, Ikeda T, Takesako S, Nagata Y. Apoptosis in placentas from human T-lymphotropic virus type I-seropositive pregnant women: a possible defense mechanism against transmission from mother to fetus. Obstet Gynecol. 1999;94:279-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Liu Y, Kuang J, Zhang R, Lin S, Ding H, Liu X. Analysis about clinical data of intrauterine infection of hepatitis B virus. Zhonghua Fuchanke Zazhi. 2002;37:465-468. |
| 16. | Shao ZJ, Xu DZ, Xu JQ, Li JH, Yan YP, Men K, Wang XP, Zhang ZY, Jiang QW, Zhang L. Maternal hepatitis B virus (HBV) DNA positivity and sexual intercourse are associated with HBV intrauterine transmission in China: a prospective case-control study. J Gastroenterol Hepatol. 2007;22:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Yin YZ, Chen XW, Li XM, Hou HY, Shi ZJ. Intrauterine HBV infection: risk factors and impact of HBV DNA. NanFang YiKe DaXue XueBao. 2006;26:1452-1454. [PubMed] |
| 18. | Best JM. Laboratory diagnosis of intrauterine and perinatal virus infections. Clin Diagn Virol. 1996;5:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Chang WH, Xu DZ, Yan YP, Du KJ, Men K, Zhang JX, Wang JJ, Xu JQ, Zhang ED, Liu C, Sun FM. Study on the presence of hepatitis B virus in first-trimester villi in pregnant women with hepatitis B surface antigen positive. Zhonghua FuChanKe ZaZhi. 2005;40:376-379. [PubMed] |
| 20. | McDonagh S, Maidji E, Chang HT, Pereira L. Patterns of human cytomegalovirus infection in term placentas: a preliminary analysis. J Clin Virol. 2006;35:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Arias RA, Muñoz LD, Muñoz-Fernández MA. Transmission of HIV-1 infection between trophoblast placental cells and T-cells take place via an LFA-1-mediated cell to cell contact. Virology. 2003;307:266-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Vidricaire G, Tardif MR, Tremblay MJ. The low viral production in trophoblastic cells is due to a high endocytic internalization of the human immunodeficiency virus type 1 and can be overcome by the pro-inflammatory cytokines tumor necrosis factor-alpha and interleukin-1. J Biol Chem. 2003;278:15832-15841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Lagaye S, Derrien M, Menu E, Coïto C, Tresoldi E, Mauclère P, Scarlatti G, Chaouat G, Barré-Sinoussi F, Bomsel M. Cell-to-cell contact results in a selective translocation of maternal human immunodeficiency virus type 1 quasispecies across a trophoblastic barrier by both transcytosis and infection. J Virol. 2001;75:4780-4791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Moussa M, Roques P, Fievet N, Menu E, Maldonado-Estrada JG, Brunerie J, Frydman R, Fritel X, Herve F, Chaouat G. Placental cytokine and chemokine production in HIV-1-infected women: trophoblast cells show a different pattern compared to cells from HIV-negative women. Clin Exp Immunol. 2001;125:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Casper C, Fenyö EM. Mother-to-child transmission of HIV-1: the role of HIV-1 variability and the placental barrier. Acta Microbiol Immunol Hung. 2001;48:545-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Jordan JA, Butchko AR. Apoptotic activity in villous trophoblast cells during B19 infection correlates with clinical outcome: assessment by the caspase-related M30 Cytodeath antibody. Placenta. 2002;23:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Burton GJ, Watson AL. The Structure of the Human Placenta: Implications for Initiating and Defending Against Virus Infections. Rev Med Virol. 1997;7:219-228. [PubMed] |
| 28. | Maidji E, McDonagh S, Genbacev O, Tabata T, Pereira L. Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor-mediated transcytosis. Am J Pathol. 2006;168:1210-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 189] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 29. | Soilleux EJ, Morris LS, Lee B, Pöhlmann S, Trowsdale J, Doms RW, Coleman N. Placental expression of DC-SIGN may mediate intrauterine vertical transmission of HIV. J Pathol. 2001;195:586-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 105] [Article Influence: 4.4] [Reference Citation Analysis (0)] |