INTRODUCTION
Total clamping of the portal triad, known as "Pringle's maneuver", is commonly used to control blood loss in liver surgery[1,2]. Additionally, total hepatic vascular exclusion (THVE), which consists of Pringle's maneuver and occlusion of the inferior vena cava below and above the liver, is used clinically for the resection of large and posterior portions of the liver[3,4]. These techniques possibly worsen postoperative liver function as a result of warm ischemia-reperfusion (I/R) injury[5]. Consequently, a reduction in the I/R injury is required for the maintenance of postoperative liver function.
Atrial natriuretic peptide (ANP) was found in mammalian atrial extracts in 1981 and characterized as a 28 amino acid circular peptide[6,7]. ANP has effects on the cardiovascular system, such as diuresis, natriuresis, and hypotension, and has been used clinically for the treatment of heart failure[8,9]. Recently, ANP was shown to have protective effects mediated via the guanylate cyclase coupled A-receptor and cGMP on hepatic I/R injury[10-16] and to activate p38 MAPK[17]. On the other hand, in our previous study, the inhibition of p38 MAPK ameliorated the I/R injury of several organs[18-22].
In this study, we evaluated the protective effects of ANP treatment on warm I/R injury using a porcine THVE model and analyzed the relationship between the role of ANP and p38 MAPK activation.
MATERIALS AND METHODS
Animals
All animals were cared for in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the guidelines set forth in the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH publication 85-23, revised 1985). The study was performed under the supervision of the Animal Care and Experimental Committee of Gunma University, Showa campus, Japan.
Reagents
We used carperitide, a recombinant human ANP (HAN®), obtained from Daiichi Asubio Pharma Co., Ltd. (Tokyo, Japan).
Operative procedure
Fourteen Mexican hairless pigs (both sexes, weighing 16-24 kg) were used in this study. They were not allowed access to food for 24 h before the experiment. After intramuscular administration of ketamine hydrochloride (250 mg) and atropine (0.5 mg), the pigs were intubated endotracheally and mechanically ventilated at a tidal volume of 25 mL/kg and a rate of 12 breaths/min. General anesthesia was maintained with a mixture of 1%-2% isoflurane and 100% oxygen during the experiment. Lactated Ringer's solution (10 mL/kg per hour) was infused via a catheter inserted into the right subclavian vein. Laparotomy was performed via midline incision. The liver was completely skeletonized by dividing all suspensory ligaments and dissecting the retrohepatic vena cava from the posterior abdominal wall. The portal vein, hepatic artery, and common bile duct were isolated, and their collaterals were occluded separately. THVE was achieved by clamping the infrahepatic and suprahepatic vena cava after clamping the portal vein and hepatic artery. An active venovenous (v-v) bypass system was started as a portosystemic shunt just before THVE to avoid congestion of the portal vein and lower body. This system comprised a centrifugal pump system (Lifestream; St. Jude Medical, Chelmsford, MA) and venous cannulae. The blood-contact surfaces of these components were heparin-coated. The v-v bypass system was established with a drain (12 Fr) inserted into the portal and the right external iliac vein for blood removal and another drain inserted into the right external jugular vein (12 Fr) for blood return. Blood from the portal vein and infrahepatic vena cava was bypassed into the right external jugular vein via a Y-shaped shunt. The bypass blood flow was maintained at more than 20 mL/kg per min under conditions of systemic heparinization (100 units/kg). Liver ischemia was induced by total exclusion of hepatic inflow for 120 min. After the clamps were released to end the ischemia, the bypass system was removed. The side hole of the portal trunk was repaired with a 5-0 polypropylene running suture after removal of the cannula. The right external iliac vein and the right external jugular vein were ligated after removal of the cannula. The measurements described below were performed, and animals were observed for 120 min after reperfusion.
Experimental design
The animals were randomly assigned to two groups. In the ANP group (n = 7), intravenous administration of ANP (0.1 μg/kg per min) was continuously performed from 30 min before the THVE to the end of the experiment. In the control group (n = 7), vehicle (saline solution) was injected in the same manner.
Monitoring and sampling
The external iliac artery was cannulated to allow monitoring of arterial blood pressure and collection of blood samples. Arterial blood pressure was directly monitored through a catheter connected to a transducer (Spectramed TA 1017; San-ei Co, Tokyo, Japan). Blood samples were collected from the same catheter before and after the procedure [before ischemia and immediately (0), 30, 60, and 120 min after reperfusion]. All samples were centrifuged at 900 ×g for 15 min at 4°C, and the serum or plasma was frozen at -80°C for later measurement.
Assays of serum AST and LDH levels and serum TNF-α levels
Serum alanine aminotransferase (AST) and lactate dehydrogenase (LDH) levels were measured at 37°C using an ultraviolet rate assay on an autoanalyzer (Hitachi 736-60; Hitachi Co. Ltd., Tokyo, Japan). Serum TNF-α levels were also measured with ELISA using a BIOSOURCETM swine TNF-α ELISA kit (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions.
Hepatic tissue blood flow
Hepatic tissue blood flow (HTBF) was measured with a laser Doppler flow meter (Laser Blood Flow Monitor MBF 3; Moor Instruments, Devon, U.K.) before ischemia and 30, 60, and 120 min after reperfusion. The laser probe was always placed on the right median lobe of the liver. The blood flow was expressed as a percentage of the flow before ischemia.
Western blot analysis of total and phosphorylated (active) p38 MAPK
The left median lobe of the liver was harvested before ischemia (pre) and immediately (0), 30, 60, and 120 min after reperfusion in both groups (n = 7 in each group) and frozen at -80°C until homogenization. The extraction buffer contained 10 mmol/L Tris-HCl (pH 7.5), 0.25 mol/L sucrose, 5 mmol/L M EDTA, 50 mmol/L NaCl, 30 mmol/L sodium pyrophosphate, 50 mmol/L NaF, 100 µmol/L Na2VO4, 1 μg/mL pepstatin A, and 2 μg/mL leupeptin. Immediately before being used, 1 mmol/L of phenylmethylsulfonyl fluoride was added. The samples were homogenized in four volumes of extraction buffer on ice. All debris and nuclei were removed by centrifugation at 900 ×g at 4°C for 10 min, and the supernatant was used for Western blot analysis. Protein concentrations were determined with bovine serum albumin as the reference standard using Bio-Rad protein assay dye reagent (Bio-Rad Laboratories, Hercules, CA)[23].
Protein (20 μg) was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore Co., Billerica, MA). Membranes were blocked with 5% skim milk in Tris-buffered saline (TBS) and washed three times in 0.05% Tween 20-TBS (TBS-T). Rabbit polyclonal antibodies were used to identify p38 MAPK and phosphorylated p38 MAPK. Immunocomplexes were then detected using chemiluminescence and exposure to radiographic film. These antibodies and chemiluminescent reagent were contained in a PhosphoPlus MAPK antibody kit (Cell Signaling Technology, Beverly, MA).
The densities of the bands in both groups, which were transferred to the same membrane at every point, were quantified with ImageJ software (National Institutes of Health, Bethesda, MD), and differences between the two groups were determined statistically. In each comparison, the value was expressed as the ratio to the basal level.
Histopathologic examination
After 120 min of reperfusion, the hepatic tissues were harvested from the left median lobe and fixed in 10% formalin. The tissues were dehydrated, embedded in paraffin, cut into 3 to 5-μm sections, and mounted. After deparaffinization, the tissues were stained with hematoxylin and eosin (HE) for histological examination. Single-blind histological examination was performed by a pathologist.
Statistical analysis
The results are expressed as the mean ± SE. Statistical analysis was performed by Instat-3 statistical software (GraphPad Software, Inc., San Diego, CA) using repeated-measures ANOVA for multiple comparisons when more than two groups were analyzed. Comparisons between the two groups at each point were calculated by the Mann-Whitney U-test. A P value of less than 0.05 was considered statistically significant.
RESULTS
All animals survived until the endpoint of the study (120 min after reperfusion). ANP treatment did not affect the systemic hemodynamics, and no significant differences were observed in heart rate and blood pressure between the two groups (data not shown). No side effects due to the administration of ANP were found.
Serum AST and LDH levels
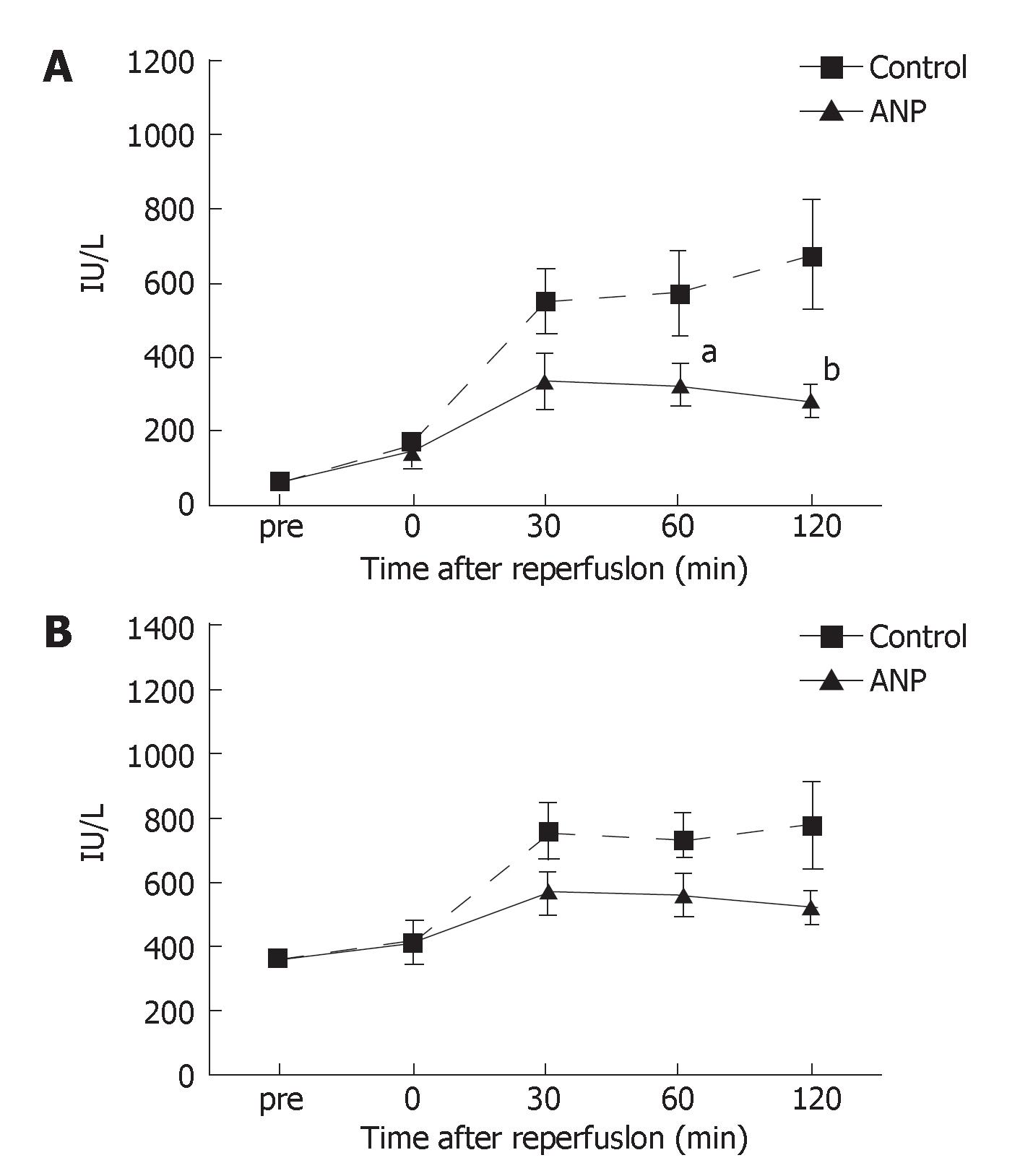
As shown in Figure 1A and B, no significant differences were detected in the serum levels of AST and LDH before ischemia between the two groups. The serum AST levels gradually increased after reperfusion in the control group; however, those in the ANP group were almost stable 30 min after reperfusion and significantly (P < 0.05 or P < 0.01) lower 60 and 120 min after reperfusion than in the control group (Figure 1A). The changes in the serum LDH levels were similar to those in the serum AST levels, while the serum LDH levels were not significantly different between the two groups (Figure 1B).
Figure 1 A: Serum AST levels before ischemia (pre) and immediately (0), 30, 60, and 120 min after reperfusion.
Data are expressed as the mean ± SE. aP < 0.05, bP < 0.01 vs control group; B: Serum LDH levels before ischemia (pre) and immediately (0), 30, 60, and 120 min after reperfusion. Data are expressed as the mean ± SE.
Hepatic tissue blood flow
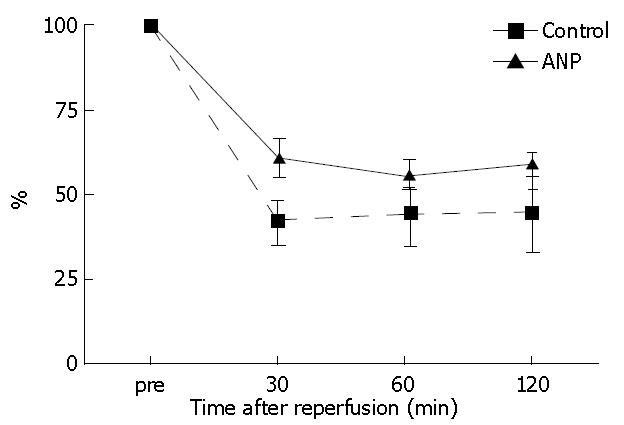
HTBF levels decreased after reperfusion in both groups. HTBF in the ANP group was maintained higher compared to the control group, although no significant differences occurred until 120 min after reperfusion (Figure 2).
Figure 2 Hepatic tissue blood flow (HTBF) levels before ischemia (pre) and 30, 60, and 120 min after reperfusion.
The HTBF levels were evaluated as a percentage of the flow levels before ischemia.
Serum TNF-αlevels
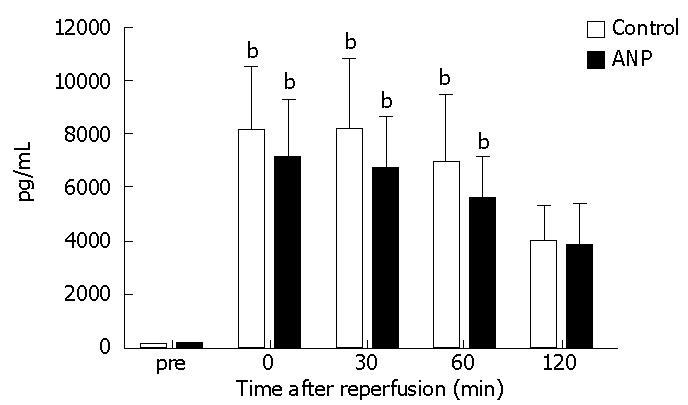
The serum TNF-α levels significantly (P < 0.01) increased 0, 30, and 60 min after reperfusion compared to those measured before ischemia in both groups, while no significant differences occurred between the two groups until 120 min after reperfusion (Figure 3).
Figure 3 Serum tumor necrosis factor-α (TNF-α) levels before ischemia (pre) and immediately (0), 30, 60, and 120 min after reperfusion.
Data are expressed as the mean ± SE. bP < 0.01 vs pre levels in each group.
Expression of total and phosphorylated (active) p38 MAPKs
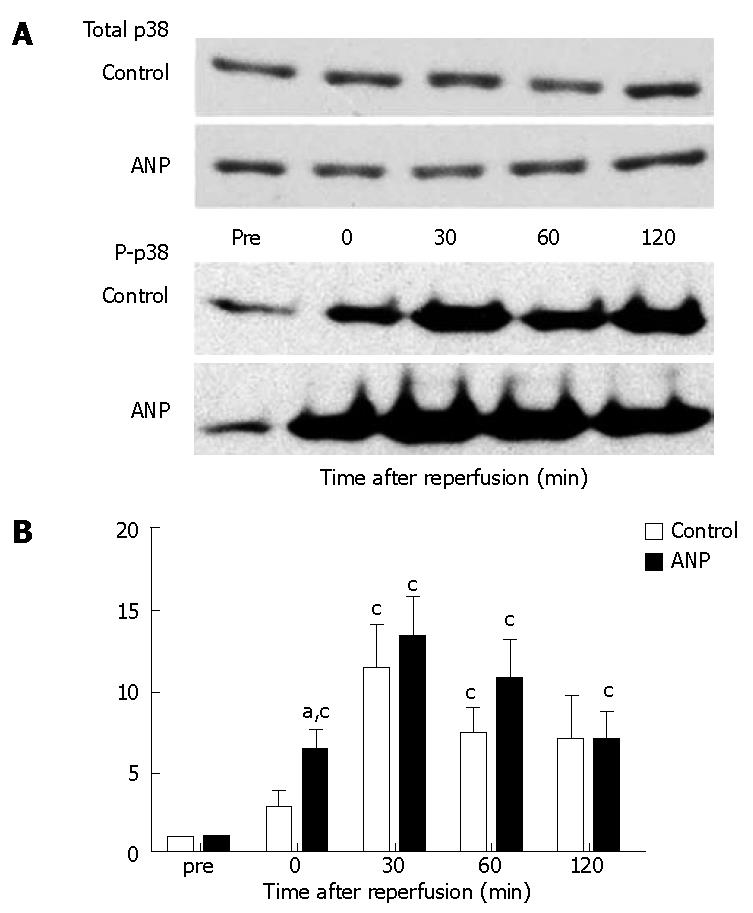
Typical examples and Image data are shown in Figure 4. The densities of bands for total p38 MAPK were similar and not statistically different between the two groups (Figure 4A). Phosphorylated p38 MAPK expression, which is an active form of p38 MAPK, was significantly (P < 0.05) elevated after reperfusion compared to data measured before ischemia in both groups. Phosphorylated p38 MAPK was higher in the ANP group than in the control group after reperfusion, and a significant (P < 0.05) difference was seen immediately after reperfusion (0 min after reperfusion) between the groups (Figure 4B).
Figure 4 Western blot analysis of ischemia-reperfusion induced phosphorylation of p38 mitogen-activated protein kinase (MAPK) in the liver before ischemia (pre) and immediately (0), 30, 60, and 120 min after reperfusion.
A: Typical images are shown. The density of the bands at each point in the control and ANP groups was quantified using the ImageJ software (NIH, Bethesda, MD); B: Data are expressed as the mean ± SE. aP < 0.05 vs control group. cP < 0.05 vs pre levels in each group.
Histopathologic findings
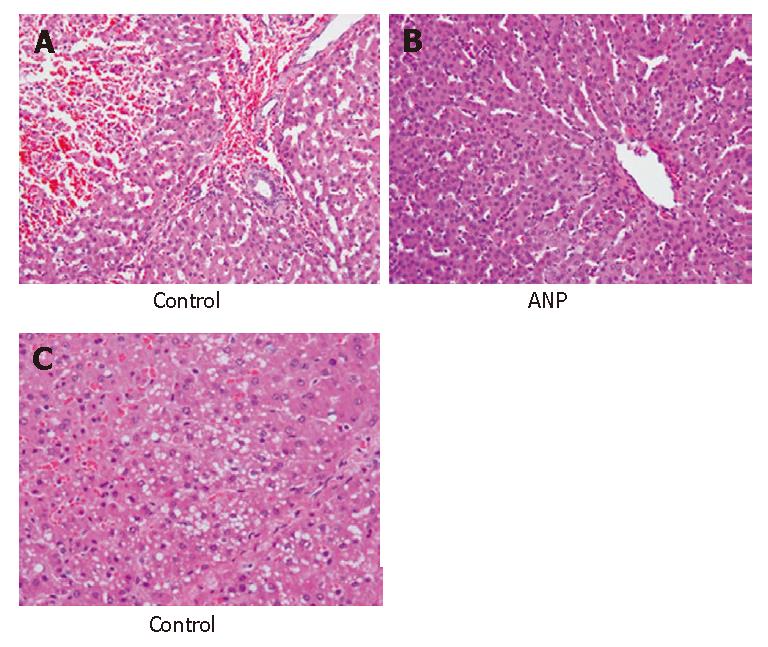
The liver tissue in the control group showed hemorrhage, infiltration of neutrophils, and sinusoidal congestion especially in the centrilobular region with acidophilic change and condensation of nuclei 120 min after reperfusion (Figure 5A). Mild microvesicular steatosis or cytoplasmic vacuolization was also observed (Figure 5B) in the control group. However, the infiltration of neutrophils was milder in the ANP group than in the control group (Figure 5A and C). In the ANP group, neither microvesicular steatosis nor cytoplasmic vacuolization was observed. Moreover, the liver tissue in the ANP group showed almost normal trabecular arrangement of hepatocytes and sinusoidal structure (Figure 5C).
Figure 5 Histopathologic findings 120 min after reperfusion in the control group (A: HE, × 200; B: HE, × 200) and ANP group (C: HE, × 400).
DISCUSSION
THVE is used clinically for the resection of large and posterior liver tumors to avoid massive hemorrhage and air embolism[3,4,23]. The maximum duration of the tolerable warm ischemic time was found to be 90 min in clinical studies in humans[24]. However, animals are known to tolerate total ischemia poorly[25]. A THVE of 30 min or more, without a portosystemic shunt, is known to cause severe liver damage after reperfusion, while THVE models with the shunt are known to tolerate longer ischemic times[18,26-28]. Nordlinger et al[29] demonstrated that a warm THVE model with the shunt was safe and tolerated for 120 min in pigs. Therefore, an active portosystemic bypass was initiated to avoid congestion of the portal vein and lower body during the 120 min of total hepatic ischemia in our study.
THVE sometimes causes serious damage such as hemodynamic instability and residual hepatic dysfunction due to I/R injury[30,31], while ANP, which regulates body fluids and blood pressure by natriuresis, diuresis, and vasodilation and has been used clinically for the treatment of heart failure[6-9], was recently shown to have organ-protective effects against I/R injury[10-16,32]. We took notice of the organ-protective role of ANP in I/R injury and evaluated the effect of ANP on I/R injury in a porcine THVE model. ANP was continuously administered at
0.1 μg/kg per min from 30 min before ischemia until the end of the experiment. The administered dose in this study was based on the clinical dose used in the treatment of heart failure, and the administration timing was based on the report that peak plasma concentrations of ANP are reached after about 20 min and maintain a plateau state in continuous infusion at 0.1 μg/kg per min[33].
As a result, serum AST and LDH levels, which were associated with hepatic parenchymal injury, were lower after reperfusion in the ANP group than in the control group. Additionally, significant differences were observed in the serum AST levels 60 and 120 min after reperfusion between the two groups. HTBF levels, which were related to hepatic sinusoidal injury, were also better maintained in the ANP group than in the control group. The microscopic histopathologic study showed that hepatocyte damage was milder in the ANP group than in the control group. Mild microvesicular steatosis or cytoplasmic vacuolization, as an indicator of degenerative cell damage, was observed in the control group. In addition, trabecular arrangement of hepatocytes and sinusoidal structure almost remained normal in the ANP group. It is often emphasized that hepatic parenchymal cell injury results in microcirculatory disturbance, which is caused by preceding sinusoidal injury[34]. Our results suggest that ANP reduces hepatic parenchymal cell injury caused by I/R injury through amelioration of the microcirculatory disturbance due to the inhibition of sinusoidal injury.
Some studies have explored the mechanisms behind the organ-protective effects of ANP in I/R injury. One of the reasons for the improvement in microcirculation is that ANP is known to be a potent vasodilator; however, one report found that the dose of ANP that was protective in I/R injury was 104-fold higher than the concentration for maximum vasodilation[35]. In our study, the serum concentration of ANP was within the ANP dose necessary for protection from I/R injury (data not shown). Therefore, other mechanisms must also be involved in the amelioration of I/R injury. The organ-protective action of ANP in I/R injury was shown to be mediated via the guanylate cyclase coupled A-receptor and cGMP[13-16,28]. Furthermore, ANP has been found to attenuate the activation of the proinflammatory transcription factors NF-κB and reduce the expression of TNF-α[16,36,37]. Although there were no significant differences in the serum TNF-α levels between the two groups in this study, the serum TNF-α levels in the ANP group tended to be lower than in the control group. Our results also suggested that the production of TNF-α was slightly inhibited by the administration of ANP, and agreed with the previous studies.
However, several studies that examined the relationship between ANP and p38 MAPK activation have indicated that protective strategies with ANP in short-time hypoxia[10] and I/R injury[38-40] result in a significant activation of p38 MAPK. Keller et al reported that preconditioning with ANP increased p38 MAPK activity in the liver[17]. Enhanced p38 MAPK activity was reported to contribute to the protection against hepatic I/R injury in models of ischemic preconditioning[38,39]. Additionally, other studies assigned an important role to p38 MAPK in protection against I/R injury in the heart[40,41]. Our results also demonstrated that p38 MAPK activation was induced to a greater extent in the ANP group. These results suggest that p38 MAPK mediated by ANP was associated with protective effects in I/R injury. However, the induction of TNF gene transcription after I/R is due to the direct activation of p38 MAPK[42]. In addition, the inhibition of p38 MAPK ameliorated the I/R injury or endotoxic injury of several organs[18-22] in our previous study. It has been reported that the role of MAPK in the stress response, either detrimental or protective, seems to depend strongly on the system[43], experimental setup, and time dependency of the activation[15]. The role of p38 MAPK is still controversial and requires further study.
In conclusion, ANP was found to have a protective role against I/R injury with p38 MAPK activation in a porcine THVE model. Our results may have clinical applications to liver surgery associated with vascular occlusion.