Published online Jul 7, 2007. doi: 10.3748/wjg.v13.i25.3493
Revised: March 7, 2007
Accepted: March 29, 2007
Published online: July 7, 2007
AIM: To investigate the effect of Platycodon grandi-florum extract (PGE) on lipid metabolism and FABP mRNA expression in subcutaneous adipose tissue of high fat diet-induced obese rats.
METHODS: PGE was treated to investigate the inhibitory effect on the pre-adipocyte 3T3-L1 differentiation and pancreatic lipase activity. Male Sprague-Dawley rats with an average weight of 439.03 ± 7.61 g were divided into four groups: the control groups that fed an experimental diet alone (C and H group) and PGE treatment groups that administered PGE along with a control diet or HFD at a concentration of 150 mg/kg body weight (C + PGE and H + PGE group, respectively) for 7 wk. Plasma total cholesterol (TC) and triglycerol (TG) concentrations were measured from the tail vein of rats. Adipocyte cell area was measured from subcutaneous adipose tissue and the fatty acid binding protein (FABP) mRNA expression was analyzed by northern blot analysis.
RESULTS: PGE treatment inhibited 3T3-L1 pre-adipocyte differentiation and fat accumulation, and also decreased pancreatic lipase activity. In this experiment, PGE significantly reduced plasma TC and TG concentrations as well as body weight and subcutaneous adipose tissue weight. PGE also significantly decreased the size of subcutaneous adipocytes. Furthermore, it significantly repressed the up-regulation of FABP mRNA expression induced by a high-fat feeding in subcutaneous adipose tissue.
CONCLUSION: PGE has a plasma lipid lowering-effect and anti-obesity effect in obese rats fed a high fat diet. From these results, we can suggest the possibility that PGE can be used as a food ingredient or drug component to therapeutically control obesity.
-
Citation: Park YS, Yoon Y, Ahn HS.
Platycodon grandiflorum extract represses up-regulated adipocyte fatty acid binding protein triggered by a high fat feeding in obese rats. World J Gastroenterol 2007; 13(25): 3493-3499 - URL: https://www.wjgnet.com/1007-9327/full/v13/i25/3493.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i25.3493
Obesity accelerates the accumulation of excessive fat, which can cause additional metabolic diseases such as fatty liver, hyperlipidemia, hypertension, arteriosclerosis, and diabetes[1-4]. To treat and control obesity, besides diet therapy and exercise, many different approaches such as drugs for weight loss or loss of appetite and food supplements are suggested to date. However, some of them have been reported to have serious side effects like vomiting, headache, stomach-ache and heart attack. Oriental medicinal herb extracts have been reported to be useful for the control of blood pressure, glucose concentration and hyperlipidemia with less significant side effects[3,4]. However, the majority of these herb extracts have yet to be scientifically or medically evaluated[5].
In line with this, we used oriental medicinal herbs that had been expected to have a lipid lowering effect based on the previous reports[6,7].
Platycodon grandiflorum is a perennial plant of the Campanulaceae family containing triterpenoid saponin, carbohydrates, and fibers. Platycodon grandiflorum is an oriental herb known to improve insulin resistance and the lipid profile in rats with diet-induced obesity[8-10]. Many previous studies have reported the lipid-lowering activity of Platycodon grandiflorum in serum and liver, and the fibers in Platycodon grandiflorum inhibited the progress of atherosclerosis by reducing cholesterol levels in rats. Regarding the mechanism of the lipid lowering effect of Platycodon grandiflorum, there is a report that saponin, a major component of Platycodon grandiflorum reduced serum cholesterol concentration by inhibiting the reuptake of bile acids in the intestine and increasing fecal excretion[5,11-13]. Considering the above reports, dietary Platycodon grandiflorum may be useful in the prevention and improvement of metabolic disorders such as NIDDM, syndrome X, and coronary artery disease, hypercholesterolemia and hyperlipidemia[6,7]. We also previously reported the effect of PGE on FABP expression along with dietary conversion from a long-term high fat diet to a normal control diet in obese rats[14].
Long-term intake of a high fat diet could alter the expression of genes governing lipid metabolism including fatty acid metabolism, and increase FABPs expression in the liver, heart, intestine and adipose tissues. One of the FABPs, also known as adipocyte lipid-binding protein (aP2), belongs to a family of intracellular lipid-binding proteins involved in the transport and storage of lipids[15], that are 14-15 kDa cytosolic fatty acid binding proteins (FABPs), and are expressed in a highly tissue specific manner[16]. FABP has been proposed to be involved in intracellular trafficking and utilization and storage of long chain fatty acids. Its expression is modulated by developmental, hormonal, pharmacological and dietary factors.
Adipocyte FABP content was increased by a high fat diet, indicating that the high fat diet increased the need for the oxidation of fatty acids and utilization in hepatocyte[17-19].
Although the lipid lowering effect of PGE in treating and preventing obesity has been reported, its underlying molecular mechanism has not been completely elucidated yet. Thus, in this study, we tried to investigate the molecular mechanism of PGE by examining its effect on FABP mRNA expression in white adipose tissue of obese rats.
Trizol reagent was obtained from GIBCO BRL (Invitrogen, Carlsbad, CA). All other chemicals and reagents, unless noted otherwise, were obtained from Sigma (St. Louis, MO). All the chemicals were of reagent grade. Laboratory diet components were purchased from CLEA Japan (Osaka Japan).
Platycodon grandiflorum, the oriental medicinal herb used in this experiment was approved as an ingredient in food and has been used in oriental medicine for the treatment of obesity in Korea. Platycodon grandiflorum was obtained from products of Yeongcheon-gun, Gyeongsangbuk-do, Korea. Platycodon grandiflorum was extracted in 5 volumes of distilled water for 2 h. The extract was collected and concentrated by vacuum evaporator (Buchi Rotavapor R-114, Switzerland) then freeze-dried for use in this experiment.
Pancreatic lipase activity in the porcine pancreas was determined by measuring the rate of release of oleic acid from triolein, as previously reported[5]. Briefly, a suspension of triolein (80 mg), phosphatidylcholine (10 mg) and taurocholic acid (5 mg) in 9 mL of 0.1 mol/L N-tris (hydroxymethyl) methyl-2-aminoethanesulfonic acid buffer (pH 7.0) containing 0.1 mol/L NaCl was sonicated for 5 min. This sonicated substrate suspension (0.1 mL) was incubated with 0.05 mL (10 U) of pancreatic lipase and 0.1 mL of various concentrations of PGE (for 30 min at 37°C in a final volume of 0.25 mL). The amount of oleic acid released was determined by the spectrophotometric method[5].
3T3-L1 preadipocytes were cultured and differentiated as previously described[20]. In short, cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) in 5% CO2 at 37°C. Two days after complete confluence was reached (d 0), differentiation was induced by changing the medium to DMEM containing 10% FBS plus 0.5 mmol/L 3-isobutyl-1-methylxanthine (MIX) (Sigma), 1 mg/mL insulin (Sigma) and 1 mmol/L dexamethasone (DEX) (Sigma). After 48 h (d 2), the medium was replaced with DMEM containing 10% FBS plus 1 mg/mL insulin. On d 4, the medium was replaced with DMEM containing only 10% FBS, this step was repeated every 2 d thereafter, until d 9. Differentiation was assessed by morphological changes and Oil red O staining method[20].
The animals used in this study were 7-wk-old male Sprague-Dawley rats (Charles River Diagnostics, Wilmington, MA) with an average body weight of 439.03 ± 7.01 g. The animals were housed two per metabolic cage and maintained with regular feeding every day during the experimental period. The animal room was maintained at 22 ± 2°C. Room lighting consisted of 12 h periods of light and dark. The diet and water were given ad libitum. After one week of adaptation, the experimental diets were given for 7 wk along with the oral administration of PGE. Body weight was measured every week. Blood was collected from the tail vein at the start and the end of the experiment. Food intake was measured every other day during the experimental period, the food efficiency ratio (FER) was calculated by the average value of food intake each group.
All the animal experimental procedures were conducted in accordance with guidelines from the Korean National Health Institute of Health Animal Facility.
The experimental diet was prepared based on AIN-93[21]. The control diet generated 11.7% of its total energy from fat. The high fat diet, in which corn oil and some portion of the corn starch in the control diet were replaced by lard, generated 59.8% of its total energy from fat. The experimental groups in this study were divided into four groups: the control (C and H groups) and PGE treatment groups that were administered PGE along with a control or high fat diet (H) at a concentration of 150 mg/kg body weight (C + PGE and H + PGE group, respectively) for 7 wk. C and H represented the control diet containing normal fat and high fat diet containing 30% lard, respectively. The composition of the experimental diet is shown in Table 1.
| Control diet (%) | HFD (%) | |
| Casein | 16 | 15 |
| Sucrose | 10 | 10 |
| Corn starch | 59 | 31 |
| Lard (80% contained) | - | 37 |
| Corn oil | 5 | - |
| Cellulose | 5 | 2 |
| Vitamin Mix | 1 | 1 |
| Mineral Mix | 3.5 | 3.5 |
| Choline bitartarate | 0.2 | 0.2 |
| DL-Methionine | 0.3 | 0.3 |
| Energy (Kcal/100 g) | 385 | 557 |
| Energy from fat (%) | 11.7 | 59.7 |
At the end of the experiment, the food was removed and experiments were performed between 9 AM and 12 PM. The rats were anesthetized with intraperitoneal injection of pentobarbital (60 mg/kg body weight). Blood samples were obtained from the tail vein of rats at the start and end of the experiment. Blood samples were rapidly centrifuged at 3000 ×g for 15 min, at 4°C and plasma was obtained and stored at -70°C before analysis. TC and TG concentrations were determined by a spectrophotometric method of a diagnostic kit from Sigma as previously described[22].
Subcutaneous adipose tissue was removed from rats and weighed. The samples were frozen in liquid nitrogen and stored at -70°C. For adipocyte staining, frozen subcutaneous adipose tissue was serially sliced at a thickness of 20 μm using a freezing microtome (Cryostat, Leica Microsystems, Nussloch GmbH, Switzerland). The samples were stained with Mayer's hematoxylin and its area was measured by using light microscopy and an image analysis program.
Total cellular RNA from homogenates of frozen subcutaneous adipose tissue was isolated using Trizol reagents, quantified by UV spectrophotometry, and stored at -70°C prior to use. Total RNA (15 μg) was electrophoresed on 1% (w/v) agarose gel and transferred to a Nylon membrane (BioRad, Hercules, CA). The cDNA fragments were prepared by PCR using specific primers. The primer pairs for PCR were as follows: for FABP (420 bp), forward 5'-AGA ACC ACA CGG CTA CCA TGC T-3', reverse 5'-TGT GTC CAT CAG CTC CAG TTG C-3'; for leptin (162 bp), forward 5'-CAG GGA GGA AAA TGT GCT GGA G, reverse 5'-CCG ACT GCG TGT GTG AAA TGT C and for LPL (198 bp), forward 5'-AGG GCT CTG CCT GAG TTG TA, reverse 5'-AGA AAT TTC GAA GGC CTG GT. The amplification of actin cDNA fragments (500 bp) was performed in a separate tube to enable semi-quantitative normalization using the following primers: forward 5'-ACC ACA GTC CAT GCC ATC AC-3', reverse 5'-TCC ACC ACC TGT TGC TGT A-3'. The PCR products were separated on 1% agarose gel, eluted and used for the hybridization probe. The probe (25 ng) was labeled with [α32P]dCTP (50 μCi) using a Random-primed DNA Labeling Kit (TAKARA Shuzo Co., Ltd.). Electroblotted RNA on a nylon membrane was hybridized with the labeled cDNA fragment at 65°C for 16 h. Hybridized bands were quantified using the Image Analyzing Device (Vilber Lourmat, France) and the bands of the images were analyzed with densitometer software (Molecular Dynamics Image Quant Version 3.3).
Statistical analysis for the experimental results were performed using SPSS (Version 11.0) program and all measurements were expressed as mean ± SE. Statistically significant differences between the groups were found using one way-ANOVA[23]. Duncan's multiple-range test was then performed on the analyzed values to identify significant differences between the average values of each treatment group. P < 0.05 was considered statistically significant.
The PGE significantly inhibited the pancreatic lipase activity in a dose dependent manner, PGE (10 mg/mL) treatment dropped the pancreatic lipase activity approximately in half (Table 2).
| Platycodon grandiflorun extract concentration (mg/mL) | Pancreatic lipase activity (% of control) |
| 0 | 100 ± 1.0a |
| 0.01 | 87.80 ± 1.08a |
| 0.1 | 85.38 ± 4.14a |
| 1 | 70.92 ± 1.59a |
| 10 | 52.30 ± 3.21a |
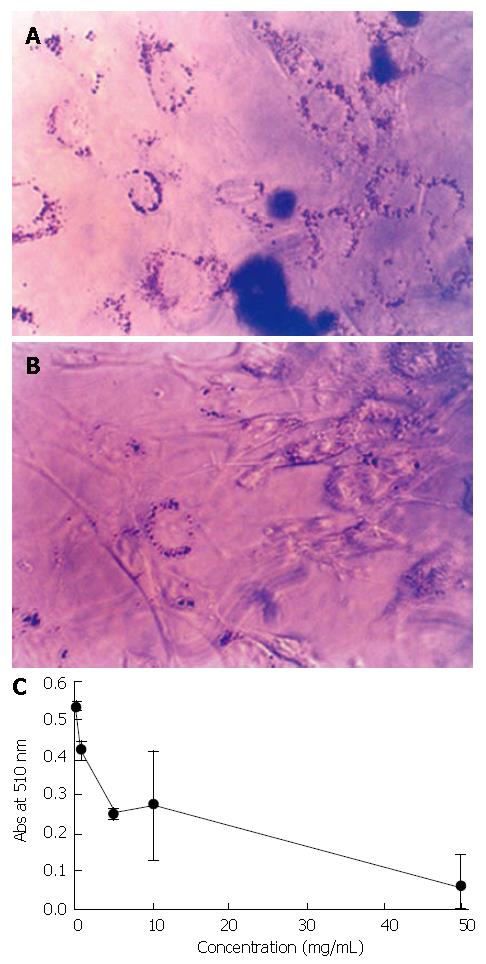
When differentiating adipocytes were stained with the fat-specific Oil Red O at d 13, a fully differentiated state, there was a significant difference between PGE treated (Figure 1B) and non-treated control cells (Figure 1A). The result of Oil Red O quantification showed PGE treatment decreased lipid accumulation. At 50 mg/mL concentration, lipid accumulation was decreased to approximately 10% compared to the PGE non-treated control group (Figure 1C).
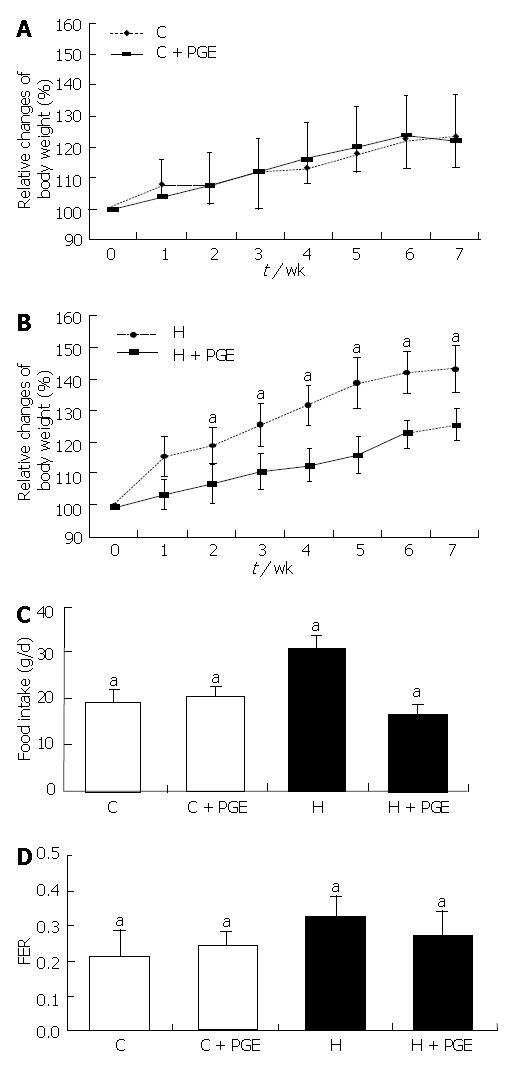
Figure 2A and B showed the changes in body weight of the control group (C) and the high-fat diet group (H), throughout the experimental period. The % changes in body weight were given in relation to the initial weight at wk 0. There were no significant differences in the average initial body weight among the groups. At the beginning of the experiment, the average body weight of the 7-wk old-rats were 439.03 ± 7.61 g and significantly increased to 516.19 ± 5.98 g after 7 wk of feeding a control diet. In the control diet groups, no significant effect was observed for PGE in reducing body weight. However, PGE treatment made a significant difference in the high fat diet groups. This was significantly inhibited by oral administration of PGE indicating it could give a beneficial effect on body weight reduction in the H groups.
Food intake was also higher in the H group than in the C group. When PGE was administered orally for 7 wk along with a high fat diet, the reduction in food intake was comparable to that of the C group (P < 0.05). The food intake ratio (food efficiency ratio, FER) showed a similar pattern as that of the food intake (Figure 2C and D).
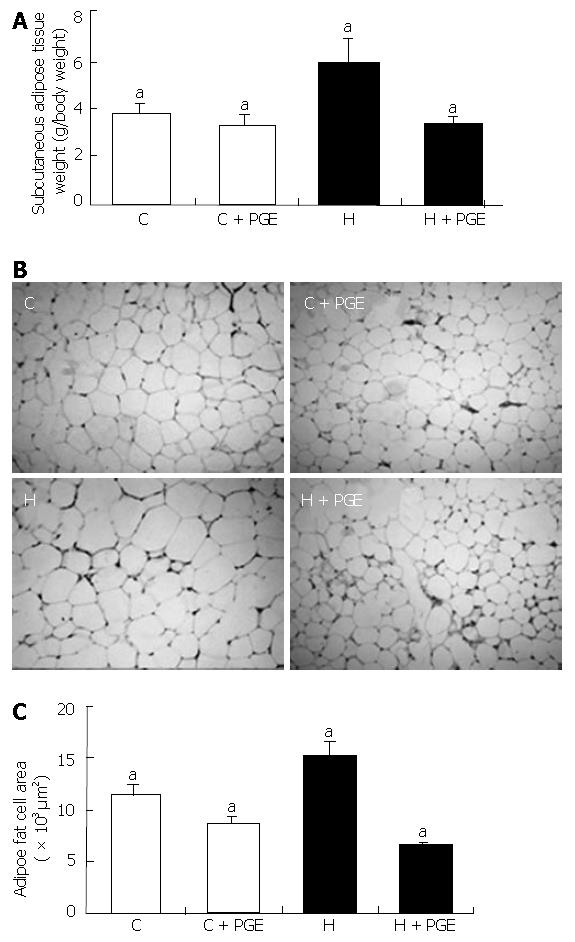
Subcutaneous adipose tissue weight was significantly higher in the H group than in the C group (5.92 ± 1.77 vs 3.81 ± 0.58). PGE also significantly decreased the subcutaneous adipose tissue weight in the H group, comparable to that of the C group (Figure 3A). This effect indicated that the administration of PGE was important in adipose tissue weight reduction as well as in body weight.
The subcutaneous adipocyte size of each group was shown in Figure 3 B and C. The size of adipocytes in the H group was significantly larger than those of the C group. When rats were fed a high fat diet and administered PGE (H + PGE group), the size of the adipocytes was significantly decreased compared to those of the control diet group (C + PGE group).
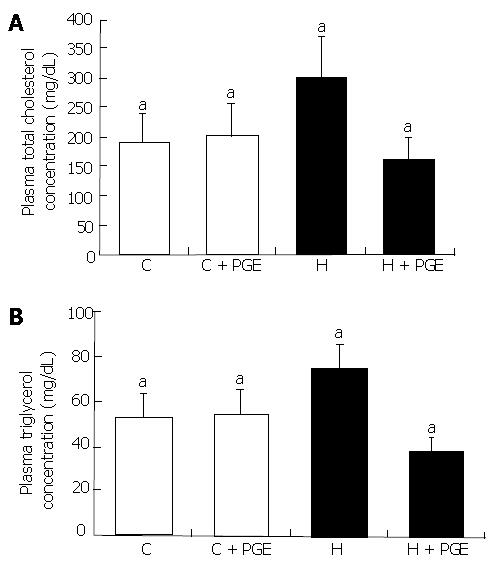
Plasma TC concentration at 7 wk in the C and H groups were 189.05 ± 51.54 mg/mL and 294.44 ± 53.5 mg/mL, respectively. Plasma TG concentration at 7 wk in the C and H groups were 54.77 ± 11.55 mg/mL and 78.25 ± 10.95 mg/mL, respectively (Figure 4). PGE treatment showed significant plasma lipid lowering effect in the H group (H + PGE groups, P < 0.05).
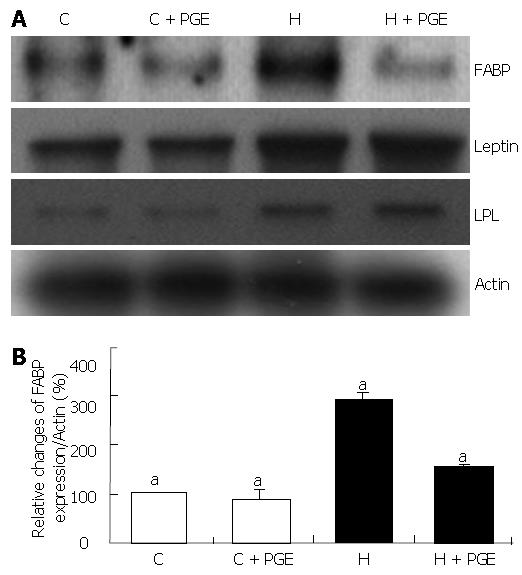
In the H group, FABP gene expression in subcutaneous adipose tissue was significantly increased approximately 290% as compared to the C group (Figure 5). This HFD induced FABP mRNA up-regulation was reduced significantly by administration of PGE (P< 0.05). However, there was no significant difference in C groups (C and C + PGE group). Although the mRNA expressions of leptin and LPL (lipoprotein lipase), a well known adipocytokine and major enzyme of lipoprotein metabolism respectively, were also significantly increased in the H group, no significant effect of PGE on their gene expressions was observed.
In the present study, we tried to investigate the effect of Platycodon grandiflorum on diet-induced obesity improvement and treatment and its underlying mechanism. PGE treatment inhibited 3T3-L1 pre-adipocyte differentiation and fat accumulation, and also decreased pancreatic lipase activity. Thus, we speculate that PGE exerts a lipid lowering effect via inhibition of pancreatic lipase activity resulting in lowered lipolysis and small intestinal absorption of dietary fat. During the digestion process dietary fat is hydrolyzed by pancreatic lipase. The two main products formed by the hydrolysis of pancreatic lipase are fatty acid and 2-monoacylglycerols. These lipolytic products are mixed with bile salts, dispersed as micelles and carried to the site of fat absorption. Lipid absorption takes place in the apical part of the plasma membrane of epithelial cells or enterocytes lining the gut[24,25]. In the process of lipid absorption, FABP regulates free fatty acid absorption, transport, storage. We also performed an animal study to confirm its effect in vivo using the concentration (150 mg/kg body weight/day) which was within the range approved by Korea Food and Drug Administration (KFDA) as a food ingredient for human.
In a long-term (7-wk) experiment, the administration of PGE also induced remarkable effects on lipid metabolism via lowering pancreatic lipase activity and adipocyte size reduction, which resulted in suppression of obesity development accompanied by significant differences in energy consumption. A previous report[7] demonstrated that high fat diet induced obese Sprague-Dawley rats fed Platycodin saponin (PS) for 4 wk were highly correlated to the food intake restriction as well as body weight reduction. In our experiment, we could also show a significant co-relation between food intake and weight loss by statistical analysis (data not shown). From these results, we could predict that PGE contained lots of saponins, the major component of the PG, which induced food intake reduction followed by weight loss and adipocyte size reduction.
Serum lipid concentration was also significantly increased in the H group agreeing with other previous studies which reported that serum lipid concentrations were improved with the administration of oriental herbs containing large amounts of saponin, a major component of Platycodon grandiflorum[8-13] and our previous report[14]. From our results, PGE triggered a significant reduction of FABP mRNA expression in subcutaneous adipose tissue, which was highly correlated to the body weight loss. These results indicate that the oral administration of PGE not only helps to suppress the accumulation of body fat but also helps to degrade accumulated fats. Adipose tissue, which stores the largest amount of energy from fat of any tissue in the body, also plays a central role in regulating fatty acid metabolism. While the immediate use of dietary nutrients and stored hepatic energy source, such as glycogen is required to satisfy short-term need, adipocytes have evolved to maintain stores of TG for the long-term energy requirements of the body. The release of fatty acid shows regional variation and is known to depend on the FABP regulation. Adipocyte FABP expression also displays regional variations[19,26-28]. Fatty acid utilization and metabolism are regulated by specific proteins. The cellular uptake of long chain fatty acids for oxidation is activated by membrane bound proteins, including fatty acid translocase (FAT/CD36)[29], plasma membrane fatty acid binding protein (FABP pm)[30], and the FA transport proteins[24,31-32]. In clinical study, mature adipocytes showed up-regulation of genes involved in lipid metabolism, such as fatty acid binding protein (FABP), adipocyte differentiation-related protein, LPL, perilipin. Damcott et al[27] reported that variation in the FABP promoter altered transcriptional activity and was associated with body composition and plasma lipid levels in human. However, there is little report on the expression and regulation of FABP in obese rats, let alone the effect of PGE.
Thus, we tried to evaluate the effect of Platycodon grandiflorum on FABP expression of subcutaneous adipose tissue in obese rats. In the H group, mRNA expression of Leptin, a well known adipocytokine, was significantly increased as expected, but PGE failed to suppress this increase. For FABP, another major target of this study, one previous study[18] reported that FABP alteration by long-term high fat feeding could modify body weight changes and lead to alterations in body composition and lipid metabolism. However, relatively little information was available regarding the role of FABP in white adipose tissue (WAT). Hence, further study was needed to elucidate the detailed mechanism of adipocyte FABP regulation.
The expression level of FABP was increased during the process of adipocyte differentiation or induced obesity. But there was no further increase after the complete differentiation of adipocytes or complete development of obesity[18,33]. Based on these results and previous reports[14], we propose that FABP is one of the main proteins regulating obesity development, and consequently, it is highly probable to treat or improve obesity by using the substances that reduce FABP expression such as Platycodon grandiflorum. The use of this PGE for the treatment and improvement of obesity could be further validated by studying its mechanisms in detail.
In conclusion, PGE has the potential to be used clinically as an ingredient of food or drug effective in obesity treatments by reducing plasma lipid concentration via fatty acid utilization without any serious side effects.
S- Editor Liu Y L- Editor Alpini GD E- Editor Ma WH
| 1. | Bray GA. A concise review on the therapeutics of obesity. Nutrition. 2000;16:953-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Greenway FL, Smith SR. The future of obesity research. Nutrition. 2000;16:976-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Kim SY, Kim HS, Su IS, Yi HS, Kim HS, Chung SY. Effects of the Feeding Platycodon Grandiflorum and Codonopsis Ianceolata on the Lipid Components of Serum and Liver in Rats. J Kor Soc Food Nutr. 1993;22:517-523. |
| 4. | Lee ES, Lim SS, Chung SH, Lee JS, Shin HD. The effect of the Banggihwanggitang on the biochemical changes of obese rats. Kor J Oriental Med. 1995;5:1-37. |
| 5. | Han LK, Xu BJ, Kimura Y, Zheng Yn, Okuda H. Platycodi radix affects lipid metabolism in mice with high fat diet-induced obesity. J Nutr. 2000;130:2760-2764. [PubMed] |
| 6. | Han LK, Zheng YN, Xu BJ, Okuda H, Kimura Y. Saponins from platycodi radix ameliorate high fat diet-induced obesity in mice. J Nutr. 2002;132:2241-2245. [PubMed] |
| 7. | Zhao HL, Sim JS, Shim SH, Ha YW, Kang SS, Kim YS. Antiobese and hypolipidemic effects of platycodin saponins in diet-induced obese rats: evidences for lipase inhibition and calorie intake restriction. Int J Obes (Lond). 2005;29:983-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Tada A, Kaneiwa Y, Shoji J, Shibata S. Studies on the saponins of the root of Platycodon grandiflorum A. De Candolle. I. Isolation and the structure of platycodin-D. Chem Pharm Bull (Tokyo). 1975;23:2965-2972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 76] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Ishii H, Tori K, Tozyo T, Yoshimura Y. Saponins from roots of Platycodon grandiflorum. Part 2: Isolation and structure of new triterpene glycosides. J Chem Soc. 1984;1:661–668. |
| 10. | Kubo M, Nagao T, Matsuda H, Namba K. Immune pharmacological studies on platycodi radix I: Effect on the phagocytosis in mouse. Shoyakugaku Zasshi. 1986;40:367–374. |
| 11. | Oakenfull DG, Fenwick DE, Hood RL, Topping DL, Illman RL, Storer GB. Effects of saponins on bile acids and plasma lipids in the rat. Br J Nutr. 1979;42:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 62] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Sidhu GS, Oakenfull DG. A mechanism for the hypocholesterolaemic activity of saponins. Br J Nutr. 1986;55:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 129] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Kim SY. Effects of the Feeding Platycodon grandiflorum and Codonopsis Ianceolata on the Fatty Acid Composition of Serum and Liver in Rats. J Kor Soc Food Nutr. 1984;13:413-420. |
| 14. | Park YS, Cha MH, Yoon YS, Ahn HS. Effects of low calorie diet and Platycodon grandiflorum extract on fatty acid binding protein expression in rats with diet-induced obesity. Nutritional Sciences. 2006;8:3-9. |
| 15. | Marr E, Tardie M, Carty M, Brown Phillips T, Wang IK, Soeller W, Qiu X, Karam G. Expression, purification, crystallization and structure of human adipocyte lipid-binding protein (aP2). Acta Crystallogr Sect F Struct Biol Cryst Commun. 2006;62:1058-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Bass NM. The cellular fatty acid binding proteins: aspects of structure, regulation, and function. Int Rev Cytol. 1988;111:143-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 208] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Ono T. Studies of the FABP family: a retrospective. Mol Cell Biochem. 2005;277:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Odani S, Nakamura J, Sato T, Fujii H. Identification of a rat 30-kDa protein recognized by the antibodies to a recombinant rat cutaneous fatty acid-binding protein as a 14-3-3 protein. J Biochem. 2001;129:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Luiken JJ, Bonen A, Glatz JF. Cellular fatty acid uptake is acutely regulated by membrane-associated fatty acid-binding proteins. Prostaglandins Leukot Essent Fatty Acids. 2002;67:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Gong H, Ni Y, Guo X, Fei L, Pan X, Guo M, Chen R. Resistin promotes 3T3-L1 preadipocyte differentiation. Eur J Endocrinol. 2004;150:885-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939-1951. [PubMed] |
| 22. | Herbert V, Lau KS, Gottlieb CW, Bleicher SJ. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965;25:1375-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2087] [Cited by in RCA: 2172] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 23. | Snedecor GW, Cochran WG. Statistical methods. Ames (Iowa): Iowa University Press 1967; 339-350. |
| 24. | Turcotte LP, Swenberger JR, Zavitz Tucker M, Yee AJ. Increased fatty acid uptake and altered fatty acid metabolism in insulin-resistant muscle of obese Zucker rats. Diabetes. 2001;50:1389-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Hernell O, Staggers JE, Carey MC. Physical-chemical behavior of dietary and biliary lipids during intestinal digestion and absorption. 2. Phase analysis and aggregation states of luminal lipids during duodenal fat digestion in healthy adult human beings. Biochemistry. 1990;29:2041-2056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 368] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 26. | Giorgino F, Laviola L, Eriksson JW. Regional differences of insulin action in adipose tissue: insights from in vivo and in vitro studies. Acta Physiol Scand. 2005;183:13-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 141] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 27. | Damcott CM, Feingold E, Moffett SP, Barmada MM, Marshall JA, Hamman RF, Ferrell RE. Variation in the FABP2 promoter alters transcriptional activity and is associated with body composition and plasma lipid levels. Hum Genet. 2003;112:610-616. [PubMed] |
| 28. | Marti A, Vaquerizo J, Zulet MA, Moreno-Aliaga MJ, Martínez JA. Down-regulation of heart HFABP and UCP2 gene expression in diet-induced (cafeteria) obese rats. J Physiol Biochem. 2002;58:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem. 1993;268:17665-17668. [PubMed] |
| 30. | Stremmel W, Strohmeyer G, Borchard F, Kochwa S, Berk PD. Isolation and partial characterization of a fatty acid binding protein in rat liver plasma membranes. Proc Natl Acad Sci USA. 1985;82:4-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 235] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 31. | Hirsch D, Stahl A, Lodish HF. A family of fatty acid transporters conserved from mycobacterium to man. Proc Natl Acad Sci USA. 1998;95:8625-8629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 326] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 32. | Stahl A, Gimeno RE, Tartaglia LA, Lodish HF. Fatty acid transport proteins: a current view of a growing family. Trends Endocrinol Metab. 2001;12:266-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 173] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 33. | Drozdowski L, Clement L, Keelan M, Niot I, Clandinin MT, Agellon L, Wild G, Besnard P, Thomson AB. Dietary lipids modify intestinal lipid-binding protein RNA abundance in diabetic and control rats. Digestion. 2004;70:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |