Published online Jul 14, 2007. doi: 10.3748/wjg.v13.i26.3592
Revised: March 14, 2007
Accepted: April 11, 2007
Published online: July 14, 2007
AIM: To explore the mechanism of intestinal endo-toxemia (IETM) formation and its changes in partially hepatectomized (PH) rats.
METHODS: One-hundred and two adult male Wistar rats were randomly divided into three groups: normal control (NC) group, partially hepatectomized (PH) group and a sham-operated (SO) group. To study the dynamic changes, rats were sacrificed before and at different time points after partial hepatectomy or the sham-operation ( 6 h, 12 h, 24 h, 36 h, 48 h, 72 h, 120 h and 168 h). NC group was used as 0h time point in observation, namely 0 h group. For each time point indicated, six rats were used in parallel. Endotoxin (ET) and diamine oxidase (DAO) levels were determined in serum using Limulus Lysate test with chromogenic substrate and spectrophotometry. Intestinal mucosa barrier was observed under optical or electron microscope. The number and functional state of Kupffer cells (KCs) in the remnant regenerating liver were measured by immunohistochemical staining.
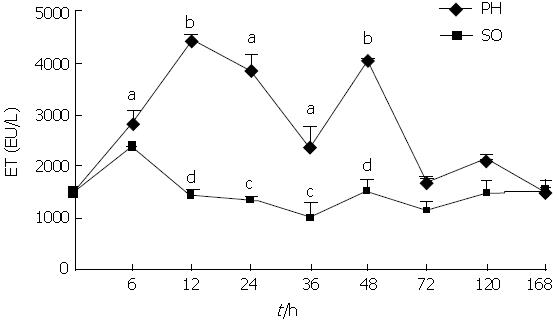
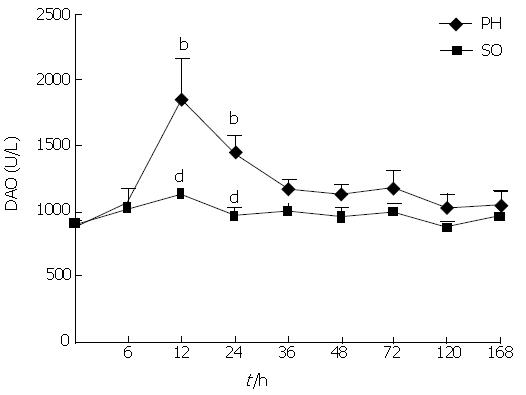
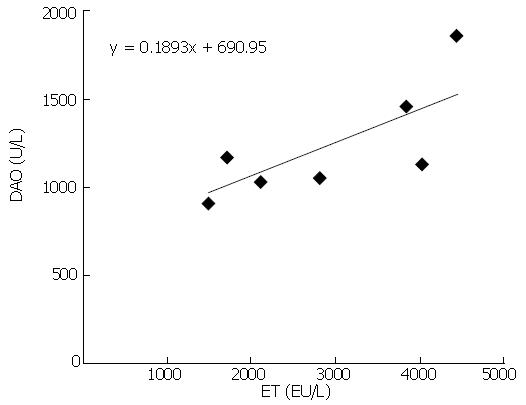
RESULTS: Serum ET levels significantly increased during 6-72 h period after PH compared with NC and SO groups, and there were two peak values at 12 and 48 h while serum DAO level significantly increased at 12 and 24 h. There was positive correlation (r = 0.757, P < 0.05) between the levels of DAO and ET dynamic changes. The optical examination showed neutrophil margination and superficial necrosis of the villi in the intestinal mucosa during 6-24 h period after PH. The penetrated electron microscope examination showed that the gaps between intestinal mucosa cells were increased and the Lanthanum (La) particles were observed among the intestinal mucosa cells during 6-48 h period. The numbers of KCs in the remnant regenerating liver were significantly increased during 24-168 h period after PH. However, the activation of KCs was predominantly observed at 48 h after PH.
CONCLUSION: The mechanism of IETM in PH rats might be the injury of intestinal mucosa barrier and the decrease of the absolute number of KCs as well as the depression of functional state of KCs. This observation is of potential value in patients undergoing liver resection.
- Citation: Xu CP, Liu J, Liu JC, Han DW, Zhang Y, Zhao YC. Dynamic changes and mechanism of intestinal endotoxemia in partially hepatectomized rats. World J Gastroenterol 2007; 13(26): 3592-3597
- URL: https://www.wjgnet.com/1007-9327/full/v13/i26/3592.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i26.3592
Gut is a vast pool of bacteria and endotoxin (ET) in the body. It has been verified that during periods of severe trauma or large operation, the intestinal mucosal barrier is damaged, and therefore the intestinal permeability increased from which bacteria and ET can easily transfer through the intestinal mucosa into portal vein and invade tissue and blood[1-3]. Endotoxemia will be generated if the level of portal vein ET surpasses the hepatic capacity for ET scavenging due to decreased phagocytic ability of liver Kupffer cells (KCs) and then ET spills over into systemic circulation. For these reasons, it is named intestinal endotoxemia (IETM), because ET comes from the gut[4]. IETM can induce the release of chemical mediators from KCs and subsequent hepatic injury[5].
Hepatectomy, both partial resection and transplantion, has greatly developed and advanced during the last years; it has also allowed wider surgical indications, including living-donor liver transplantation. Classical PH is a major operation losing nearly 70% liver volume. It means that intestinal mucosal barrier might be damaged and the absolute number of KCs in the remnant liver is abruptly decreased. Therefore, PH could result in IETM. Some researches suggested that the serum ET level was elevated in rats after PH, which was shown to be involved in the pathogenic process of liver injury and regeneration[6,7]. However, whether IETM actually occurred after PH, and what are its dynamic changes and formative mechanism during liver regeneration after PH, are still not clear. To further confirm the existence of IETM and to explore its mechanism, we observed systematically the dynamic changes of serum ET, intestinal mucosa barrier, and the number and functional state of KCs after PH in rats.
One-hundred and two adult healthy male Wistar rats weighing 180-220 g, obtained from the Animal Center of Shanxi Medical University, were employed in the present study. All animals received humane care during the study under a protocol that was in accordance with institutional guidelines for animal research and was approved by the Ethics and Research Committee of Shanxi Medical University. Experimental animals were randomly divided into three groups: normal control (NC, n = 6) group, partial hepatectomy (PH, n = 48) group and sham-operation (SO, n = 48) group. Rats were sacrificed at 6 h, 12 h, 24 h, 36 h, 48 h, 72 h, 120 h and 168 h after the partial hepatectomy or sham-operation. NC group was used as 0 h time point in observation, namely 0 h group. For each time point indicated, six rats (n = 6) were used in parallel.
Rats were maintained on a 12:12 h light-dark cycle and standard feeding. All surgical procedures were performed under strict sterile conditions. Surgery was performed between 8 and 10 AM. Rats were fasted 12 h before surgery and anesthetized with pentobarbital sodium (30 mg/kg) intra-abdominally. PH rats model (two-thirds hepatectomy) was established as described by Higgins and Anderson, i.e. a mid laparotomy was performed with removal of the left lateral hepatic lobe and mid lobe; blood vessels were identified and cut, and both lobes were ligated and resected, thus making up the classically so-called 70% hepatectomy. The laparotomy was stitched up and rats were placed in recovery cupboards under appropriate temperature and humidity conditions. They returned to their standard feeding. The SO rat model was established exactly as indicated for PH, except that the livers of SO rats were only manipulated but not resected. After 6 h, 12 h, 24 h, 36 h, 48 h, 72 h, 120 h, and 168 h, respectively, PH and SO rats underwent relaparotomy again under anesthesia with pentobarbital sodium (20 mg/kg) intra-abdominally. Abdomen aorta blood was drawn from rats in sterile tubes, centrifuged at 1000 ×g for 10 min at 4°C and stored at -70°C for serum ET and diamine oxidase (DAO) assay. Segments were taken from distal ileum and rinsed with ice-cold normal saline, fixed in 10% buffered formalin and 3% glutaraldehyde, respectively, for light and electron microscopic assay. Livers were removed and fixed in 10% buffered formalin. The fixed livers were dehydrated through increasing concentrations of ethanol in xylene and embedded in paraffin. Livers embedded in paraffin were sectioned at 4μm for immunohistochemistry.
ET levels were measured using Limulus test kit (Shanghai Yi Hua Scientific, Inc) according to manufacturer's instructions. DAO levels were measured using Li's method by spectrophotometry (UV-2102C, Shanghai)[8].
The resected segments of ileum fixed in 10% buffered formalin were opened lengthwise, embedded in paraffin after 24 h, sectioned (4μm), and stained with hematoxylin-eosin (HE). The sections were analyzed under a light microscope (Olympus, Japan) for histological examination of distal ileum lesions.
