Published online Jul 14, 2007. doi: 10.3748/wjg.v13.i26.3581
Revised: March 19, 2007
Accepted: April 11, 2007
Published online: July 14, 2007
AIM: To investigate the effect of compound Danshen injection on lipopolysaccharide (LPS)-induced rat mesenteric microcirculatory dysfunctions and the underlying possible mechanism by an inverted intravital microscope and high-speed video camera system.
METHODS: LPS was continuously infused through the jugular artery of male Wistar rats at the dose of 2 mg/kg per hour. Changes in mesenteric microcirculation, such as diameters of arterioles and venules, velocity of RBCs in venules, leukocyte rolling, adhesion and emigration, free radicals released from post-capillary venules, FITC-albumin leakage and mast cell degranulation, were observed through an inverted intravital microscope assisted with CCD camera and SIT camera. Meanwhile, the expression of adhesion molecules CD11b/CD18 and the production of free radical in neutrophils, and the expression of intercellular adhesion molecule 1 (ICAM-1) in human umbilical vein endothelial cells (HUVECs) were quantified by flow cytometry (FACS) in vitro.
RESULTS: The continuous infusion with LPS resulted in a number of responses in microcirculation, including a significant increase in the positive region of venule stained with Monastral blue B, rolling and adhesion of leukocytes, production of oxygen radical in venular wall, albumin efflux and enhanced mast cell degranulation in vivo, all of which, except for the leukocyte rolling, were attenuated by the treatment with compound Danshen injection. Experiments performed in vitro further revealed that the expression of CD11b/CD18 and the production of oxygen free radical in neutrophils, and the expression of ICAM-1 in HUVECs were increased by exposure to LPS, and they were attenuated by compound Danshen injection.
CONCLUSION: These results suggest that compound Danshen injection is an efficient drug with multi-targeting potential for improving the microcirculatory disturbance.
- Citation: Han JY, Horie Y, Miura S, Akiba Y, Guo J, Li D, Fan JY, Liu YY, Hu BH, An LH, Chang X, Xu M, Guo DA, Sun K, Yang JY, Fang SP, Xian MJ, Kizaki M, Nagata H, Hibi T. Compound Danshen injection improves endotoxin-induced microcirculatory disturbance in rat mesentery. World J Gastroenterol 2007; 13(26): 3581-3591
- URL: https://www.wjgnet.com/1007-9327/full/v13/i26/3581.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i26.3581
It is now well-recognized that stimulation with lipopoly-saccharide (LPS) leads to a number of responses in microcirculation, such as endothelial cell damage, an increase in rolling, adhesion and emigration of leukocytes in post-capillary venules, enhanced oxygen-free radical production, mast cell degranulation and protein efflux[1-6]. Furthermore, it is demonstrated that LPS stimulation evokes an increase in the expression of adhesion molecules, including L-selectin[7] and CD11b/CD18 in leukocytes, E-selectin and intercellular adhesion molecule 1 (ICAM-1) in endothelial cells[7-13]. In addition, LPS stimulation can cause the release of pro-inflammatory mediators and vasoactive substances, such as tumor necrosis factor-alpha (TNF-α) and histamine, with the mast cell degranulation[14]. Therefore, many investigators have made much effort to abolish or reduce the LPS-elicited injury. For instance, it was reported that application of antibodies against adhesion molecules could inhibit LPS-induced leukocyte adhesion to post-capillary venules[15]. However, there are few in vivo studies about any treatment which attenuates microcirculatory disturbance via both scavenging the oxygen-free radicals and reducing the adhesion between leukocytes and vascular endothelium.
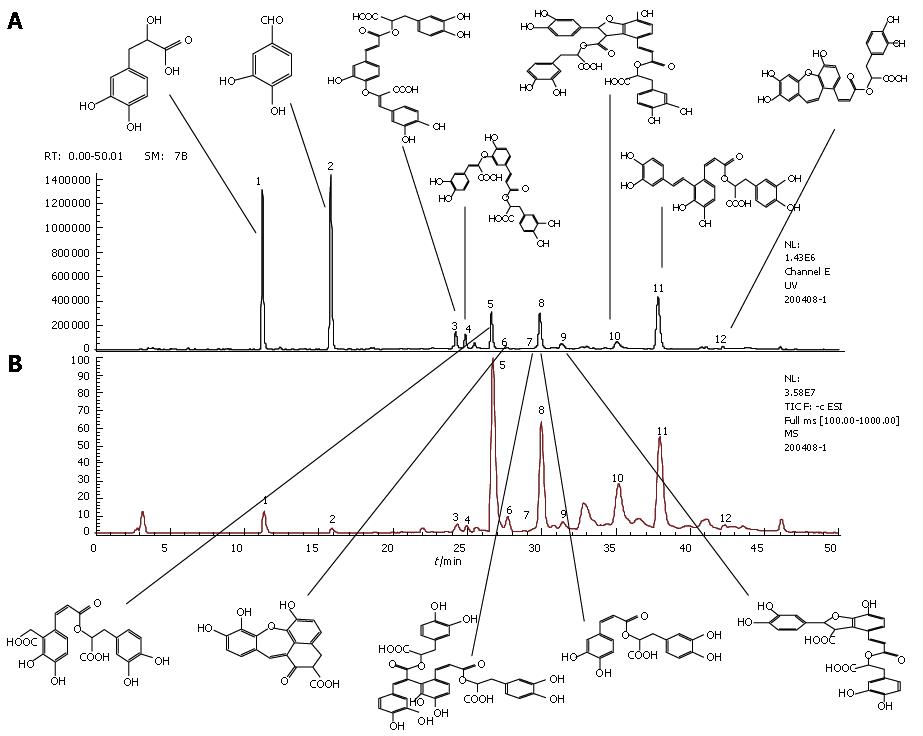
Compound Danshen injection is made from the aqueous extracts of Danshen (Salvia miltiorrhiza), and it has been clinically used for treatment of various vascular diseases in China. There are twelve ingredients in compound Danshen injection (Figure 1), which are 3, 4-dihydroxy-phenyl lactic acid (DLA), protocatechu aldehyde (Pal), salvianolic acid I (SalI), salvianolic acid H (SalH), salvianolic acid D (SalD), salvianolic acid G (SalG), salvianolic acid E (SalE), rosmarinic acid (RA), lithospermic acid (LsA), salvianolic acid B (SalB), salvianolic acid A (SalA), and isosalvianolic acid C (ISalC). It is thus interesting to know the physiological actions of this medicine, especially at molecular level. Several lines of data have accumulated so far in this field. To mention a few, for example, pretreatment with Cardiotonic Pills (CP, the major ingredients of which are DLA and SalB) attenuated the gut ischemia/reperfusion-induced leukocyte adhesion in the liver sinusoids and plasma TNF-α and endotoxin[16], and diminished the thrombi induced by photochemical stimulation in rat mesenteric venules, as demonstrated in our previous study[17]. CP was reported to inhibit the expression of ICAM-1 and vascular cell adhesion molecule 1 (VCAM-1) in endothelial cells[18]. Aqueous extract of Danshen has been shown to inhibit the expression of ICAM-1 and this effect may be due to its capacity to block the activation of nuclear factor kappa-B (NF-κB) of endothelial cells[19,20]. DLA and SalB were also shown to inhibit TNF-α-induced NF-κB secretion in human umbilical vein endothelial cells (HUVECs)[20]. Kim et al[21] revealed that Danshen water-soluble extract abrogated the LPS-induced NF-κB signaling pathway by targeting the I-κB-α kinase (IKK) complex in intestinal epithelial cells. Aqueous extract of Danshen was also found to reduce the oxidative stress in endothelial cell[22] and lipid peroxidation in hepatocytes and hepatic stellate cells[23]. DLA can effectively scavenge superoxide anion generated from the xanthin-xanthin oxidase system and protect the myocardial mitochondria from lipid peroxidation[24].
However, little is known about the action of compound Danshen injection on leukocyte-endothelial cell interaction during endotoxin-induced microcirculatory disturbances in vivo. Therefore, in the present study, an LPS-induced rat mesentery microcirculatory disturbance model was used and an inverted intravital microscope assisted with the CCD camera, SIT camera, high-speed video camera system was utilized, in attempt to explore the dynamics of microcirculation alterations occurred in vivo and the inherent mechanism. The parameters examined in vivo included the diameters of arterioles and venules, velocity of RBCs in venules, leukocyte rolling, adhesion and emigration, free radicals released from post-capillary venules, FITC-albumin leakage and mast cell degranulation. Meanwhile, the expression of adhesion molecules CD11b/CD18 and production of free radical in neutrophils, and expression of ICAM-1 in HUVEC were quantified by flow cytometry (FACS) in vitro.
Dihydrorhodamine 123 (DHR) was obtained from Molecular Probes Ltd. (Eugene, OR, USA). FITC-albumin, monastral blue B (MBB), lipopolysaccharide (LPS, O55:B5), toluidine blue were obtained from Sigma Chemical Co. (St Louis, MO, USA). FITC-labeled anti-CD11b, FITC-labeled anti-CD18 antibodies, FITC-labeled mouse IgGκ and FITC-labeled mouse IgA1 were purchased from BD Biosciences Pharmingen (USA). Haemolysin was purchased from BD Biosciences Immunocytometer Systems (USA).
Compound Danshen injection ample was obtained from Shanghai First Pharmaceutical Co. (Shanghai, China). For determination of phenolic acids, 1 mL of compound Danshen injection sample was directly diluted to 4 mL with deionized water, and the resulting solution was filtered through a membrane (0.45 μm pore size) and then injected into HPLC. The analyses were performed using an Agilent series 1100 HPLC system (Agilent, Waldronn, Germany). In order to identify the structures of main constituents in compound Danshen injection, the sample was analyzed by HPLC-MS techniques[24]. Twelve common peaks in compound Danshen injection were designated (Figure 1), on which twelve different phenolic acids were identified and presented (Figure 2). One milliliter of compound Danshen injection contains 3.25 mg of DLA, 0.51 mg of Pal, 0.30 mg of RA, 0.17 mg of LsA, 0.15 mg of SalB and 0.08 mg of SalA.
All studies were approved by and all animals were handled according to the guidelines of the Keio University Animal Research Committee. Male Wistar rats, weighing 200-250 g, (Saitama Experimental Animals Supply Co. Ltd, Saitama, Japan) were fasted for 12 h before the experiment, allowing for free access to water.
Surgical procedure in the present study was almost same as our previous study[25]. Briefly, rats were anesthetized and the right jugular vein and artery were cannulated for the infusion of compound Danshen injection or LPS. After abdomen was opened, ileocecal portion of the mesentery was gently mounted on a transparent plastic stage designed for the rat. Microcirculation hemodynamics in the mesentery was observed by a transillumination method using an inverted microscope (Diaphot TMD-2S; Nikon, Tokyo, Japan). The mesentery was transilluminated with a 12-V, 100-W, direct current-stabilized light source. Video images were recorded with a video-cassette recorder[25]. Single unbranched venules with diameters ranging between 25 and 40 μm and length longer than 200 μm were selected for study.
In the control group, physiological saline (6 mL/kg body weight per hour) was continuously infused through the jugular vein. In the LPS group, LPS in saline was continuously infused through the jugular artery at the dose of 2 mg/kg body weight per hour. In the LPS plus compound Danshen injection group, compound Danshen injection was diluted 5-fold with physiological saline and infused by the jugular vein (6 mL/kg body weight per hour), which started 10 min before the LPS infusion.
The diameters of mesenteric arterioles or venules were determined using a video measuring gauge (IV-560; Hoei, Tokyo, Japan).
The velocity of RBCs in the venule was recorded at a rate of 1000 frames/s using a high-speed video camera system (Ektapro 1000; Kodak, San Diego, CA, USA), and the recordings were replayed at a rate of 30 frames/s from the high-speed stored images. RBC velocity in the venule was measured with a temporal correlation velocimeter (CapiFlow, Kista, Sweden)[25].
Monastral Blue B (MBB) solution (0.1 mL/100 g of body weight) was administered via the jugular vein immediately before the starting of LPS infusion. The microvascular images of the mesentery were recorded with the video recording system (CC-090; Flovel, Tokyo, Japan). The length of microvascular wall stained with MBB was traced using the stored microcirculatory images in the video tape and estimated as MBB-positive length along a 200-μm long segment of venule[5].
The number of leukocytes that were rolling, adherent and emigrated was determined off-line during play-back of videotaped images. The rolling leukocytes were defined as those that could be seen moving within a small (200 μm in length) viewing area of the vessel for 10 s with the same area used throughout the experiment. The adherent leukocytes to the venule walls were identified as cells that attached to the same site for more than 30 s judging from the replayed video images. The number of adherent leukocytes was counted along venules (25-40 μm in diameter, 200 μm in length) randomly selected from the videotape images recorded and expressed as the number per 200 μm of venule length[25]. Leukocyte emigration was quantified as the number of leukocytes per field of view surrounding the venule.
To evaluate the albumin leakage across mesenteric venules, the animals were intravenously injected with 50 mg/kg of FITC-labeled bovine serum albumin 10 min before each experiment, as described previously[25]. Fluorescence intensity of FITC-albumin on the venular wall (excitation 420 to 490 nm; emission 520 nm) was determined using a silicon-intensified target camera (C-2400-08; Hamamatsu Photonics, Hamamatsu, Japan), and measured with an image processor[25].
In an other set of experiments, the oxidant-sensitive fluorescent probe dihydrorhodamine 123 (DHR; Molecular probes) was added to the mesenteric superfusate (10 μmol/L) to assess the oxidant stress in venular walls, as described previously[25]. DHR fluorescence intensity on the venular wall was observed and estimated with an image processor[25].
Mast cells were identified by vital staining with topical application of 0.1% toluidine blue to the mesentery at 60 min after the LPS infusion. The numbers of both non-degranulated mast cells and degranulated mast cells were scored from the CCD video images, and the ratio of the number of degranulated mast cells to the total number of mast cells was calculated and expressed as the degranulated mast cell ratio[25].
The mesentery in each experiment condition was also prepared for electron microscopy. Briefly, the animals were divided randomly into 3 groups, 3 rats each, and continuously infused for 60 min with physiological saline, LPS or LPS plus compound Danshen injection, respectively. Immediately after infusion, the rats, maintained under deep anesthesia, underwent perfusion through the left ventricle with physiological saline followed by 120 mL of phosphate-buffered 40 g/L paraformaldehyde plus 20 g/L glutaraldehyde at a speed of 3 mL/min. The mesentery tissues were then removed, localized and further fixed by immersion in phosphate-buffered 30 g/L glutaraldehyde for 1 h. The tissues were routinely processed for transmission electron microscopy and examined in JEM 1230 (JEOL, Japan).
Blood was taken from the abdominal aorta of normal rats and anticoagulated with heparin. In control group (n = 6), no LPS or drugs were added. In LPS group (n = 6), the samples were treated with LPS (2 μg/mL). In the compound Danshen injection-treated group (n = 6), both LPS (2 μg/mL) and compound Danshen injection were added to 200 μL blood, the final concentration of the latter was either 20 μL/mL or 30 μL/mL. All the preparations were maintained at 37°C for 2 h, and afterward incubated with FITC-labeled anti-CD11b (5 μg/mL), FITC-labeled anti-CD18 (5 μg/mL) antibodies or corresponding FITC-labeled mouse isotype (5 μg/mL) for 20 min at room temperature. The erythrocyte lysis was accomplished using haemolysin according to the manufacturer's instruction, and the cells were washed twice with PBS. The mean fluorescence intensity was accessed with flow-cytometry (FACS Calibur; BD Company, USA). Neutrophils were then sorted by characteristic forward-/side-scatter expression as reported previously[26]. Five thousands of neutrophils were evaluated for each sample.
In a separated group of rats, blood samples were taken from the abdominal aorta and anticoagulated with heparin. Neutrophils were isolated using Mono-poly resolving medium following the manufacturer's instruction, and suspended in RPMI 1640 medium. Cells were preloaded with 25 μmol/L dihydrorhodamine 123 (DHR) at 37°C for 20 min. The DHR passively diffuses across the cell membranes, where it is oxidized to cationic rhodamine 123 and localizes in the mitochondria. After labeling, the cells were treated with both compound Danshen injection (0.2 μL/mL, 0.5 μL/mL and 1.0 μL/mL, respectively) and LPS (2 μg/mL) for 20 min on ice, the production of hydrogen peroxide was then determined by flow cytometry by measuring emission at 590 nm for DHR[27].
Human umbilical vein endothelium cells (HUVECs) were provided by Cascade Company, and 3 passages were used. The confluent monolayer of HUVECs was incubated with 5 μL/mL, 10 μL/mL or 20 μL/mL of compound Danshen injection for 4 h, followed by addition of LPS (0.1 μg/mL) and incubation for further 12 h. Afterwards, HUVECs were digested and collected, and incubated with PE-labeled antibody against ICAM-1. The mean fluorescence intensity was accessed by flow cytometry (FACS Calibur; BD Company, USA).
The data were analyzed by one-way ANOVA and Fisher's post test. All values were expressed as mean ± SD of values from 6 rats. P < 0.05 was considered statistically significant.
No significant alteration was observed in the diameter of either arterioles or venules in mesenteric microcirculation during 60 min of LPS infusion, and the situation remained unchanged by continuous treatment with compound Danshen injection (data was not shown).
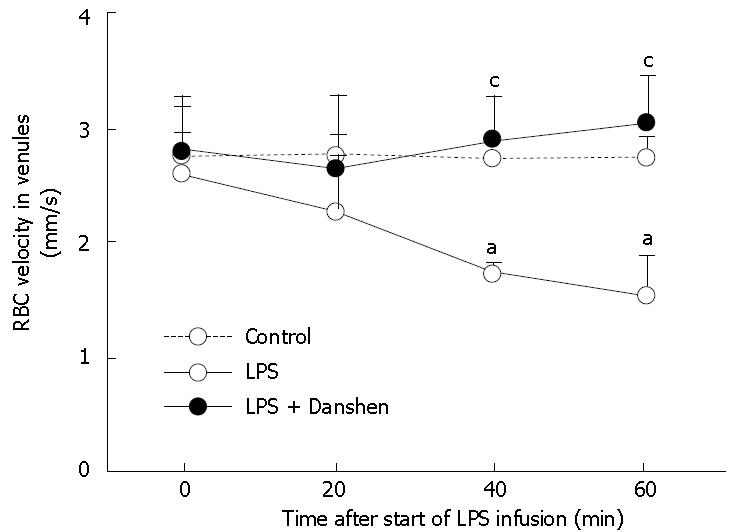
The examination of velocity of RBCs in venules revealed that there was no significant difference among the groups prior to LPS infusion. LPS infusion gave rise to a significant decrease in the velocity of RBCs in venules from 40 min to 60 min of LPS infusion than that at 0 min. Continuous treatment with compound Danshen injection significantly attenuated the LPS-induced decrease in the velocity of RBCs in venules (Figure 2).
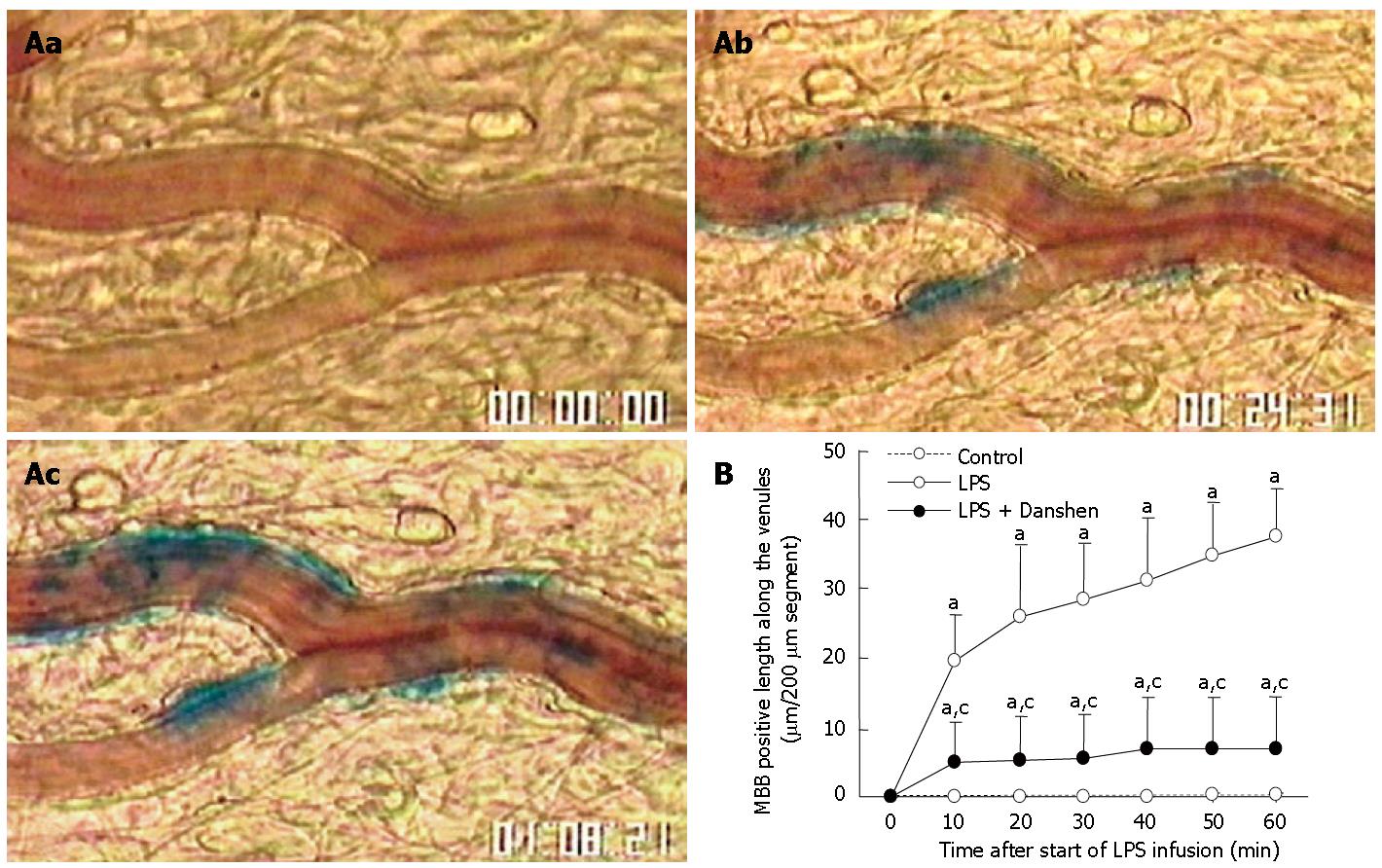
No MBB staining was observed in venular wall before LPS infusion (Figure 3Aa). After LPS infusion, numerous MBB staining areas were detected in venular wall at 20 min and 60 min (Figure 3Ab and Ac). The time course of changes in the length of venular wall stained with MBB is shown in Figure 3B. It was found that LPS infusion resulted in a progressive increase in the length of venular wall stained with MBB, starting from 10 min and keeping increase until 60 min of LPS infusion. These MBB-positive reactions along the venules were markedly suppressed by the continuous treatment with compound Danshen injection.
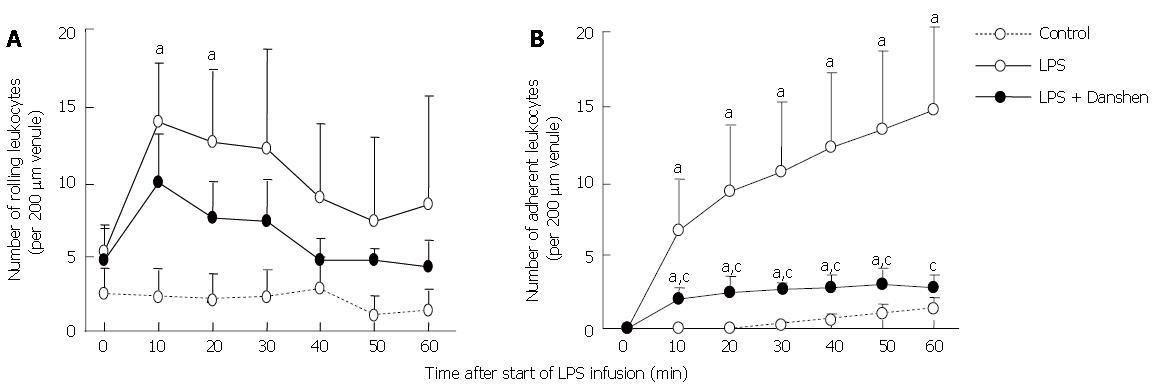
The result concerning the time course of changes in the number of rolling leukocytes along venular walls is summarized in Figure 4A. At baseline, the number of rolling leukocytes along venular wall of 200 μm in length was less than 5 per 10 s, and no apparent difference among the control group, LPS group, and LPS plus compound Danshen injection group could be detected. The number of rolling leukocytes along venular walls was significantly increased from 10 min to 20 min after LPS infusion. A similar alteration was observed in the LPS plus compound Danshen group without significant difference in comparison with LPS exposure alone.
The time course of changes in the number of leukocytes adherent to venular wall was examined and the result is depicted in Figure 4B. Obviously, the increase in the number of leukocytes adherent to venules initiated from 10 min after LPS infusion and remained until 60 min of LPS infusion. On the other hand, continuous treatment with compound Danshen injection caused a profound attenuation in leukocyte adhesion in venules induced by LPS infusion.
The number of leukocytes emigrated from venules was evaluated. No leukocyte emigration was detected in the control group in so far as the areas examined. However, after 40 min of LPS infusion, the emigrated leukocytes, although of a small number, could be observed. By contrast, in the LPS infusion plus compound Danshen injection group, few, if any, of leukocytes could be found emigrated from venules (data not shown).
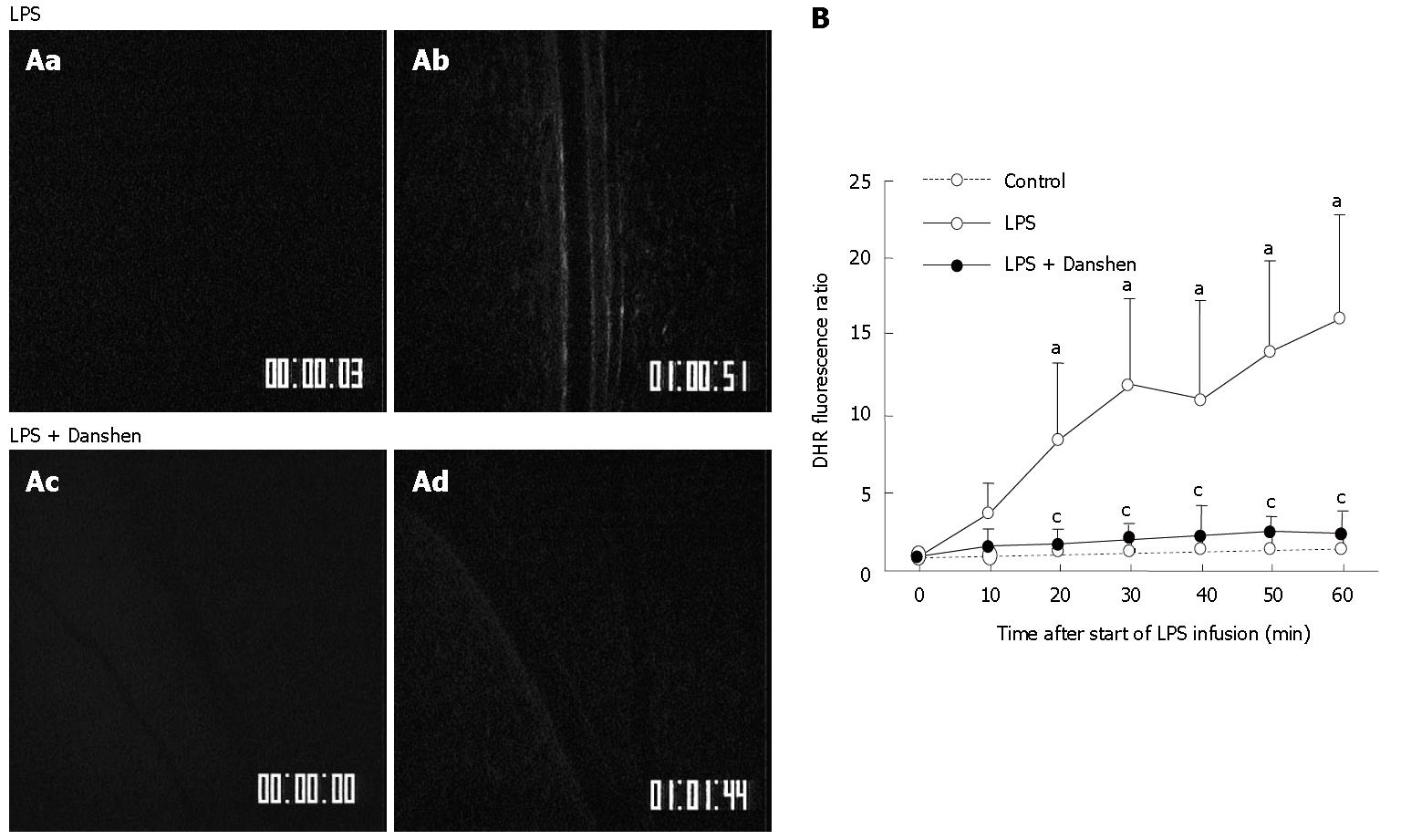
An experiment was carried out to determine the effect of treatment with compound Danshen injection on the fluorescence intensity of the H2O2-sensitive probe DHR in rat mesentery venular walls challenged by LPS (Figure 5A). Before LPS infusion (0 min), no detectable DHR florescence was observed in either LPS group (Figure 5Aa) or LPS plus compound Danshen injection group (Figure 5Ac). The fluorescence-stained venular wall was observed at 60 min after LPS infusion (Figure 5Ab). Treatment with compound Danshen injection, apparently attenuated the LPS-induced DHR fluorescence on the venular walls (Figure 5Ad).
The time course of changes in DHR fluorescence ratio on venular walls is presented in Figure 5B. Here the fluorescence ratio is defined as the ratio of the fluorescence intensity of a time point of LPS infusion to the baseline fluorescence intensity. The result demonstrated that DHR fluorescence ratio on the venular wall had no significant change in control group throughout the period of observation as compared with the baseline. On the contrary, the intensity of DHR fluorescence significantly increased in the LPS infusion group from 20 min and this tendency persisted till 60 min of LPS infusion. Continuous treatment with compound Danshen injection caused a significant attenuation in the increased DHR fluorescence induced by LPS infusion.
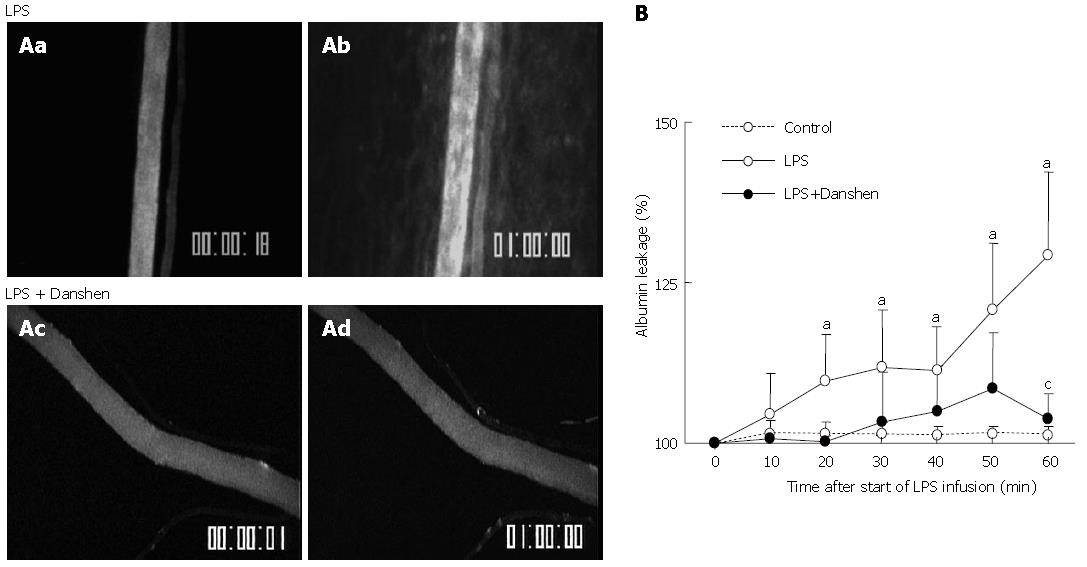
The albumin leakage from the rat mesentery venular walls in the LPS group and LPS plus compound Danshen injection group was evaluated at baseline (0 min) and 60 min after LPS infusion, respectively, and the representative fluorescence images are illustrated in Figure 6. Before LPS infusion, no obvious albumin leakage was detected in either LPS group (Figure 6Aa) or LPS plus compound Danshen injection group panel (Figure 6Ac). The albumin leakage from venular wall was observed at 60 min after LPS infusion (Figure 6Ab). The LPS-induced albumin leakage from venule was apparently suppressed by the treatment with compound Danshen injection (Figure 6Ad).
The quantitative evaluation of the results are presented as a percentage of albumin leakage changed with time and shown in Figure 6B. It is evident that the baseline albumin leakage was undetectable in all the three conditions studied. In control group, the albumin leakage from the venular wall maintained non-significant alteration over the observation period. The increase in albumin leakage became significant after 20 min of the LPS infusion, which was more pronounced at 60 min. This increase was inhibited by the treatment with compound Danshen injection at 60 min after the LPS infusion.
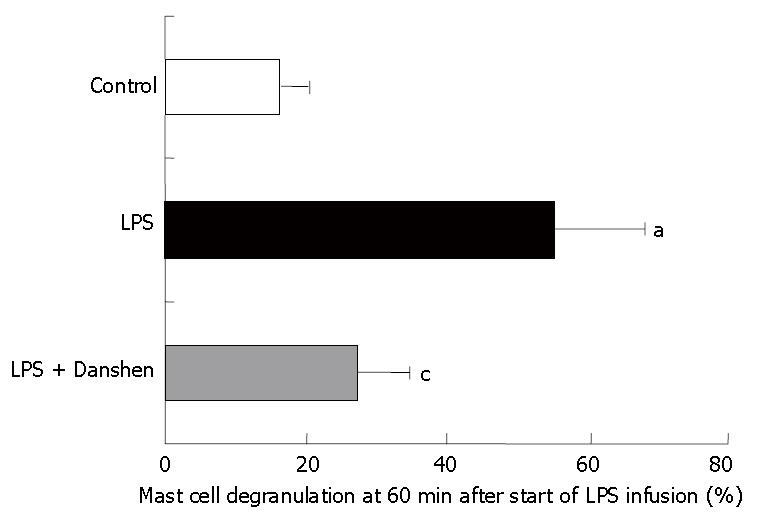
Mast cell degranulation was examined after 60 min of LPS infusion in various conditions, and the results were quantified as the ratio of the number of degranulated mast cells to the total number of mast cells examined (Figure 7). We found that LPS infusion resulted in an apparent increase in the mast cell degranulation in comparison with the control (55.3% ± 12.9% vs 22% ± 4.1%), and this increase was suppressed significantly by compound Danshen injection (27.5% ± 7.1%).
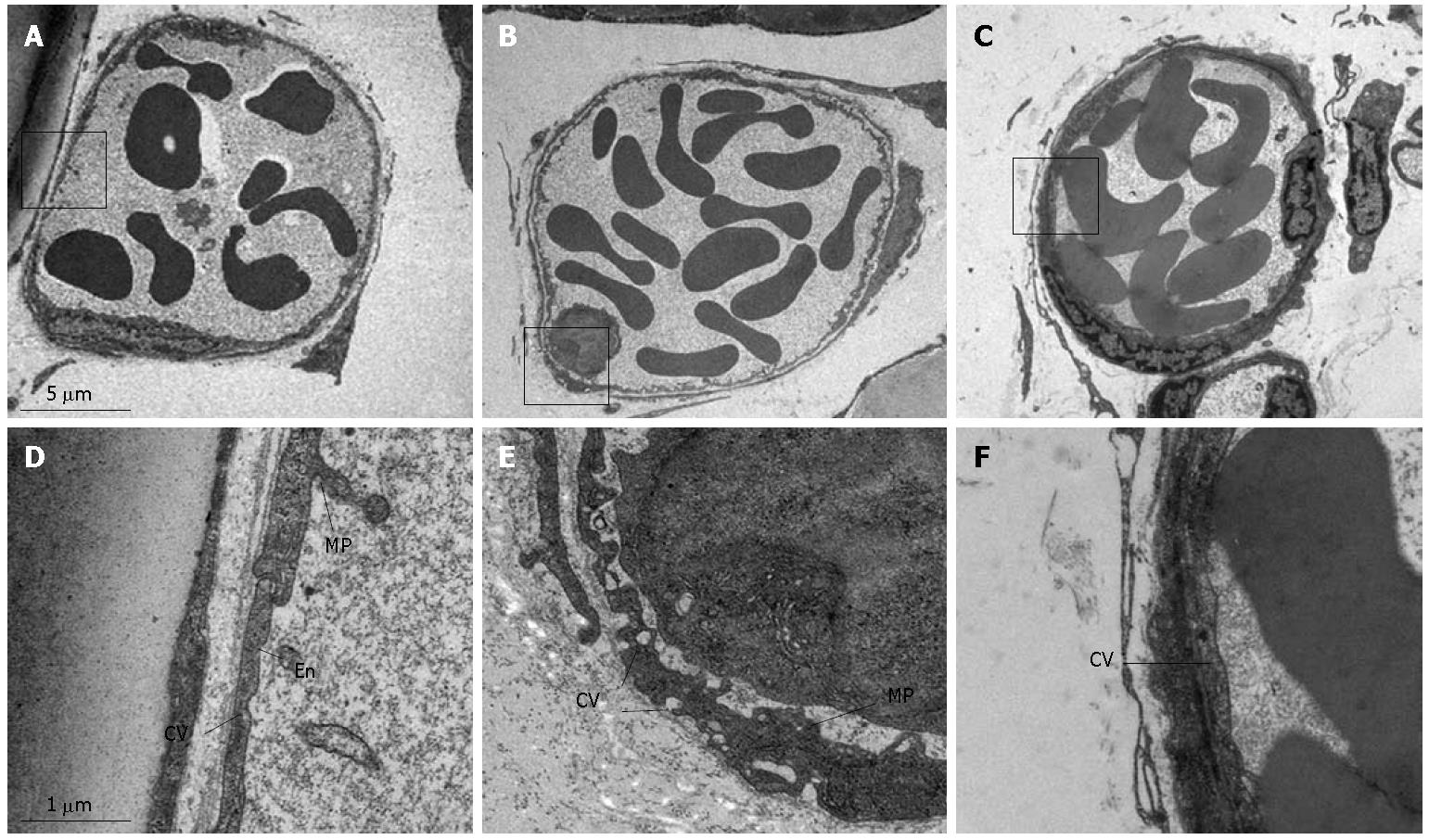
The representative electron micrographs of post-capillary venules in rat mesentery in control, LPS infusion and LPS plus compound Danshen injection groups are presented in Figure 8. The lumen of venules in normal rat mesentery was lined by a layer of endothelial cells, which exhibited rather smooth inner face with occasionally occurred microprojections on the surface and vesicles in the cytoplasm (Figure 8A and D). LPS infusion led to an apparent alteration in the ultrastructure of the endothelium of post-capillary venules, which were characterized by plentiful vesicles of different size in the cytoplasm and multiple cytoplasmic projections on the inner surface (Figure 8B and E). Some of the vesicles in the cytoplasm were located beneath or directly connected to both sides of the plasma membrane, indicating their possible function for transendothelial traffic. Adherent leukocytes were frequently observed, which were attached to the venular wall through the cytoplasmic projections of the endothelium. The LPS-induced alterations in the ultrastructures of endothelium were significantly abated by treatment with compound Danshen injection (Figure 8C and F).
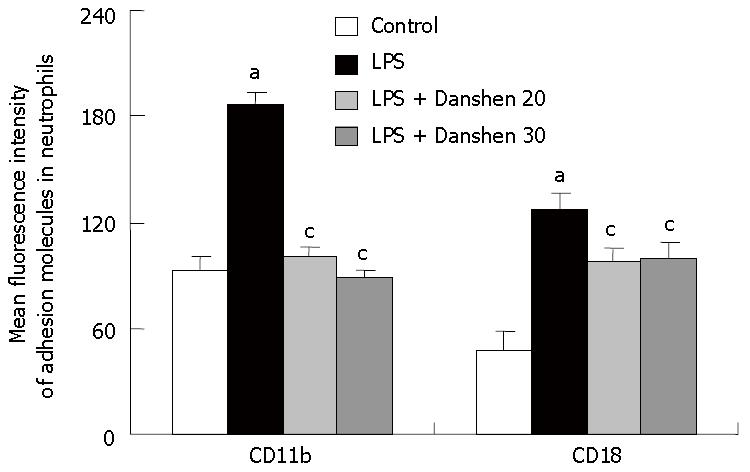
An in vitro study was performed to determine the fluorescence intensity of adhesion molecules CD11b and CD18 in neutrophils (Figure 9). The fluorescence intensity of CD11b and CD18 was significantly increased by LPS stimulation compared to the control group. Treatment with compound Danshen injection of 20 μL/mL or 30 μL/mL significantly inhibited the fluorescence intensity of CD11b and CD18 in neutrophils induced by LPS stimulation.
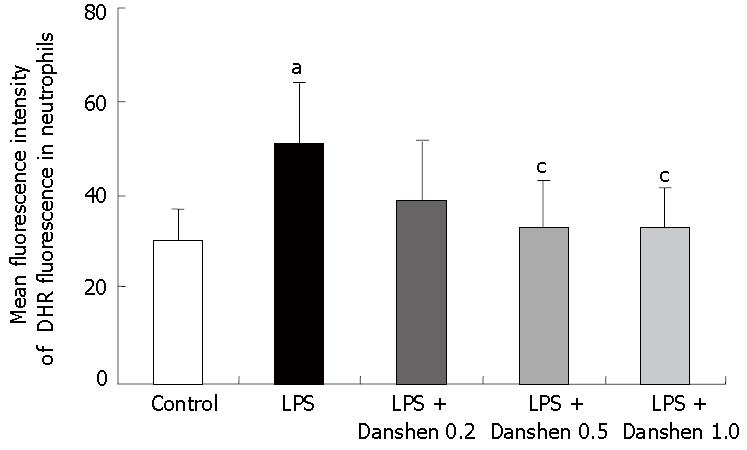
The intensity of DHR fluorescence in neutrophils was determined in vitro. LPS exposure significantly increased the fluorescence intensity of DHR in neutrophils (51.12 ± 13.20) compared with control group (30.52 ± 6.57), and this increase was almost completely abolished by the treatment with compound Danshen injection when the concentration was 0.5 μL/mL (33.57 ± 9.92) and 1.0 μL/mL (33.58 ± 8.17) (Figure 10).
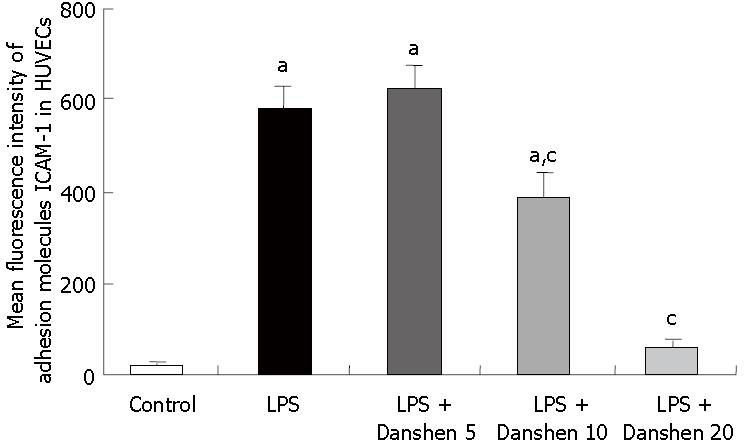
The fluorescence intensity of adhesion molecule ICAM-1 in HUVECs is illustrated in Figure 11. In comparison with control group, the fluorescence intensity of adhesion molecule ICAM-1 was significantly enhanced by stimulation with LPS and this LPS-elicited increase in fluorescence intensity of adhesion molecule ICAM-1 was inhibited profoundly by treatment with compound Danshen injection of either 10 μL/mL or 20 μL/mL, but not of 5 μL/mL.
In the present study, compound Danshen injection was found to reduce the rat mesenteric microcirculatory disturbance induced by LPS continuous infusion except for an increase in the leukocyte rolling. Up-regulation of a variety of adhesion molecules was evoked following the LPS stimulation, mediating different phases of leukocyte recruitment in microvessels, with L-selectin in leukocytes and E-selectin in endothelial cells[7,28,29] being involved in leukocyte rolling along vascular endothelium[7], and CD11b/CD18 in leukocytes and ICAM-1 in endothelial cells implicated in firm adhesion of leukocytes to vascular wall[5,15,30,31]. The fact that compound Danshen injection did not display any effect on the LPS-induced leukocyte rolling implies that it might reduce leukocytes adhering to venular wall through its inhibitory effect on the expression of adhesion molecules either CD11b/CD18 in neutrophils or ICAM-1 in endothelial cells or both rather than selectins. It was claimed that the LPS-induced expression of adhesion molecules CD11b/CD18 in neutrophils was related to the translocation of CD11b/CD18 from secretory vesicles and specific granules to the plasma membrane[10] as well as the conformation change in I-domain, the active region of CD11b/CD18 in neutrophils[31-33]. On the other hand, the expression of adhesion molecules ICAM-1 in endothelial cells was modulated by NF-κB[34], and conformation change in I-domain in endothelial cells[30,35,36]. Previous in vitro studies demonstrated that aqueous extract of Danshen, the main ingredients of compound Danshen injection, inhibited the expression of ICAM-1 and activation of NF-κB in endothelial cells[19,20]. Likewise, DLA and SAB, the main ingredients of Danshen, were reported to inhibit TNF-α-induced NF-κB secretion in HUVECs[20]. Kim et al[21] revealed that Danshen water-soluble extract abrogated the LPS-induced NF-κB signaling pathway by targeting the IKK complex in intestinal epithelial cells. However, the mechanism whereby compound Danshen injection inhibits the expression of adhesion molecules of leukocytes and endothelial cells needs further research.
It was reported that leukocyte adhesion to the venules triggered the release of oxygen-free radicals from leukocytes[4]. DHR, an H2O2-sensitive fluorescent probe successfully used to measure intracellular H2O2 levels, allowed us to monitor the dynamic alteration in the oxidative stress[25]. DHR fluorescence intensity in venules was significantly increased starting from 20 min of LPS infusion, and this increase was suppressed by the treatment with compound Danshen injection. It is unable to distinguish the DHR fluorescence of leukocytes from that of endothelial cells due to the limitation of resolution. However, the continuous linear appearance of the fluorescence images observed in the present study seems to represent H2O2 level in venular wall, especially in the endothelium. Nevertheless, the result from our in vitro experiment documented that compound Danshen injection significantly inhibited the production of H2O2 in neutrophils induced by LPS, indicating that it scavenged oxygen-free radicals from both endothelial cells and neutrophils.
It is well recognized that mast cell degranulation induced by LPS leads to the release of pro-inflammatory mediator and vasoactive substances, such as histamine, IL-5, IL-6 and TNF-α through TLR-4 receptor[3,37-39]. These pro-inflammatory mediator and vasoactive substances attack vessels from outside[40], promote the expression of VCAM-1 and E-selectin on endothelial cells[41], increase the rolling and adhesion of leukocytes, and enhance the albumin leakage[39,41,42]. In the present study, the degranulation of mast cells induced by LPS infusion was found to be markedly inhibited by the treatment with compound Danshen injection in terms of the degranulated mast cell ratio, suggesting that compound Danshen injection is also capable of protecting the microvessels by blockage of vasoactive substances from outside.
MBB is a macromolecule pigment which is able to immerge into the impaired vascular endothelium as well as intercellular spaces but unable to penetrate the endothelium basal lamina, and thus can be used as a marker to identify the injury sites of blood vessel43]. Pre-treatment of samples with MBB made it possible to monitor the vascular endothelium injury occurring with time[44]. The result of our present study showed that the significant increase in MBB staining area initiated at 10 min after LPS infusion, and the increase trend persisted until 60 min. Interestingly, a similar time course of change was noted in the LPS-induced increase in the number of adherent leukocytes and the production of oxygen-free radicals. Thus, the vascular endothelium injury appears to be associated with both the interaction between leukocytes and endothelium and the pernicious effect of oxygen-free radicals. Compound Danshen injection significantly reduced the area of MBB staining elicited by LPS infusion, suggesting that compound Danshen injection was potentially able to protect vascular endothelium from injury.
The pathway for the albumin leakage from mesentery venules found upon LPS stimulation is still to be explored. It was documented in the lung[45] and myocardium[46] that the increased vascular permeability induced by inflammatory stimulations was due to the enhanced transendothelial traffic, which was carried out by a population of cytoplasm vesicles. In line with these findings, the result of electron microscopy in the present study showed that the number of vesicles in cytoplasm of endothelial cells of venules was increased apparently by LPS continuous infusion, whereas the endothelial cells themselves and the intercellular junctions remained intact, implying that the LPS-induced albumin leakage observed in the present situation was accomplished by the transendothelial route. Treatment with compound Danshen injection reduced the number of endothelium cytoplasm vesicles as well as FITC-labeled albumin leakage from venular wall induced by LPS, suggesting that it inhibited albumin leakage from venular wall by modulation of the transendothelial traffic.
In summary, our present study demonstrated that compound Danshen injection significantly attenuated the LPS-induced microcirculatory disturbances, including endothelial cells damage, adhesion of leukocytes to venular wall, production of oxygen-free radical in venular wall, albumin leakage from venules and mast cell degranulation. Compound Danshen injection reduced adhesion of leukocytes to venular wall through inhibition of the expression of CD11b/CD18 in neutrophils or ICAM-1 in HUVECs or both. Furthermore, the protective effect of compound Danshen injection on the endothelium may ascribe to its diverse actions, including scavenging oxygen-free radicals from both leukocytes and endothelial cells, the attenuation of leukocyte adhesion, and the inhibition of the mast cell degranulation. Overall, the results of our present study revealed the multi-targeting potential of compound Danshen injection in the improvement of the LPS-induced microcirculatory disturbance. Experiments are currently being undertaken in our laboratory to discriminate among the constituents present in the compound Danshen injection which are responsible for the respective actions exerted by this medicine.
S- Editor Liu Y L- Editor Kumar M E- Editor Ma WH
| 1. | Darcissac EC, Bahr GM, Parant MA, Chedid LA, Riveau GJ. Selective induction of CD11a,b,c/CD18 and CD54 expression at the cell surface of human leukocytes by muramyl peptides. Cell Immunol. 1996;169:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Harris NR, Russell JM, Granger DN. Mediators of endotoxin-induced leukocyte adhesion in mesenteric postcapillary venules. Circ Shock. 1994;43:155-160. [PubMed] |
| 3. | Ikeda T, Funaba M. Altered function of murine mast cells in response to lipopolysaccharide and peptidoglycan. Immunol Lett. 2003;88:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Kurose I, Suematsu M, Miura S, Fukumura D, Sekizuka E, Nagata H, Oshio C, Tsuchiya M. Oxyradical generation from leukocytes during endotoxin-induced microcirculatory disturbance in rat mesentery--attenuating effect of cetraxate. Toxicol Appl Pharmacol. 1993;120:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Suzuki H, Suematsu M, Miura S, Liu YY, Watanabe K, Miyasaka M, Tsurufuji S, Tsuchiya M. Rat CINC/gro: a novel mediator for locomotive and secretagogue activation of neutrophils in vivo. J Leukoc Biol. 1994;55:652-657. [PubMed] |
| 6. | Wu X, Yoshida A, Sasano T, Iwakura Y, Endo Y. Histamine production via mast cell-independent induction of histidine decarboxylase in response to lipopolysaccharide and interleukin-1. Int Immunopharmacol. 2004;4:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Davenpeck KL, Steeber DA, Tedder TF, Bochner BS. P- and L-selectin mediate distinct but overlapping functions in endotoxin-induced leukocyte-endothelial interactions in the rat mesenteric microcirculation. J Immunol. 1997;159:1977-1986. [PubMed] |
| 8. | Sanz MJ, Alvarez A, Piqueras L, Cerdá M, Issekutz AC, Lobb RR, Cortijo J, Morcillo EJ. Rolipram inhibits leukocyte-endothelial cell interactions in vivo through P- and E-selectin downregulation. Br J Pharmacol. 2002;135:1872-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Lorenzon P, Vecile E, Nardon E, Ferrero E, Harlan JM, Tedesco F, Dobrina A. Endothelial cell E- and P-selectin and vascular cell adhesion molecule-1 function as signaling receptors. J Cell Biol. 1998;142:1381-1391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 166] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Moreland JG, Bailey G, Nauseef WM, Weiss JP. Organism-specific neutrophil-endothelial cell interactions in response to Escherichia coli, Streptococcus pneumoniae, and Staphylococcus aureus. J Immunol. 2004;172:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Moreland JG, Fuhrman RM, Pruessner JA, Schwartz DA. CD11b and intercellular adhesion molecule-1 are involved in pulmonary neutrophil recruitment in lipopolysaccharide-induced airway disease. Am J Respir Cell Mol Biol. 2002;27:474-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Moudry R, Spycher MO, Doran JE. Reconstituted high density lipoprotein modulates adherence of polymorphonuclear leukocytes to human endothelial cells. Shock. 1997;7:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Smith CW, Rothlein R, Hughes BJ, Mariscalco MM, Rudloff HE, Schmalstieg FC, Anderson DC. Recognition of an endothelial determinant for CD 18-dependent human neutrophil adherence and transendothelial migration. J Clin Invest. 1988;82:1746-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 443] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 14. | Iuvone T, Den Bossche RV, D'Acquisto F, Carnuccio R, Herman AG. Evidence that mast cell degranulation, histamine and tumour necrosis factor alpha release occur in LPS-induced plasma leakage in rat skin. Br J Pharmacol. 1999;128:700-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Wang JH, Sexton DM, Redmond HP, Watson RW, Croke DT, Bouchier-Hayes D. Intercellular adhesion molecule-1 (ICAM-1) is expressed on human neutrophils and is essential for neutrophil adherence and aggregation. Shock. 1997;8:357-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Horie Y, Han JY, Mori S, Konishi M, Kajihara M, Kaneko T, Yamagishi Y, Kato S, Ishii H, Hibi T. Herbal cardiotonic pills prevent gut ischemia/reperfusion-induced hepatic microvascular dysfunction in rats fed ethanol chronically. World J Gastroenterol. 2005;11:511-515. [PubMed] |
| 17. | Wang F, Liu YY, Liu LY, Guo J, Sun K, Wang CS, Fan JY, Han JY. Inhibition effect of cardiotonic pills on venous thrombosis induced in rat mesentery by photochemical reaction. Clin Hemorheol Microcirc. 2006;34:131-138. [PubMed] |
| 18. | Ling S, Dai A, Guo Z, Yan X, Komesaroff PA. Effects of a Chinese herbal preparation on vascular cells in culture: mechanisms of cardiovascular protection. Clin Exp Pharmacol Physiol. 2005;32:571-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Ding M, Zhao GR, Yuan YJ, Guo ZX. Aqueous extract of Salvia miltiorrhoza regulates adhesion molecule expression of tumor necrosis factor alpha-induced endothelial cells by blocking activation of nuclear factor kappaB. J Cardiovasc Pharmacol. 2005;45:516-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Ding M, Zhao GR, Ye TX, Yuan YJ, Guo ZX. Salvia miltiorrhiza protects endothelial cells against oxidative stress. J Altern Complement Med. 2006;12:5-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Kim JS, Narula AS, Jobin C. Salvia miltiorrhiza water-soluble extract, but not its constituent salvianolic acid B, abrogates LPS-induced NF-kappaB signalling in intestinal epithelial cells. Clin Exp Immunol. 2005;141:288-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Lin YL, Wu CH, Luo MH, Huang YJ, Wang CN, Shiao MS, Huang YT. In vitro protective effects of salvianolic acid B on primary hepatocytes and hepatic stellate cells. J Ethnopharmacol. 2006;105:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Zhao BL, Jiang W, Zhao Y, Hou JW, Xin WJ. Scavenging effects of salvia miltiorrhiza on free radicals and its protection for myocardial mitochondrial membranes from ischemia-reperfusion injury. Biochem Mol Biol Int. 1996;38:1171-1182. [PubMed] |
| 24. | Zhang JL, Cui M, He Y, Yu HL, Guo DA. Chemical fingerprint and metabolic fingerprint analysis of Danshen injection by HPLC-UV and HPLC-MS methods. J Pharm Biomed Anal. 2005;36:1029-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 25. | Han JY, Miura S, Akiba Y, Higuchi H, Kato S, Suzuki H, Yokoyama H, Ishii H. Chronic ethanol consumption exacerbates microcirculatory damage in rat mesentery after reperfusion. Am J Physiol Gastrointest Liver Physiol. 2001;280:G939-G948. [PubMed] |
| 26. | Sun K, Wang CS, Guo J, Liu YY, Wang F, Liu LY, He JG, Fan JY, Han JY. Effect of Panax notoginseng saponins on lipopolysaccharide-induced adhesion of leukocytes in rat mesenteric venules. Clin Hemorheol Microcirc. 2006;34:103-108. [PubMed] |
| 27. | Becker S, Dailey LA, Soukup JM, Grambow SC, Devlin RB, Huang YC. Seasonal variations in air pollution particle-induced inflammatory mediator release and oxidative stress. Environ Health Perspect. 2005;113:1032-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 211] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 28. | Davenpeck KL, Zagorski J, Schleimer RP, Bochner BS. Lipopolysaccharide-induced leukocyte rolling and adhesion in the rat mesenteric microcirculation: regulation by glucocorticoids and role of cytokines. J Immunol. 1998;161:6861-6870. [PubMed] |
| 29. | Klintman D, Li X, Thorlacius H. Important role of P-selectin for leukocyte recruitment, hepatocellular injury, and apoptosis in endotoxemic mice. Clin Diagn Lab Immunol. 2004;11:56-62. [PubMed] |
| 30. | McDowall A, Leitinger B, Stanley P, Bates PA, Randi AM, Hogg N. The I domain of integrin leukocyte function-associated antigen-1 is involved in a conformational change leading to high affinity binding to ligand intercellular adhesion molecule 1 (ICAM-1). J Biol Chem. 1998;273:27396-27403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Mesri M, Plescia J, Altieri DC. Dual regulation of ligand binding by CD11b I domain. Inhibition of intercellular adhesion and monocyte procoagulant activity by a factor X-derived peptide. J Biol Chem. 1998;273:744-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Balsam LB, Liang TW, Parkos CA. Functional mapping of CD11b/CD18 epitopes important in neutrophil-epithelial interactions: a central role of the I domain. J Immunol. 1998;160:5058-5065. [PubMed] |
| 33. | Hogg N, Leitinger B. Shape and shift changes related to the function of leukocyte integrins LFA-1 and Mac-1. J Leukoc Biol. 2001;69:893-898. [PubMed] |
| 34. | Jersmann HP, Hii CS, Ferrante JV, Ferrante A. Bacterial lipopolysaccharide and tumor necrosis factor alpha synergistically increase expression of human endothelial adhesion molecules through activation of NF-kappaB and p38 mitogen-activated protein kinase signaling pathways. Infect Immun. 2001;69:1273-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Van der Vieren M, Crowe DT, Hoekstra D, Vazeux R, Hoffman PA, Grayson MH, Bochner BS, Gallatin WM, Staunton DE. The leukocyte integrin alpha D beta 2 binds VCAM-1: evidence for a binding interface between I domain and VCAM-1. J Immunol. 1999;163:1984-1990. [PubMed] |
| 36. | Zhang F, Marcus WD, Goyal NH, Selvaraj P, Springer TA, Zhu C. Two-dimensional kinetics regulation of alphaLbeta2-ICAM-1 interaction by conformational changes of the alphaL-inserted domain. J Biol Chem. 2005;280:42207-42218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | McCurdy JD, Lin TJ, Marshall JS. Toll-like receptor 4-mediated activation of murine mast cells. J Leukoc Biol. 2001;70:977-984. [PubMed] |
| 38. | Vannier E, Miller LC, Dinarello CA. Histamine suppresses gene expression and synthesis of tumor necrosis factor alpha via histamine H2 receptors. J Exp Med. 1991;174:281-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 117] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Varadaradjalou S, Féger F, Thieblemont N, Hamouda NB, Pleau JM, Dy M, Arock M. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human mast cells. Eur J Immunol. 2003;33:899-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 204] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 40. | Leskinen MJ, Heikkilä HM, Speer MY, Hakala JK, Laine M, Kovanen PT, Lindstedt KA. Mast cell chymase induces smooth muscle cell apoptosis by disrupting NF-kappaB-mediated survival signaling. Exp Cell Res. 2006;312:1289-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | van Haaster CM, Derhaag JG, Engels W, Lemmens PJ, Gijsen AP, Hornstra G, van der Vusse GJ, Duijvestijn AM. Mast cell-mediated induction of ICAM-1, VCAM-1 and E-selectin in endothelial cells in vitro: constitutive release of inducing mediators but no effect of degranulation. Pflugers Arch. 1997;435:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | Kurose I, Pothoulakis C, LaMont JT, Anderson DC, Paulson JC, Miyasaka M, Wolf R, Granger DN. Clostridium difficile toxin A-induced microvascular dysfunction. Role of histamine. J Clin Invest. 1994;94:1919-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Joris I, DeGirolami U, Wortham K, Majno G. Vascular labelling with monastral blue B. Stain Technol. 1982;57:177-183. [PubMed] |
| 44. | Yoshida M, Wakabayashi G, Ishikawa H, Kawachi S, Tanabe M, Otani Y, Shimazu M, Kubota T, Kitajima M. Arteriovenous shunting blood flow is intravitally observed in the stomach after thermal injury in rats. Keio J Med. 2002;51:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Heckel K, Kiefmann R, Dörger M, Stoeckelhuber M, Goetz AE. Colloidal gold particles as a new in vivo marker of early acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2004;287:L867-L878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 46. | Yamaji T, Fukuhara T, Kinoshita M. Increased capillary permeability to albumin in diabetic rat myocardium. Circ Res. 1993;72:947-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |