INTRODUCTION
Debilitating pain is very common in patients with pancreatic cancer and chronic pancreatitis. Up to 70%-80% of patients with pancreatic cancer have pain at the time of diagnosis, which may increase to 90% as the disease advances[1,2]. Despite treatment options such as surgery, radiation and chemotherapy the prognosis remains poor[3,4]. Therefore, an important focus is improving the quality of life by optimal management of the symptoms[5,6]. However, despite adherence to the World Health Organization analgesic ladder consisting of medication titration (i.e., progressing from nonsteroidal anti-inflammatory drugs to narcotics), pain remains difficult to treat and frequently requires the use of high-dose narcotics. This results in unwanted side effects related to narcotic use such as constipation, nausea, vomiting, somnolence, confusion, and drug dependence and addiction[2,7-11].
The potential causes of pain in pancreatic cancer and chronic pancreatitis are poorly understood[12]. Possible etiologies include celiac plexus invasion by tumor infiltration, pancreatic duct obstruction and distention, inflammation and ischemia[7-9,13]. Overall, cancer-related pain is likely multidimensional[14]. This has led to different approaches to providing pain relief and reduction in drug dependence, including celiac plexus block and neurolysis.
ANATOMY
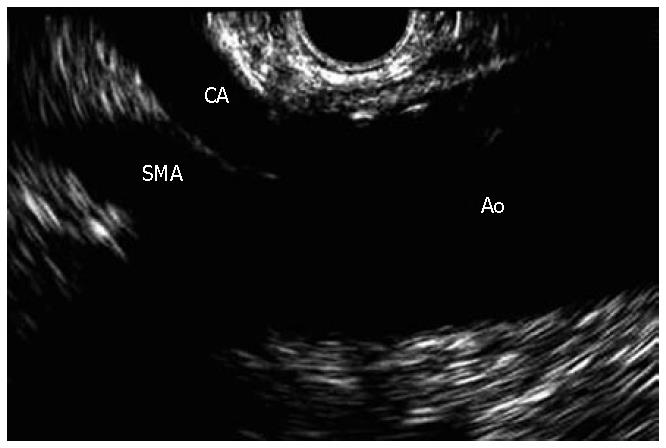
The celiac plexus is composed of a right and left ganglion, located anterolateral to the aorta at the level of the celiac trunk. In general, the crura of the diaphragm and the L1 vertebral body are located posterior to the celiac plexus, while the kidneys, adrenals and the inferior vena cava are present laterally, and the pancreas covers the celiac plexus anteriorly. However, the location of the celiac plexus in relation to the celiac trunk is the most reliable landmark, which is very helpful since the celiac ganglia are not easily identified on endoscopic ultrasound (EUS) (Figure 1)[7,8,10,15].
Figure 1 Linear EUS images of the aorta (Ao), celiac axis (CA), and superior mesenteric artery (SMA).
On average, the left and the right ganglion are located 0.9 cm and 0.6 cm inferior to the celiac artery respectively (Figure 2). Autonomic pancreatic nerves carry visceral afferent signals to the celiac plexus, and the central transmission occurs via the splanchnic nerves. Signals from the splanchnic nerves travel to the T5-T12 dorsal root ganglia, which are then carried up the spinal cord to the cerebral cortex[7,8,13,16,17].
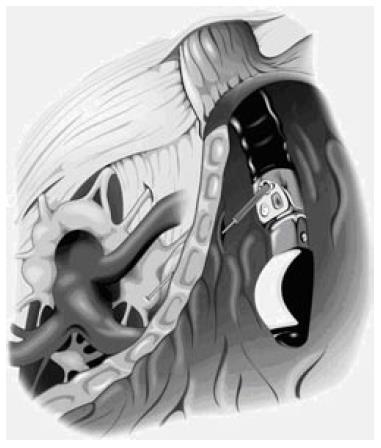
Figure 2 Illustration of the position of the celiac plexus, celiac trunk, and stomach when performing EUS-guided celiac injection.
OVERVIEW OF CELIAC PLEXUS BLOCK AND NEUROLYSIS
Celiac plexus block (CPB) has been used in the management of pancreatic pain since it was first described by Kappis in 1914[7,16-20]. CPB refers to the temporary inhibition of the celiac plexus often achieved with a corticosteroid injection in patients with benign pancreatic diseases like chronic pancreatitis. A local anesthetic such as bupivacaine is often used in combination with the steroid injection to provide a more prolonged analgesic effect compared to the local anesthetic alone[7,8]. Celiac plexus neurolysis (CPN) refers to the ablation of the plexus, often achieved with alcohol or phenol administered with a local anesthetic, such as bupivicaine which is injected first to prevent pain associated with the alcohol injection. CPN with alcohol is not routinely used in benign diseases given the risk of retroperitoneal fibrosis, which would render any subsequent pancreatic surgery more difficult[8].
Until the recent development of EUS, using curved-array linear echoendoscopes and the widespread availability of cross sectional imaging, CPB/CPN was limited to anesthesiologists, surgeons and interventional radiologists who used the posterior approach or employed intraoperative splanchnicectomy[21]. With the posterior technique, a needle is introduced posterolateral to the L1 vertebra alongside the vertebral body, and various approaches were used including transaortic, retrocrural and transcrural to reach the celiac plexus[17,23-27]. However, serious complications occur in 1% patients, including paraplegia, as a result of the needle entering a spinal artery or piercing the dura mater, and pneumothorax from piercing the diaphragm[7,8,18,22,28,29].
These complications have led to the development of an anterior approach under the guidance of transcutaneous ultrasound, computed tomography or EUS (Figure 2)[18,30-32,60]. EUS allows for real-time imaging of the celiac space for CPB and CPN as well as fine needle aspiration (FNA) for diagnostic purposes and tumor staging[10,34-38,71]. EUS-guided fine needle injection has been used even in patients with enternal self-expandable stents[39]. In a small prospective randomized study on 22 patients, Gress et al compared EUS-versus CT-guided CPB in the treatment of chronic pancreatitis pain. Nearly 50% patients who underwent EUS-guided CPB experienced significant improvement in pain scores allowing for reduction in the pain medications, compared with only a 25% reduction in pain relief in patients who had CT-guided CPB. Also, 40% and 30% of the EUS-guided CPB patients had continued benefit at 8 wk and 24 wk, respectively, compared to only 12% of the CT-guided CPB patients at 12 wk. Three patients failed to experience pain relief with either EUS- or CT-guided CPB. These patients were treated surgically with partial pancreatectomy, but failed to experience any pain improvement[19].
Although no serious complications were observed with either technique, EUS was preferred by patients who had experienced both procedures. This was attributed to the lack of any back discomfort associated with the CT-guided approach, as well as superior sedation with EUS. Moreover, EUS was found to be more cost effective[19]. Overall, both these procedures which utilize the anterior approach appear to provide greater benefit, given the decreased risk of any major neurologic complications. However, the anterior approach is also associated some serious complications including retroperitoneal abscess formation[40].
CPN FOR PANCREATIC CANCER PAIN
Most of the current experience with CPN has been obtained in the management of pancreatic cancer pain. Given the overall poor outcome of pancreatic cancer, one of the main goals of treatment is palliation. Pain in pancreatic cancer occurs frequently and can be very frustrating both for the patient and the physician. High dose narcotics are often unsatisfactory because of the impact on the quality of life due to their adverse effects. Therefore, one of the goals of CPN is to improve the quality of life in these patients through superior pain control. Eisenberg et al published a meta-analysis of 24 published articles (21 retrospective, 1 prospective and 2 randomized controlled trials) involving 1145 patients treated with percutaneous CPN for cancer pain. The majority (68%) of the percutaneous CPN procedures were performed radiologically, with the bilateral posterior approach being the most common. The type of cancer was reported in 1117 of the 1145 patients, and 63% were pancreatic cancer. After the first 2 wk, 89% patients reported good to excellent pain relief, with partial to complete pain relief being maintained in 90% patients at 3 mo, and in 70%-90% beyond 3 mo. It was noted that patients with pancreatic cancer had a similar response to treatment with CPN as patients with other intraabdominal malignancies. However, several severe side effects were reported, which included neurologic complications (1%), such as lower extremity weakness and parasthesia, and nonneurologic complications (1%) such as a pneumothorax[22].
Other studies have compared CPN with analgesics in patients with pancreatic cancer. In 1993, Mercadante evaluated 20 patients with pancreatic cancer and compared CPN plus analgesics versus analgesics alone. CPN use was associated with lower opioid consumption and better pain control. In the group treated with analgesics alone, pain control required higher opioid consumption and had more drug-related adverse effects[41]. In 2003, Mercadante et al reported a multicenter study on 22 patients with pancreatic cancer who underwent CPN and were followed until death. The therapeutic effects of CPN lasted for 4-5 wk post procedure, and although improved analgesia was attributed to CPN over the 4-5 wk period, narcotic use increased as the disease progressed. None of the variables examined (such as age, gender, initial cancer site, sites of pain, peritoneal involvement or technique) influenced the effect of CPN[42].
A study by Kawamata et al in 1995 compared CPN (percutaneous posterior approach with x-ray guidance) with narcotics in 21 patients with pancreatic cancer pain. There was significantly superior pain control in the CPN group during the first 4 wk compared with patients who received analgesia alone. Moreover, morphine consumption was significantly lower in the CPN treated patients, and continued to remain so in weeks 4-7. After 7 wk, although morphine consumption continued to be lower in the CPN group, the difference was not significant. Both groups achieved satisfactory pain relief, but given the lower narcotic consumption, CPN use was associated with less deterioration in the quality of life secondary to narcotic side effects[43]. These findings are consistent with the results of a randomized, double-blind study by Polati et al that looked at CPN (posterior approach under fluoroscopy) versus analgesic therapy in 24 patients with pancreatic cancer pain. Findings of immediate pain relief and decrease in the mean consumption of analgesics were seen in the CPN group leading to reduction in medication-induced adverse effects. Also, CPN combined with drug therapy led to complete pain relief until death in 75% of the patients compared with 58% of patients treated with analgesics alone[44].
Several studies have assessed the impact of CPN on the quality of life and life expectancy in pancreatic cancer. Lillemoe et al in 1993 in a double-blind randomized study on 139 patients with unresectable pancreatic cancer, compared surgical splanchnicectomy with 50% alcohol in saline (65 patients) with sham saline block (72 patients). Pain relief was significantly superior at 2, 4 and 6 mo in the CPN group compared with placebo. More importantly, patients who received alcohol injection showed significant improvement in survival compared with the controls (P < 0.0001)[45]. In 2001, Staats et al used the data from the Lillemoe et al study to explore the effects of negative mood state on pain status and longevity. Data obtained from 130 patients showed that higher pain intensity was associated with greater interference with patient activity and higher negative mood state. When compared with the placebo group, the CPN group had less pain, better mood state, reduced interference with daily activity, and an overall increase in life expectancy. Possible explanations for the improvement in longevity include improvement in the immune system because of superior control of pain and depression, increased ambulation secondary to reduction in pain leading to fewer complications such as DVTs and pulmonary emboli, and better compliance and adherence to palliative treatment[46]. In another study, celiac ganglion destruction in patients with advanced pancreatic cancer was found to improve immunity at the cellular level[47].
However, other studies have not shown improvement in quality of life and life expectancy. Wong et al in a randomized controlled trial examined the effect of CPN (posterior approach under fluoroscopic guidance) on pain relief, survival and quality of life in patients with unresectable pancreatic cancer. Data from the 100 patients showed that CPN provided better pain relief, but had no effect on opioid consumption or quality of life. A trend towards improved survival was seen in the CPN group, but did not reach statistical significance[48]. Yan et al in 2007 included this data in a meta-analysis of five randomized controlled trials on the effect of CPN in pain control in unresectable pancreatic cancer. CPN use was associated with a significant reduction in pain intensity and narcotic use, however, survival was not impacted. The effect on quality of life could not be adequately analyzed because of the differences in the outcome scales in the studies. Also of note, constipation improved with reduced narcotic usage, but other narcotic-related side effects like nausea, vomiting and lethargy did not improve[49]. These findings are in conflict with some previous nonrandomized studies that showed an overall improvement in the quality of life and survival[46].
Patient selection appears to be an important factor in pain reduction by CPN in pancreatic cancer patients. It has been observed that patients with pancreatic head tumors are more likely to achieve pain reduction from CPN than patients with tumors in the pancreatic body or tail. This seems intuitive since most patients with tumors in their pancreatic body and tail are diagnosed in more advanced stages[50]. However, other studies have not confirmed this observation[42].
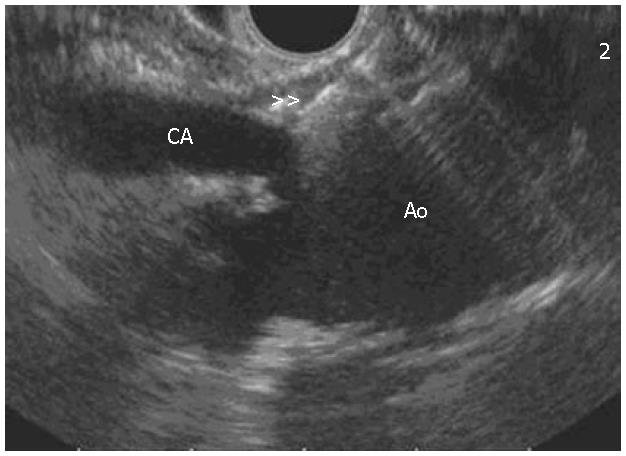
Another factor that may improve pain scores post CPN is needle position. De Cicco et al in 1997 studied the effects of needle position with CPN via an anterior approach using computed tomography needle guidance. All patients had normal celiac area anatomy. It was observed that the needle position cephalad to the celiac artery achieved wider distribution of neurolysis, and therefore longer lasting pain control[51].This finding is consistent with the observation in EUS CPN or CPB where the injection is given into the cephalad area of the celiac trunk (Figure 3).
Figure 3 EUS image of the needle (double arrows) located at the celiac plexus in relation to the celiac axis (CA) and aorta (Ao).
Even though all these studies have shown reduction in pain with CPN in patients with pancreatic cancer, the majority have used the posterior approach for CPN. Although, the posterior approach is usually well tolerated, 1% of patients develop serious complications such as lower extremity weakness, paresthesias or pneumothorax[18,20,22,28,29]. These complications can be avoided by using EUS, which allows direct access to the celiac plexus.
EUS has been shown to be an effective method for providing pain control in pancreatic cancer patients. Wiersema in 1996 studied EUS-guided CPN in 30 patients with intraabdominal malignancy (25 with pancreatic cancer). Improvement in pain at 2, 4, 8 and 12 wk post CPN was noted in 79%-88% of patients[52]. In 2001, Gunaratnam et al reported 58 patients with unresectable pancreatic cancer who underwent EUS-guided CPN; the patients were followed for 6 mo. CPN reduced pain in 78% patients, however, narcotic usage did not alter significantly. After CPN, 14% patients received chemotherapy with gemcitabine alone, while 17% received chemotherapy (5-fluorouracil) plus radiation within 3 wk of CPN. Patients who received adjuvant therapy along with CPN had superior pain reduction compared with patients who did not receive adjuvant therapy[20]. These results are in conflict with the findings by Wiersema et al[52] who showed no pain reduction with chemotherapy and/or radiation.
It has been reported in several studies that EUS-guided CPN should be considered early in pancreatic cancer. Pain in pancreatic cancer is more visceral in nature earlier in the disease process. As the cancer progresses and more advanced tumor infiltration occurs, the pain becomes multifactorial involving somatic and neuropathic pathways along with other visceral pathways besides the celiac plexus[19,43,48,53]. However, a study published by de Oliveira et al in 2004 showed no difference in pain reduction in early versus late neurolytic plexus block in 25 patients with intraabdominal cancer. Patients that achieved early and late neurolytic plexus block had a significant reduction in their pain, improved quality of life and decreased narcotic consumption[1]. Therefore, even though pain reduction can still occur with late neurolytic plexus block, the earlier that the neurolytic block occurs, the better off the patient because of fewer adverse effects secondary to reduction in narcotic consumption.
Overall, EUS-guided CPN is a safe and effective procedure in providing palliative care in patients with pancreatic cancer. Besides utilizing the anterior approach and avoiding the diaphragm as well as the spinal arteries and nerves, EUS is able to utilize continuous, real-time imaging for direct access to the celiac plexus, and in addition provides FNA sampling and tumor staging[10,34-38]. EUS-guided CPN should be considered as an adjuvant therapy in the management of pain in all patients with pancreatic cancer.
CPB FOR CHRONIC PANCREATITIS PAIN
Pain in chronic pancreatitis likely has similar pathways as pancreatic cancer pain and a visceral component is definitely present as CPB has been successfully used to reduce pain in chronic pancreatitis. However, the role of CPB in the treatment of pancreatitis pain is more controversial compared with pancreatic cancer pain[15]. It has been suggested that in chronic pancreatitis, EUS CPB should be limited to patients whose pain has not responded to other modalities or for the treatment of flares of chronic pain[54-56]. Some authors have advocated surgical bypass or resection as the primary treatment of pain in chronic pancreatitis[57]. Leung et al in 1983 compared percutaneous neurolysis in pancreatitis and pancreatic cancer and observed that 84.6% (11 out of 13) patients with pancreatic cancer had complete pain relief initially, while only 52% (12 of 23) patients with pancreatitis had initial complete pain relief. Patients with chronic pancreatitis remained pain free for a mean of 2 mo, while 53.8% of the patients with pancreatic cancer remained pain free until death[58]. As with the use of CPN in pancreatic cancer pain, some studies have advocated CPB early in the management of pain in pancreatitis, especially before the patient becomes dependent on narcotics[59].
One of the first studies on EUS-guided CPB in pancreatitis management was reported by Faigel et al in 1996. These workers reported a patient with chronic pancreatitis who was treated with EUS and fluoroscopic injection of a bupivacaine and epinephrine mixture. The patient obtained initial pain relief which returned to the baseline state shortly after the injection[60].
Gress et al in 2001 reported 90 patients with chronic pancreatitis pain who had failed previous treatments. All patients underwent EUS-guided CPB; pain reduction was achieved in 55% patients at a mean follow-up of 8 wk, while 10% experienced persistent benefit at 24 wk. It was observed that patients younger than 45 years and those with a prior history of surgery for chronic pancreatitis were less likely to respond to CBD. There was no correlation between the response to CPB and the etiology of pancreatitis[61].
Basinski et al in 2005 compared celiac plexus block with videothoracoscopic splanchnicectomy (VSPL) with respect to pain relief and quality of life in patients with chronic pancreatitis. Both procedures had a positive effect with reduction in pain medications and improvement in fatigue and emotional well-being. Celiac plexus block was superior in terms of social support compared with VSPL[62]. Like CPN, VSPL has also been shown to improve the quality of life and reduce pain in patients with inoperable pancreatic cancer[63].
EUS BENEFITS AND COMPLICATIONS
As with CPN in the treatment of pain in pancreatic cancer, CPB is just one of several effective treatments in pancreatitis pain. EUS-guided CPB and CPN appear to be as effective and safe as other techniques, while being more cost effective since biopsy, FNA and staging can be obtained at the same sitting. Transient diarrhea, pain and hypotension are the common complications associated with EUS-guided CPB and CPN. Transient diarrhea and hypotension have been reported in 44% and 38%, respectively, in patients undergoing CPN, given the unopposed parasympathetic activity[13,15,22,64]. However, severe chronic diarrhea has also been reported post CPB[65,66]; potential treatments include intravenous atropine and octreotide[67,68]. Transient hypotension is minimized by infusion of normal saline while the patient is recovering from conscious sedation[33]. Transient pain has been reported, but in the Gunaratnam et al[20] study only 9% of patients experienced an increase in the pain, which lasted 48 h. Gastroparesis has also been reported as a rare complication of CPB[69]. The anterior approach utilized by EUS reduces the risk of major complications such as paraplegia, but other serious complications such as retroperitoneal hemorrhage and peripancreatic abscess formation have been reported[70]. The risk of abscess formation has led to the recommendations of antibiotic coverage prior to EUS CPB, but its use remains operator dependent. Antibiotics are not routinely required in CPN as the bactericidal effects of alcohol appear to be adequate.
CONCLUSION
EUS guided CPB and CPN are valuable tools for pain management in patients with pancreatic pain. EUS-guided CPN should be considered as first line therapy in patients with pain due to pancreatic cancer. It provides superior pain control compared to the traditional management with narcotics. A trend for improved survival in pancreatic cancer patients treated with CPN has been reported, but larger studies are required to confirm this finding. At this time, the use of EUS-guided CPB cannot be recommended as routine therapy for pain in patients with chronic pancreatitis. Only one-half of the patients experience pain reduction and the beneficial effect tends to be short lived. EUS-guided CPB and CPN should be used as part of a multidisciplinary team approach for pain management.
S- Editor Ma N L- Editor Anand BS E- Editor Wang HF