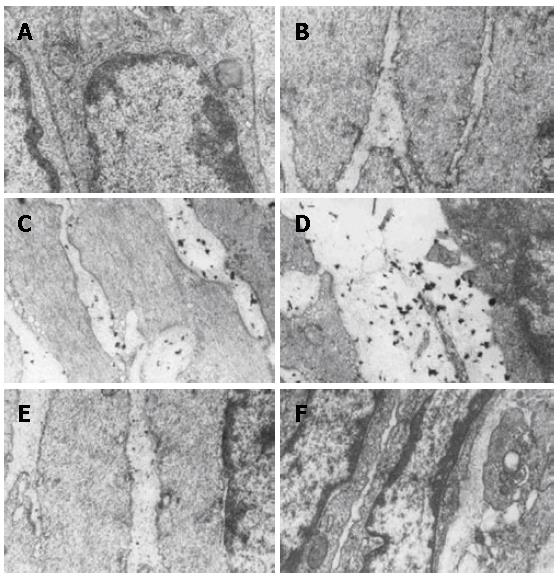
Ultra-structural and permeability changes of ileum were observed through the leakage of La particle. The resected samples of ileum were fixed for 2 h at 4°C with 3% glutaraldehyde in 0.1 mol/L cacodylate buffer (pH 7.2) containing La, then washed three times with 0.1 mol/L cacodylate buffer (pH 7.2) and stained with osmium tetroxide containing La, dehydrated through ethanol, embedded in epoxy resin 618, and sectioned extra thin. Sections were examined under a JEM 100CX transmission electron microscope. Increased ileum permeability was defined by a La particle[9,10].
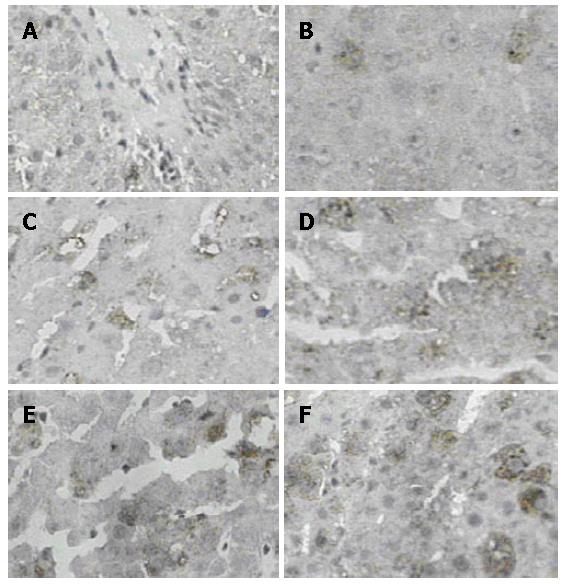
Immunohistochemical staining was performed by SABC (SABC Kit, Wuhan Boster, China) method as described in detail below. Paraffin remnant liver sections (4μm) were dewaxed and dehydrated in graded alcohols. Endogenous peroxidase activity was quenched with 2% H2O2 in PBS for 30 min at room temperature. Non-specific staining was blocked with 5% BSA for 20 min at room temperature. After moving extra liquid, a polyclonal rabbit anti-LYZ primary antibody (Wuhan Boster, China)at a dilution 1:100 was applied in PBS and incubated overnight at 4°C. The following day, sections were washed (2 × 3 min in PBS) and incubated with biotinylated goat-anti-rabbit Ig (Wuhan Boster, China) for 30 min, then washed (3 ×2 min in PBS) and incubated for 30 min with SABC, and washed again (3 × 2 min) before each section underwent DAB (Wuhan Boster, China) staining according to the manufacturer's instruction, resulting in the formation of a brown reaction product. Finally, sections were briefly counterstained with hematoxylin, then dehydrated, cleared and mounted in neutral gum under cover slips. Controls consisted of omitting the primary and secondary antibody to perform negative control staining. A known positive staining specimen (rat liver) was used as a positive control. LYZ staining was assessed by two independent observers who were blinded to the origin of the sections. The cells with cytoplasm showing brown grain under the optical microscope were defined as positive cells. The number of cytoplasm stained positive cells were calculated by randomly choosing 10 fields under medium power (× 200) microscope and counting average value.
Results were evaluated using analysis of variance and correlated with SPSS11.0 software. All values were expressed as the mean ± SD. P value of 0.05 or less was considered to be statistically significant. The linear trends were measured by corresponding tests.
As shown in Figures 1 and 2, serum ET and DAO levels in rats of PH group were increased significantly compared with NC and SO groups during the early post-PH period. The serum ET levels were elevated obviously in the PH group 6, 12, 24 and 48 h after PH, and there are two peak values at 12 h and 48 h. The serum DAO levels rose obviously in the PH group 12 h and 24 h after PH. The serum levels of ET and DAO at the different time points of SO group were not significantly different from the NC group. There was positive correlation (r = 0.757, P < 0.05) between the dynamic changes of ET and DAO levels in PH group (Figure 3).
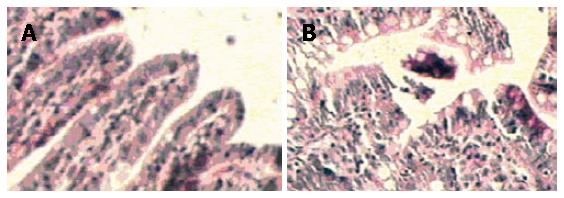
The NC and SO specimens showed normal and typical leaf-like villi crypts. In contrast, the specimens from the PH groups showed different degrees of structural changes ranging from swelling and degeneration of villous epithelial cells to extensive denudation and collapse of the villi. At 6 h after PH, an increased degree of mucosal injury was observed (Figure 4A and B).The major injury occurred at 24 h after PH.
Ultra-structural changes of small intestine were observed through the staining with La, which was used as a tracer to demonstrate the increased intestinal mucosa permeability. Electron microscopic examination of the intestinal mucosa revealed some changes as follows (Figure 5A-F). No La particles and widen cell gap were found in the intestinal epithelium of tight junction among the cells in NC group. Few La particles and slightly widen cell gap were found in SO group at 6 h. Lots of La particles and clear widen cell gap were found in PH group at 6-48 h, and La particles diffused between the cells gap as well as in the cytoplasm. The morphologic changes of intestinal mucosa were dramatic following the liver regeneration after PH. Morphologic changes in these periods included widen cells gap and recovery, Good correlation between cells gap changes and La particles permeability was noted in this period.
To investigate the dynamic changes of KCs number and functional state in remnant liver after PH, the immunostaining was carried out using a specific anti-LYZ antibody. LYZ was used in this study as a marker to demonstrate the KCs number and shape in the liver for LYZ is a reliable and stable marker for mononuclear-phagocyte system, which only exists in the KCs in the liver. The change of KCs shape and LYZ content in KCs illustrate the change of KCs function[11]. Activated KCs appeared round in shape with increasing LYZ content in the cytoplasm. As evident from Figure 6A-F, the different staining intensities of LYZ positive cells were observed in the cytoplasm of NC, SO and PH group rat liver, surrounding the central vein(CV) and portal triad(PT). There was no significant difference in the number of LYZ positive staining cells at different time points of SO group compared with NC group (Table 1), and activated KCs were not shown in SO and NC groups. However, there was significant difference in the number of LYZ positive staining cells during 24-168 h period in PH groups compared with NC and SO, and activated KCs were predominantly observed at 48 h after PH. There was no correlation between the dynamic changes of the KCs number in remnant liver and of the ET levels in serum.
It has been verified that during the period of severe trauma or large operations, the intestinal mucosal barrier was damaged, and therefore the intestinal permeability increased, resulting in IETM[1,2]. Because IETM can activate KCs and injure the liver, living donor liver transplantion is an effective therapy for deteriorative liver disease, the liver graft is from healthy adults, and there are unknown risks for donors,it is necessary to explore the mechanism of IETM formation in PH.
Previous studies have noted that the blood level of gut-derived ET was elevated during liver regeneration after PH[6,7]. As indicated by the data of this investigation, the serum ET levels increased obviously in the PH group at 6, 12, 24 and 48 h. These findings suggested that the PH rats formed a sustained and changed endotoxemia. But where is ET mainly from?
A major function of the gut is to prevent the absorption of toxins, antigens, protease and micro-organisms across the intestinal wall. Epithelial cells cover the surface of the gastrointestinal tract, serve as a barrier between the luminal and tissue compartments[12,13]. Maintenance of the intestinal barrier function depends on the integrity of cellular plasma membrane, tight junctions as well as the elaboration of endothelial and epithelial secreting products[14]. Whether the mucosal barrier works well or not is closely related to mucosal injury and intestinal permeability. In this study, light microscopy for histological examination and electron microscopy for intestinal mucosa permeability assay using La staining were used. These methods can determine whether and when a mucosal injury and an increase of intestinal permeability appear, and can find how and when the degrees of mucosal injury and permeability increase correlate with the changes of serum ET levels after PH. In this study, the optical examination showed that neutrophil margination and superficial necrosis of the villi at 6-24 h appeared in intestinal mucosa after PH, penetrated electron microscopy showed the gaps between intestinal mucosa cells were increased and the La particles were observed among the intestinal mucosa cells within 48 h after PH. It is similar with a previous experimental study that 48 h after PH there is a trend for decreased injury of intestinal mucosa[15]. It is suggested that elevated serum ET appeared to be consistent with the degree of intestinal mucosal barrier injury. A preliminary conclusion that the serum ET in PH rats mainly coming from gut could be deduced by these data.
DAO exists in high concentrations in the intestinal mucosa. Most DAO in the blood comes from the intestine. The serum DAO is reported to be proportional to the amount of intestinal DAO, and it is a reliable marker of intestinal mucosal integrity[16]. In order to further confirm the degree of intestinal mucous damage, the dynamic changes of serum DAO levels were measured. In the present study, serum DAO levels significantly increased in the PH group at 12 and 24 h, which was positively correlated with the dynamic changes of serum ET closely. This correlation was related to the fact that high serum ET might come from gut. Together with the findings of intestinal mucosal injury under light and electron microscopy, it is strongly suggested that intestinal mucosal barrier function was damaged and IETM appeared after PH. The mechanism of the intestinal mucosal barrier function injury after PH might be: (1) PH as a surgical stress losing 70% liver tissues, leading to swollen mucosa and weakened intestinal movement as well as silt and decreased secretion of bile, which bring about the massive bacterial overgrowth and bacterial translocation, and cause the invasion of enteric ET into blood[17,18]; (2) PH could result in a condition of incomplete ischemia of the gut. Ischemia/reperfusion injury of the gut lead to epithelial injury and increased intestinal mucosal permeability[19].
The mononuclear-phagocyte system of the liver is important in clearing organisms from the portal circulation. In recent years, more attention has been paid to the role of KCs in liver. Studies have showed that intestinal mucosal barrier injury causes transient IETM, only when KCs function is depressed, which will result in sustained IETM[20]. PH is an operation losing nearly 70% liver volume, it causes an abrupt decrease of the absolute number of KCs in remnant liver. In an attempt to clarify the dynamic changes of KCs number and functional state, the immunostaining was carried out using a specific anti-LYZ antibody to investigate the expression and number of KCs. The numbers of KCs in remnant regenerating liver were significantly increased during 24-168 h period after PH compared with NC and SO groups. However, the activation of KCs was predominantly observed at 48 h after PH. This is later than the time point of serum ET first peak value at 12 h. It is suggested that the main reason of sustained ET level increase before 48 h could be the depression of KCs function as well as the decrease of KCs number; and the the ET level decreased quickly after 48 h possibly due to the activation of KCs function and the increase of KCs number.
In summary, these findings indicated that there have been increased levels of ET in serum and damage in enteric wall as well as the changes of KCs in number and functional state in the remaining liver during the liver regeneration after PH. In conclusion, the mechanism of IETM in PH rats might be the injury of intestinal mucosal barrier and the decrease of KCs absolute number as well as the depression of KCs functional state. This observation might be of potential value in patients undergoing liver resection. Further studies are required to assess whether the IETM can cause postoperative septic complications.
Hepatectomy, both partial resection and transplantion, has greatly developed and advanced during the last years; it has also allowed a wider surgical indications, including living-donor liver transplantation.
Living donor liver transplantation is an effective therapy for deteriorative liver disease. Liver graft in clinics is from healthy adults. There are unknown risks for donors. Intestinal endotoxemia can cause the liver injury.
It is the first report to characterize the dynamic changes of serum endotoxin, intestinal mucosal barrier, and the number and functional state of Kupffer cells in PH rats.
This observation might be of potential value in patients undergoing liver resection.
This paper presented an outstanding original overview about the pathophysiology of intestinal endotoxemia after partial hepatectomy in rats. It may represent a milestone in understanding the physiopathology of IETM in humans undergoing a liver resection or a segmental liver transplantation.
S- Editor Liu Y L- Editor Ma JY E- Editor Wang HF
| 1. | Yao YM, Yu Y, Sheng ZY, Tian HM, Wang YP, Lu LR, Yu Y. Role of gut-derived endotoxaemia and bacterial translocation in rats after thermal injury: effects of selective decontamination of the digestive tract. Burns. 1995;21:580-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Bahrami S, Redl H, Yao YM, Schlag G. Involvement of bacteria/endotoxin translocation in the development of multiple organ failure. Curr Top Microbiol Immunol. 1996;216:239-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Rodríguez Sanz MB, Alarcón García J, del Riego Tomás FJ, Vaquero Puerta C. Effects of partial hepatectomy on the distal ileum in rats. Rev Esp Enferm Dig. 2004;96:185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Han DW. Intestinal endotoxemia as a pathogenetic mechanism in liver failure. World J Gastroenterol. 2002;8:961-965. [PubMed] |
| 5. | Han DW. Studies on pathogenesis of hepatic failure: hypothesis of intestinal endotoxemia. Zhughua Ganzangbing Zazhi. 1995;3:134-137. |
| 6. | Cornell RP. Gut-derived endotoxin elicits hepatotrophic factor secretion for liver regeneration. Am J Physiol. 1985;249:R551-R562. [PubMed] |
| 7. | Tsuchiya H, Kaibori M, Yanagida H, Yokoigawa N, Kwon AH, Okumura T, Kamiyama Y. Pirfenidone prevents endotoxin-induced liver injury after partial hepatectomy in rats. J Hepatol. 2004;40:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Li JY, Sun D, Lu Y, Jin H, Jiang XG, Hu S, Sheng ZY. Change in intestinal function in sepsis in rat. Zhongguo WeiZhon Bing JiJiu YiXue. 2004;16:352-354. [PubMed] |
| 9. | Xia YY. Exploring ways of Lanthanum techniques used as a tracer to demonstrate the extracellular gaps. Dier Junyi Daxue Xuebao. 1987;8:282-283. |
| 10. | Han YS, Li H. Using domestically produced lanthanum nitrate for electron microscopic specimen preparation techniques. Zhonghua Wuli Yixue Zazhi. 1983;5:157-158. |
| 11. | Decker K. The response of liver macrophages to inflammatory stimulation. Keio J Med. 1998;47:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Baumgart DC, Dignass AU. Intestinal barrier function. Curr Opin Clin Nutr Metab Care. 2002;5:685-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 173] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Gewirtz AT, Liu Y, Sitaraman SV, Madara JL. Intestinal epithelial pathobiology: past, present and future. Best Pract Res Clin Gastroenterol. 2002;16:851-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Nusrat A, Turner JR, Madara JL. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am J Physiol Gastrointest Liver Physiol. 2000;279:G851-G857. [PubMed] |
| 15. | Assimakopoulos SF, Alexandris IH, Scopa CD, Mylonas PG, Thomopoulos KC, Georgiou CD, Nikolopoulou VN, Vagianos CE. Effect of bombesin and neurotensin on gut barrier function in partially hepatectomized rats. World J Gastroenterol. 2005;11:6757-6764. [PubMed] |
| 16. | Luk GD, Bayless TM, Baylin SB. Diamine oxidase (histaminase). A circulating marker for rat intestinal mucosal maturation and integrity. J Clin Invest. 1980;66:66-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 174] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Wang XO, Sun ZW, Soltesz V, Deng XM, Andersson R. The role of intravenous administration of dextran 70 in enteric bacterial translocation after partial hepatectomy in rats. Eur J Clin Invest. 1997;27:936-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Okay E, Karadenizli A, Müezzinoglu B, Zeybek U, Arzu Ergen H, Isbir T. N-acetylcysteine attenuates bacterial translocation after partial hepatectomy in rats. J Surg Res. 2005;127:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Moore-Olufemi SD, Kozar RA, Moore FA, Sato N, Hassoun HT, Cox CS, Kone BC. Ischemic preconditioning protects against gut dysfunction and mucosal injury after ischemia/reperfusion injury. Shock. 2005;23:258-263. [PubMed] |
| 20. | Enomoto N, Ikejima K, Bradford BU, Rivera CA, Kono H, Goto M, Yamashina S, Schemmer P, Kitamura T, Oide H. Role of Kupffer cells and gut-derived endotoxins in alcoholic liver injury. J Gastroenterol Hepatol. 2000;15 Suppl:D20-D25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 3.8] [Reference Citation Analysis (0)] |