Published online Jul 7, 2007. doi: 10.3748/wjg.v13.i25.3478
Revised: February 10, 2007
Accepted: March 31, 2007
Published online: July 7, 2007
AIM: To investigate the effects of heme oxygenase-1 (HO-1) against oxidant-induced injury caused by bile duct ligation (BDL).
METHODS: Either cobalt protoporphyrin (CoPP), a HO-1 inducer, or saline were injected intraperitoneally in male SD-rats. Three days later, BDL or sham-operations were performed. Rats were sacrificed 3 wk after BDL and livers were harvested for histology. Fibrosis was evaluated by sirius red staining and image analysis. Alpha-smooth muscular actin, which indicates activation of stellate cells, was detected by immunohistochemical staining, and cytokine and collagen-Iα (Col-Iα) mRNA expression was detected using RNase protection assays.
RESULTS: Serum alanine transaminase increased 8-fold above normal levels one day after BDL. Surprisingly, enzyme release was not reduced in rats receiving CoPP. Liver fibrosis was evaluated 3 wk after BDL and the sirius red-positive area was found to be increased to about 7.8%. However, in CoPP pretreated rats sirius red-positive areas were increased to about 11.7% after BDL. Collagen-Iα and TGF-β mRNA increased significantly by BDL. Again, this effect was increased by HO-1 overexpression.
CONCLUSION: Hepatic fibrosis due to BDL is not reduced by the HO-1 inducer CoPP. In contrast, HO-1 overexpression increases liver injury in rats under conditions of experimental chronic cholestasis.
- Citation: Froh M, Conzelmann L, Walbrun P, Netter S, Wiest R, Wheeler MD, Lehnert M, Uesugi T, Scholmerich J, Thurman RG. Heme oxygenase-1 overexpression increases liver injury after bile duct ligation in rats. World J Gastroenterol 2007; 13(25): 3478-3486
- URL: https://www.wjgnet.com/1007-9327/full/v13/i25/3478.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i25.3478
Chronic cholestatic liver diseases are one of the leading indications for liver transplantation in children and adults[1,2]. Various drugs, total parenteral nutrition, chronic liver transplant rejection, and graft-vs-host disease can also produce chronic cholestasis[3-5]. This leads to liver injury and will finally progress to portal fibrosis, cirrhosis and end-stage liver disease necessitating liver transplantation. Ursodeoxycholic acid is currently the most promising therapy for chronic cholestatic liver diseases[6]; however, it cannot prevent fibrosis[7]. How cholestasis induces liver injury and fibrosis remains unclear. One possible mechanism is that accumulation of hydrophobic bile acids causes oxidative stress in the liver[8]. Previous studies showed that hepatic mitochondria generate reactive oxygen species when isolated hepatocytes are exposed to hydrophobic bile acids[8,9]. This mitochondrial free radical production may be an important mechanism of cholestatic liver injury. However oxypurinol, an inhibitor of xanthine oxidase, decreases hepatocelluar injury without decreasing lipid peroxidation in mitochondria and microsomes, suggesting that mitochondrial oxidative stress does not play an important role in cholestatic liver injury[10]. Therefore, the major source of free radicals remains unclear. In biliary obstructed rats, hepatic glutathione levels and activities of antioxidant enzymes decrease, whereas 4-hydroxynonenal and malondialdehyde levels increase[9,11,12]. These findings also support the involvement of oxidative stress in cholestatic stress.
Heme oxygenases (HOs) are ubiquitous enzymes that catalyze the initial and rate-limiting steps in heme catabolism[13,14] yielding equimolar amounts of biliverdin, carbon monoxide, and free divalent iron[15-18]. Biliverdin is subsequently reduced to bilirubin by biliverdin reductase, and the free iron is used in intracellular metabolism or sequestered into ferritin. The HO-system consists of three isoforms (HO-1, HO-2, and HO-3) as the products of separate genes[14,19,20]. HO-1, also known as heat shock protein 32, is an inducible isoform activated by most oxidative stress inducers and cytokines[17]. Constitutively expressed HO-2 is unresponsive to any of the known HO-1 inducers[17,20,21]. The HO-3 isozyme has 90% homology in the amino acid sequence with HO-2 and is nearly devoid of catalytic activity, serving mainly as heme-sensing/binding protein[19]. The mechanism by which HO-1 confers protection against oxidative stress has not yet been fully understood. It has been suggested that HO-1 possesses antioxidant activity deriving from the elimination of prooxidant heme and from the biological activities of its reaction products: biliverdin, bilirubin and iron[22-24]. Both biliverdin and bilirubin possess antioxidant properties[25], whereas iron released during heme catabolism can stimulate ferritin synthesis[26].
This study was designed to test whether an increased HO-1 expression by the HO-1 inducer cobalt protoporphyrin (CoPP) would minimize oxidative stress caused by experimental cholestasis and thereby decrease liver fibrosis.
Adult male Sprague-Dawley rats (200-250 g) were housed four to a cage in a facility approved by the Association for the Accreditation and Assessment of Laboratory Animal Care International. Three days before surgery, rats were randomly assigned to two experimental groups and received either 0.5 mL of lactated Ringer's solution or CoPP (Frontier Scientific Inc., Logan, UT, USA) at a concentration of 5 mg/kg diluted in 0.5 mL of lactated Ringer's solution intraperitoneally. After surgery, rats were allowed free access to standard laboratory chow and tap water. All animals received humane care in compliance with guidelines approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
Rats underwent bile duct ligation (BDL) and transection or sham operation under ether anesthesia, as described elsewhere[27].
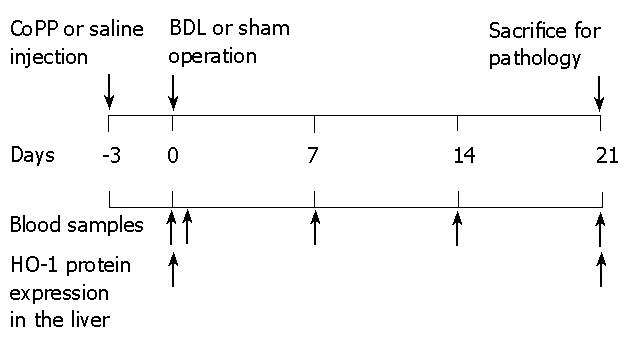
Blood samples were collected from the tail vein at times indicated in Figure 1. Serum enzymes were measured using analytic kits from Sigma (St. Louis, MO, USA). For histology, the liver was rinsed with normal saline, followed by slow infusion of 5 mL 10% buffered formaldehyde. After 48 h in fixative, paraffin sections were prepared and stained with hematoxylin-eosin or 0.1% Sirius red and Fast green FCF (Sigma)[28]. Areas in sections stained for collagens by Sirius red were quantified by image analysis using an Universal Imaging Image-1/AT image acquisition and analysis system (West Chester, PA, USA) incorporating an Axioskop 50 microscope. Detection thresholds were set for the red color of stained collagen based on an intensely labeled point and a default color threshold range that was assigned. The degree of labeling in each section was determined from the area within the color range divided by the total cellular area.
Immunohistochemistry for α-smooth muscle actin (α-SMA) was performed on 6 μm sections of formalin-fixed, paraffin-embedded liver-sections according to the supplier's instructions. Sections were deparaffinized in xylene, rehydrated in a series of graded alcohol concentrations, and placed in phosphate-buffered saline with 1% Tween 20. Staining was performed with monoclonal primary antibodies (DAKO, Carpinteria, CA, USA) against α-SMA and followed by peroxidase labeling using an EnVision kit (DAKO). The primary antibody was diluted 1:200 with 1% bovine albumin (Sigma) in phosphate-buffered saline. After immunohistochemistry, samples were lightly counterstained with hematoxylin.
Whole liver tissue was homogenized in lysis buffer with protease inhibitors at 4°C. The supernatant was collected and used as the source of protein for Western blots, as described elsewhere[29,30]. Protein was analyzed using a 1:1000 dilution of anti-HO-1 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and a 1:5000 dilution of horseradish peroxidase-conjugated antibody (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Measurement of AP-1 and NF-κB by EMSA was performed, as described in detail elsewhere[29-31].
Total RNA was isolated from liver tissue using RNA STAT 60 (Tel-Test, Friendswood, TX, USA). The RNA was extracted and purified following standard phenol/chloroform extraction and ethanol precipitation. RNase protection assays were performed using the RiboQuant multiprobe assay system (Pharmingen, San Diego, CA, USA) or an individual probe[29,30]. Gels were visualized by autoradiography[32]. Bands corresponding to the protected labeled fragment were quantitated by scanning densitometry software (ImageQuant 5.0), and where statistical data are given, they were normalized to the level of GAPDH as the housekeeping gene.
Data are presented as mean ± SD. ANOVA and the Student-Newman-Keuls post hoc tests were used to determine statistical significance between treatment groups. A P value less than 0.05 was selected before the study as the level of significance.
Experimental design is depicted in Figure 1. To confirm that CoPP pretreatment induced HO-1 overexpression, HO1 protein expression in whole livers was studied by immunoblotting analysis. As expected, HO-1 protein was identified in saline pretreated rats 3 wk after BDL (Figure 2), confirming previously published data[33]. Almost no HO-1 protein signal was detected in sham-operated rats pretreated with saline (Figure 2). In contrast, CoPP pretreatment produced a strong HO-1 protein signal in sham-operated and bile duct-ligated rats, indicating an increased HO-1 expression thru CoPP (Figure 2).
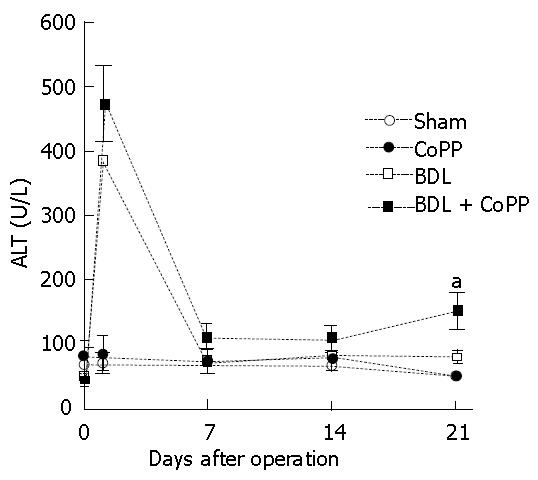
In untreated rats that were fed a standard chow diet, serum alanine aminotransaminase (ALT) levels averaged 70 U/L (Figure 3) and were not significantly altered by CoPP pretreatment and sham operation. After BDL, ALT increased to 386 U/L after 1 d (Figure 3), indicating liver injury. ALT levels decreased afterward and reached a new steady-state level of about 80 U/L after 1 wk (Figure 3). When rats were pretreated with the HO-1 inducer CoPP, ALT levels increased to 472 U/L one day after BDL(Figure 3). ALT levels decreased also afterward and reached a steady-state level of about 110 U/L after 1 wk before they significantly increased to 148 U/L after 3 wk (Figure 3).
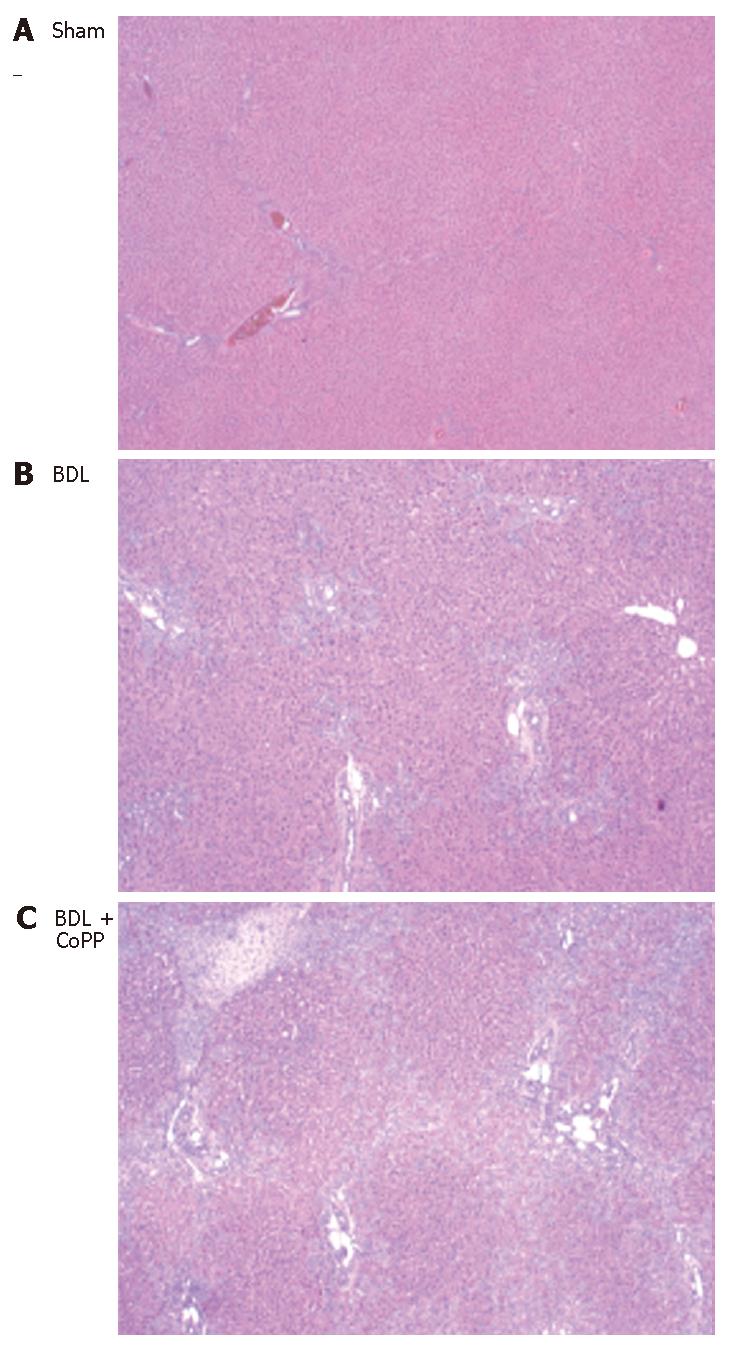
Normal liver architecture was observed after saline or CoPP pretreatment and sham operation (Figure 4A). Three weeks after BDL characteristic morphology of obstructive biliary cirrhosis, including bile duct proliferation, diffuse micronodules, focal necrosis and white blood cell infiltration were observed in livers from rats receiving saline pretreatment (Figure 4B). These pathological changes were increased by rats receiving CoPP pretreatment (Figure 4C).
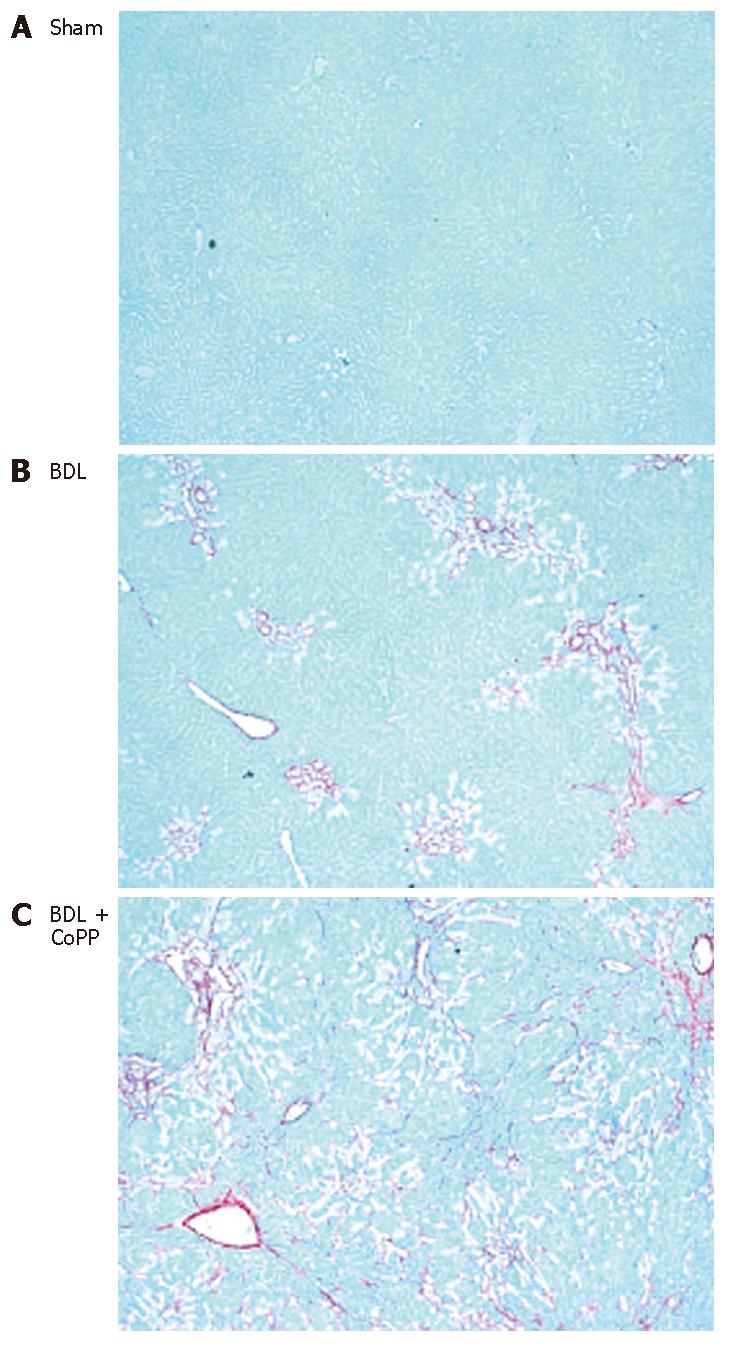
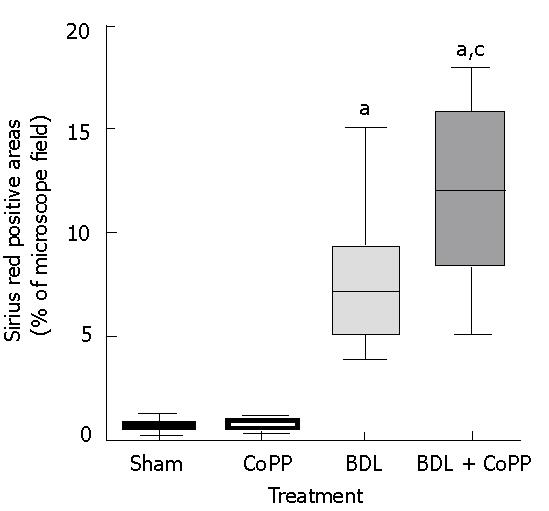
To evaluate the effects of HO-1 overexpression on liver fibrosis after BDL, liver sections were stained with Sirius red for collagen. Independently of pretreatment (saline or CoPP), no fibrosis was observed in livers from sham-operated rats (Figure 5A). In rats receiving normal saline, hepatic fibrosis developed within 2 wk after BDL (data not shown) and was severe after 3 wk (Figure 5B). When rats pretreated with CoPP were subjected to BDL, histology revealed increased fibrosis (Figure 5C). Image analysis revealed that Sirius red stained an area of about 0.7% of liver sections from sham-operated rats, independently of pretreatment (Figure 6). Sirius red staining increased to 7.8% after 3 wk following BDL (Figure 6). Pretreatment with CoPP raised this increase in Sirius red staining significantly after BDL to 11.7% of the measured areas (Figure 6).
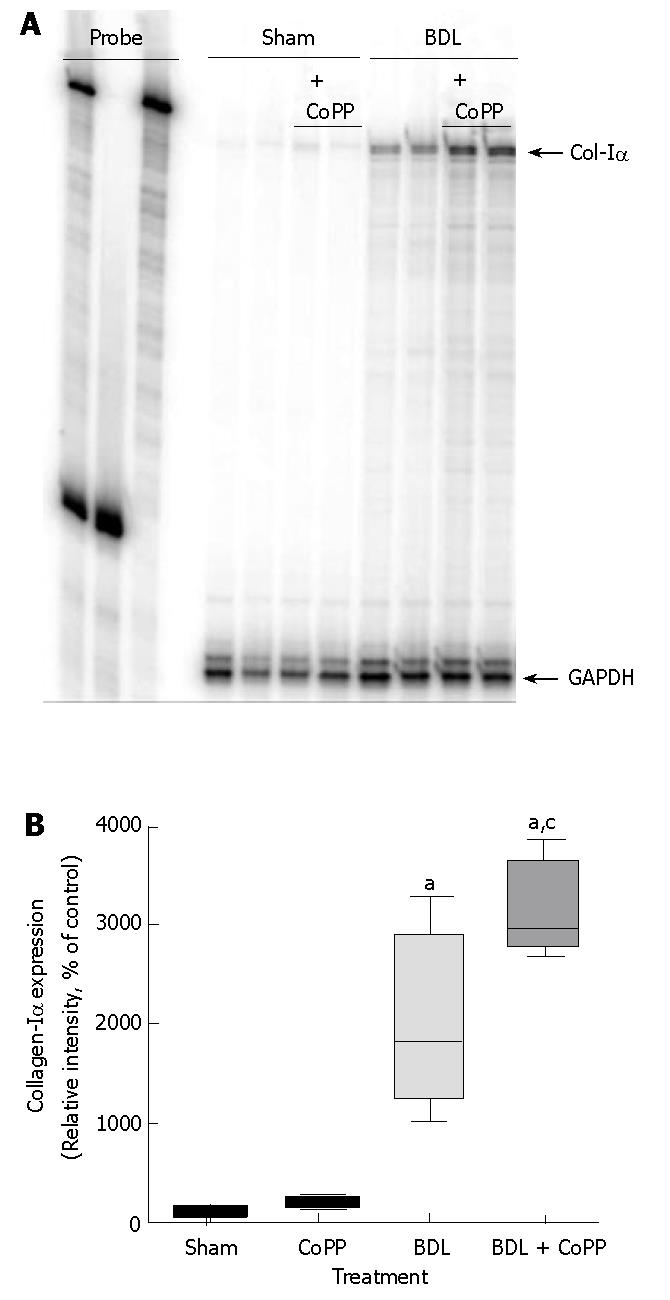
Collagen gene expression was evaluated by RNase protection assay for collagen-Iα mRNA and was barely detectable in livers from sham-operated rats, independently of pretreatment (Figure 7A). Three weeks after BDL, however, collagen-Iα mRNA expression increased to about 2000% compared with sham-operated groups (Figure 7A and B). Pretreatment with CoPP worsen the increase in collagen-Iα mRNA expression to about 3000% (Figure 7A and B).
Immunohistochemical staining forα-SMA
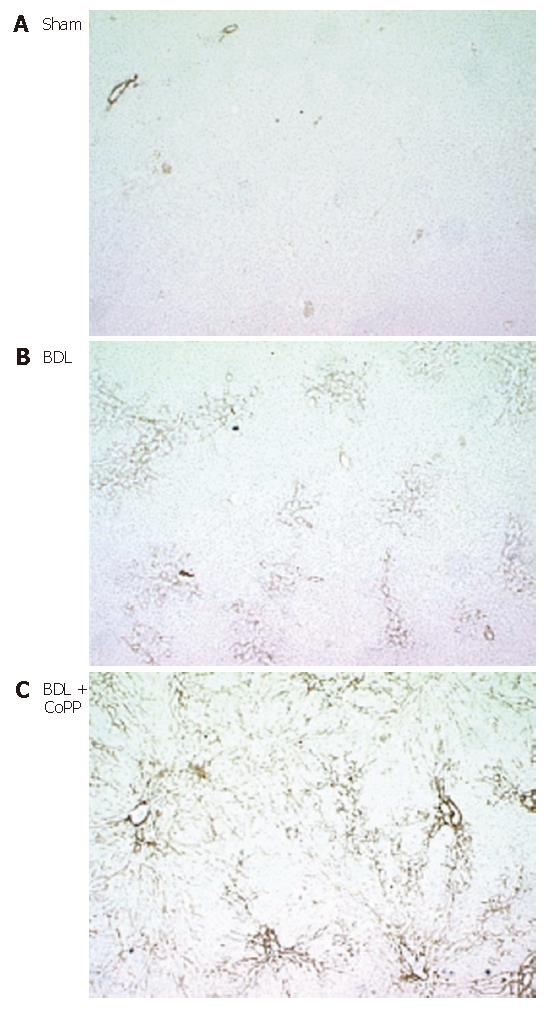
Activated stellate cells are the major source of matrix proteins in diseased liver[34]. Accordingly, we evaluated α-SMA, an indicator of stellate cell activation, by immunohistochemical staining after BDL. In livers from sham-operated rats (independently of pretreatment), small amounts of α-SMA were detected in the smooth muscle and endothelium of blood vessels (Figure 8A). After BDL, α-SMA increased markedly in perisinusoidal cells consistent with stellate cell activation in cholestatic livers (Figure 8B). Again, HO-1 overexpression by CoPP treatment before BDL significantly increased the amount of α-SMA-positive areas, indicating an increase of stellate cell activation (Figure 8C).
NF-κB/DNA complexes were barely detectable in livers from sham-operated rats (independently of pretreatment) but increased around 3.5-fold after BDL, consistent with our previous studies[29,30]. CoPP pretreatment did not suppress this activation (data not shown). A small amount of AP-1/DNA complex was detected in livers from sham-operated rats. Three weeks after BDL, however, AP-1/DNA complex levels increased around 2-fold, indicating activation of AP-1[29,30]. Again, HO-1 overexpression thru CoPP pretreatment did not suppress this activation (data not shown).
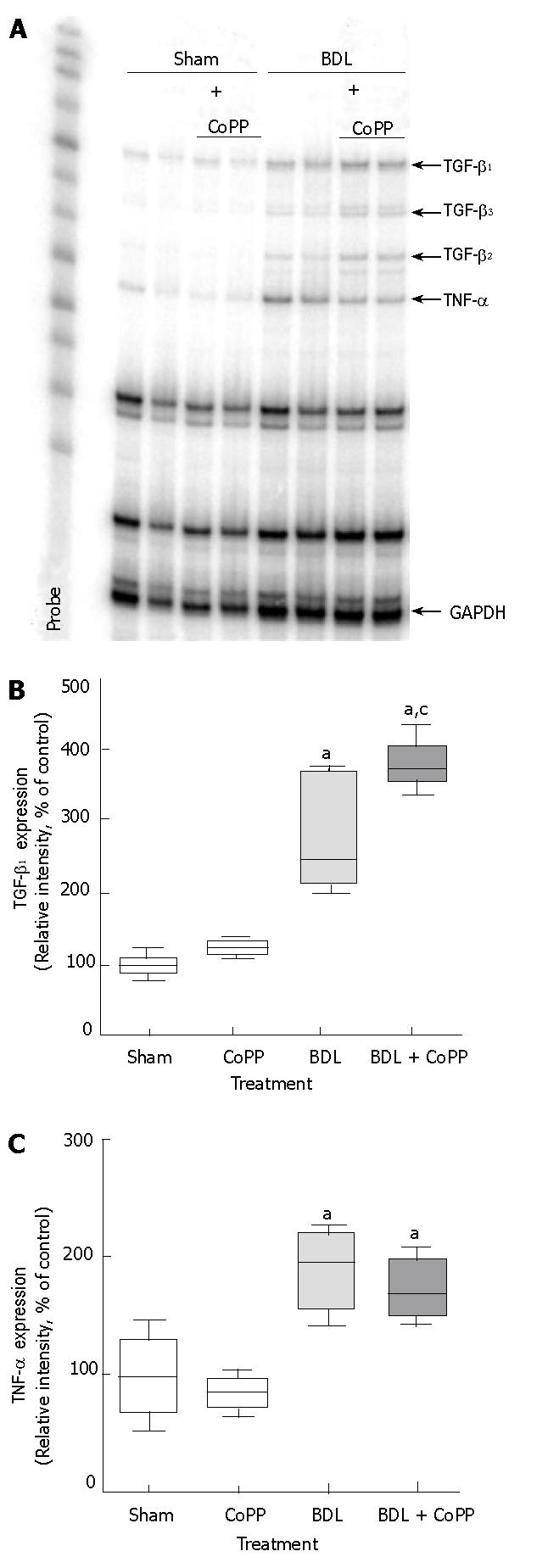
Minimal amounts of TGF-β1 mRNA were detected in livers from rats after sham operation. After BDL, TGF-β1 mRNA expression significantly increased by around threefold (Figure 9A and B). CoPP pretreatment did not suppress these increases in TGF-β expression. In contrast, TGF-β1 mRNA expression was even more increased in rats receiving CoPP before BDL (Figure 9B). TNF-α mRNA was barely detectable in livers from sham-operated rats (Figure 9A and C) but was increased around twofold after BDL. HO-1 overexpression thru CoPP pretreatment did not suppress this increase in TNF-α mRNA expression (Figure 9C).
Cholestasis leads to liver injury and will finally progress to fibrosis necessitating liver transplantation[1,35]. Current therapy for cholestasis, such as ursodeoxycholic acid, does not prevent fibrosis[7]. Therefore, new strategies to prevent cholestasis-induced liver injury and fibrosis are needed. Previous work suggest that oxidative stress occurs during cholestasis and probably plays a role in cholestasis-induced liver injury[8,9,36]. Accordingly, antioxidant therapy represents a potential strategy to prevent liver injury and fibrosis.
There are several reports suggesting protective effects of HO-1 against oxidant-induced injury and the induction of HO-1 as an adaptive response against oxidative damage. In detail, HO-1 overexpression can protect steatotic rat liver from ischemia reperfusion injury[37], prolong cardiac allograft[38], prevent arteriosclerosis and cardiac fibrosis in cardiac transplantation[39], be neuroprotective[40], attenuate chronic rejection of renal transplantation[41], and protect animals from endotoxic shock[42]. Based on this data, we assumed that HO-1 overexpression leading to less oxidative damage reduced cholestasis-induced liver injury and fibrosis. Accordingly, we assessed the effect of CoPP, a HO-1 inducer, leading to a stable HO-1 overexpression[37] in a rat model of cholestasis induced by BDL.
In confirmation of previous work from our and several other laboratories[11,12,27,29,30], BDL caused hepatic ALT release (Figure 3), cell necrosis (Figure 4) and fibrosis (Figures 5-7) as expected. Surprisingly, enzyme release and histopathological changes were not reduced in rats receiving CoPP. Moreover, a significant increase in ALT levels (Figure 3) and pathology (Figure 4) could be demonstrated 3 wk after BDL for CoPP pretreated vs bile duct-ligated rats receiving saline. Furthermore, HO-1 overexpression significantly deteriorated fibrosis caused by cholestasis, as shown by Sirius red staining and collagen-Iα mRNA expression (Figures 5-7).
Beside protective effects of upregulation of the HO-1 pathway there is increasing evidence that HO-1 overexpression and activity is not exclusively cytoprotective. Recent studies indicate that the protection might be restricted to a narrow threshold of HO-1 overexpression[43-46]. High levels of HO-1 may even sensitize the cell to oxidative stress, as demonstrated in fibroblast cell cultures, where low HO-1 induction was shown to be cytoprotective, whereas excessive activation of HO-1 resulted in the accumulation of free divalent iron and increased oxidative injury[43]. Moreover, increased HO-1 expression was shown in human NASH patients, reflecting the severity of the disease and the progression of liver failure[44]. Furthermore, a dual role for HO-1 expression in human liver transplants, with either cytopro-tection or increased cytotoxicity, depending on the initial level of overexpression could be demonstrated[45]. These results point towards the potentially different effects of HO-1, which may be neither exclusively cytoprotective nor exclusively cytotoxic.
Normally, factors of an increased HO-1 expression in a model of experimental cirrhosis may include NO and oxidative stress[9,47,48], as well as especially increased cytokine and endotoxin levels[49]. It is known, that HO-1 expression and activity is greatly enhanced in cirrhotic rat livers[33,50,51], suggesting an important role in cirrhosis development. In BDL livers, HO-1 was induced especially in hepatocytes but it was also enhanced in Kupffer cells[33,51]. Accordingly, the additionally exogenous induction of HO-1 in a model of hepatic fibrosis due to BDL could further increase an already elevated HO-1 expression, resulting in potentially detrimental effects instead of cytoprotection.
Oxidant-induced cell death may also cause exaggerated repair leading to fibrosis. Therefore, non-prevention of cell death may increase subsequent fibrosis. There are numerous reports indicating anti-apoptotic abilities of HO-1[39,52-54] by suppressing one or several signaling pathways of the pro-apoptotic stimuli. In contrast to these findings, we found that HO-1 overexpression increased ALT release and necrosis of parenchymal cells (Figures 3 and 4), as well as maximizing fibrosis (Figures 5-7) in this model of chronic cholestasis due to BDL.
Activation of hepatic stellate cells appears to be a critical step in hepatic fibrogenesis that is regulated by several factors, including cytokines and oxidative stress[34,55,56]. Previous studies could demonstrate HO-1 overexpression as a negative regulator in the control of fibrogenic activities of hepatic stellate cells[57,58]. Therefore it was hypothesized that HO-1 overexpression may prevent fibrosis by inhibiting oxidant-dependent activation and proliferation of stellate cells. In contrast to this hypothesis, α-SMA, a marker of stellate cell activation, dramatically increased after BDL (Figure 8), an effect that was significantly worsen by CoPP pretreatment. Activated stellate cells also produce TGF-β1, which causes further proliferation of stellate cells and strongly stimulates production of collagens[59,60]. Oxidative stress not only increases production of TGF-β1 but also activates latent TGF-β1[61,62]. Additionally, H2O2 activates transcriptional factors such as AP-1 and NF-κB[63] that are involved in stellate cell activation and synthesis of TGF-β1[55,63]. Consistent with these earlier findings, activation of AP-1, NF-κB, and increases in TGF-β1 mRNA were all observed in the present study after BDL (Figure 9), supporting the hypothesis that oxidative stress stimulates TGF-β1 production, leading to collagen gene and protein expression (Figure 7). But in contrast, CoPP pretreatment before BDL did not blocked activation of transcription factors and cytokine production. Furthermore, HO-1 overexpression induced TGF-β1 (Figure 9B) expression after BDL even more, leading to a significant increase in collagen-Iα mRNA and α-SMA protein expression (Figures 7 and 8). These findings also point to the potential pro-oxidant consequences of an increased HO-1 expression and activity.
Another possibility is that elevated HO-1 over-expression aggravates the activation of Kupffer cells, thus increasing formation of inflammatory and fibrogenic mediators. Previous reports identified Kupffer cells as the main source of HO-1 expression in human livers[45,64] in contrast to controversial data from rat livers, where considerable expressions of HO-1 has also been found in hepatocytes[51,65]. Besides the large body of evidence demonstrating cytoprotective effects of HO-1 upregulation in transplant models in animals[37,66,67], Geuken et al[45] suggested that high HO-1 overexpression in Kupffer cells in human liver allografts may contribute to an increased cell injury. In general, the role of Kupffer cells in fibrosis is controversial. Destruction of Kupffer cells attenuated liver fibrosis caused by carbon tetrachloride[68]. By contrast, in a rat model of reversible biliary obstruction, inactivation of Kupffer cells impaired collagen metabolism and inhibited the resolution of fibrosis[69]. Kupffer cells release many mediators that activate stellate cells, including TNF-α, TGF-β, human growth factor, PDGF, and reactive oxygen species[60,70]. TNF-α production and NF-κB activation increase during cholestasis[71,72]. Activation of NF-κB, probably due to oxidative stress, could lead to expression of TNF-α. An inhibition of proinflammatory cytokine production as a result of HO-1 overexpression, as reported for several other liver injury models[73-75], could not be demonstrated in the present study. In addition, we have shown that activation of NF-κB and expression of TNF-α and TGF-β were increased by cholestasis and that these effects were not blocked by HO-1 overexpression (Figure 9). In contrast, TGF-β1 mRNA expression was significantly increased in rats receiving CoPP before BDL (Figure 9B) demonstrating an additional accumulation of one key mediator in fibrogenesis. However, in a previous study we could demonstrate that suppression of Kupffer cell function with GdCl3, a treatment that blocks carbon tetrachloride-induced fibrosis[68], did not attenuate fibrosis caused by cholestasis[29]. This might indicate that Kupffer cells likely do not play a prominent role in cholestasis-induced fibrosis in vivo and that HO-1 up-regulation does not work exclusively by increasing Kupffer cell activation.
In conclusion, we could demonstrate that hepatic fibrosis due to BDL is not reduced by HO-1 overexpression. In contrast, CoPP dependent HO-1 overexpression increases liver injury in rats under conditions of experimental chronic cholestasis. In conjunction with previous reports, these findings indicate that increased HO-1 activity is not exclusively protective. Rather, the HO-1 induced cytoprotection might be restricted to a narrow threshold of overexpression in the injury model used.
S- Editor Liu Y L- Editor Roberts SE E- Editor Ma WH
| 1. | Starzl TE, Demetris AJ, Van Thiel D. Liver transplantation (1). N Engl J Med. 1989;321:1014-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 393] [Article Influence: 10.9] [Reference Citation Analysis (3)] |
| 2. | Whitington PF, Balistreri WF. Liver transplantation in pediatrics: indications, contraindications, and pretransplant management. J Pediatr. 1991;118:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 95] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Engelhardt JF, Ye X, Doranz B, Wilson JM. Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc Natl Acad Sci USA. 1994;91:6196-6200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 433] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 4. | Tyler DD. Polarographic assay and intracellular distribution of superoxide dismutase in rat liver. Biochem J. 1975;147:493-504. [PubMed] |
| 5. | Qureshi WA. Intrahepatic cholestatic syndromes: pathogenesis, clinical features and management. Dig Dis. 1999;17:49-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Stiehl A, Rudolph G, Raedsch R, Möller B, Hopf U, Lotterer E, Bircher J, Fölsch U, Klaus J, Endele R. Ursodeoxycholic acid-induced changes of plasma and urinary bile acids in patients with primary biliary cirrhosis. Hepatology. 1990;12:492-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 95] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Skulina D, Owczarek D, Ciećko-Michalska I, Szczepański W, Tetnowski J, Garlicka M, Janas-Skulina U. The influence of ursodeoxycholic acid on some biochemical, immunologic and histopathologic parameters in patients with primary biliary cirrhosis. Przegl Lek. 1999;56:201-204. [PubMed] |
| 8. | Sokol RJ, Winklhofer-Roob BM, Devereaux MW, McKim JM. Generation of hydroperoxides in isolated rat hepatocytes and hepatic mitochondria exposed to hydrophobic bile acids. Gastroenterology. 1995;109:1249-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 206] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Pastor A, Collado PS, Almar M, González-Gallego J. Antioxidant enzyme status in biliary obstructed rats: effects of N-acetylcysteine. J Hepatol. 1997;27:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 111] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Sokol RJ, Devereaux MW, Khandwala R. Effect of oxypurinol, a xanthine oxidase inhibitor, on hepatic injury in the bile duct-ligated rat. Pediatr Res. 1998;44:397-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Parola M, Leonarduzzi G, Robino G, Albano E, Poli G, Dianzani MU. On the role of lipid peroxidation in the pathogenesis of liver damage induced by long-standing cholestasis. Free Radic Biol Med. 1996;20:351-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 126] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Sokol RJ, Devereaux M, Khandwala RA. Effect of dietary lipid and vitamin E on mitochondrial lipid peroxidation and hepatic injury in the bile duct-ligated rat. J Lipid Res. 1991;32:1349-1357. [PubMed] |
| 13. | Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557-2568. [PubMed] |
| 14. | Maines MD, Trakshel GM, Kutty RK. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J Biol Chem. 1986;261:411-419. [PubMed] |
| 15. | Tenhunen R, Marver HS, Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969;244:6388-6394. [PubMed] |
| 16. | Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA. 1968;61:748-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1280] [Cited by in RCA: 1348] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 17. | Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1997] [Cited by in RCA: 1975] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 18. | Abraham NG, Lin JH, Dunn MW, Schwartzman ML. Presence of heme oxygenase and NADPH cytochrome P-450 (c) reductase in human corneal epithelium. Invest Ophthalmol Vis Sci. 1987;28:1464-1472. [PubMed] |
| 19. | McCoubrey WK, Huang TJ, Maines MD. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur J Biochem. 1997;247:725-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 614] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 20. | Shibahara S, Yoshizawa M, Suzuki H, Takeda K, Meguro K, Endo K. Functional analysis of cDNAs for two types of human heme oxygenase and evidence for their separate regulation. J Biochem. 1993;113:214-218. [PubMed] |
| 21. | McCoubrey WK, Maines MD. The structure, organization and differential expression of the gene encoding rat heme oxygenase-2. Gene. 1994;139:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Wunder C, Potter RF. The heme oxygenase system: its role in liver inflammation. Curr Drug Targets Cardiovasc Haematol Disord. 2003;3:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 913] [Cited by in RCA: 967] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 24. | Alcaraz MJ, Fernández P, Guillén MI. Anti-inflammatory actions of the heme oxygenase-1 pathway. Curr Pharm Des. 2003;9:2541-2551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 184] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 25. | Baranano DE, Rao M, Ferris CD, Snyder SH. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci USA. 2002;99:16093-16098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 800] [Cited by in RCA: 830] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 26. | Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton JW, Vercellotti GM. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem. 1992;267:18148-18153. [PubMed] |
| 27. | Symonidis A, Trams EG. Morphologic and functional changes in the livers of rats after ligation or excision of the common bile duct. Am J Pathol. 1957;33:13-27. [PubMed] |
| 28. | López-De León A, Rojkind M. A simple micromethod for collagen and total protein determination in formalin-fixed paraffin-embedded sections. J Histochem Cytochem. 1985;33:737-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 474] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 29. | Zhong Z, Froh M, Lehnert M, Schoonhoven R, Yang L, Lind H, Lemasters JJ, Thurman RG. Polyphenols from Camellia sinenesis attenuate experimental cholestasis-induced liver fibrosis in rats. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1004-G1013. [PubMed] |
| 30. | Zhong Z, Froh M, Wheeler MD, Smutney O, Lehmann TG, Thurman RG. Viral gene delivery of superoxide dismutase attenuates experimental cholestasis-induced liver fibrosis in the rat. Gene Ther. 2002;9:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Zabel U, Schreck R, Baeuerle PA. DNA binding of purified transcription factor NF-kappa B. Affinity, specificity, Zn2+ dependence, and differential half-site recognition. J Biol Chem. 1991;266:252-260. [PubMed] |
| 32. | Gilman M. Ribonuclease protection assay. Current Protocols im Molecular Biology. New York: Wiley and Sons 1999; 4.7.1-4.7.8. |
| 33. | Wei CL, Lee KH, Khoo HE, Hon WM. Expression of haem oxygenase in cirrhotic rat liver. J Pathol. 2003;199:324-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Friedman SL, Roll FJ, Boyles J, Bissell DM. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci USA. 1985;82:8681-8685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 651] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 35. | Poupon R, Chazouillères O, Poupon RE. Chronic cholestatic diseases. J Hepatol. 2000;32:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 122] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 36. | Alptekin N, Mehmetçik G, Uysal M, Aykaç-toker G. Evidence for oxidative stress in the hepatic mitochondria of bile duct ligated rats. Pharmacol Res. 1997;36:243-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen XD, Zhao D, Zaky J, Melinek J, Lassman CR. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest. 1999;104:1631-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 406] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 38. | Woo J, Iyer S, Cornejo MC, Mori N, Gao L, Sipos I, Maines M, Buelow R. Stress protein-induced immunosuppression: inhibition of cellular immune effector functions following overexpression of haem oxygenase (HSP 32). Transpl Immunol. 1998;6:84-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Hancock WW, Buelow R, Sayegh MH, Turka LA. Antibody-induced transplant arteriosclerosis is prevented by graft expression of anti-oxidant and anti-apoptotic genes. Nat Med. 1998;4:1392-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 383] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 40. | Panahian N, Yoshiura M, Maines MD. Overexpression of heme oxygenase-1 is neuroprotective in a model of permanent middle cerebral artery occlusion in transgenic mice. J Neurochem. 1999;72:1187-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 314] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 41. | Parry N, Buelow R, Jiang J, Garcia B, Chakrabarti S, Zhong R. A rationally designed immunomodulatory peptide upregulates expression of hemeoxygenase-1 and attenuates chronic rejection in a rat renal allograft model. Transplantation. 1999;67:252. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 42. | Otterbein L, Sylvester SL, Choi AM. Hemoglobin provides protection against lethal endotoxemia in rats: the role of heme oxygenase-1. Am J Respir Cell Mol Biol. 1995;13:595-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 241] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 43. | Suttner DM, Dennery PA. Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. FASEB J. 1999;13:1800-1809. [PubMed] |
| 44. | Malaguarnera L, Madeddu R, Palio E, Arena N, Malaguarnera M. Heme oxygenase-1 levels and oxidative stress-related parameters in non-alcoholic fatty liver disease patients. J Hepatol. 2005;42:585-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 45. | Geuken E, Buis CI, Visser DS, Blokzijl H, Moshage H, Nemes B, Leuvenink HG, de Jong KP, Peeters PM, Slooff MJ. Expression of heme oxygenase-1 in human livers before transplantation correlates with graft injury and function after transplantation. Am J Transplant. 2005;5:1875-1885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 46. | Yet SF, Pellacani A, Patterson C, Tan L, Folta SC, Foster L, Lee WS, Hsieh CM, Perrella MA. Induction of heme oxygenase-1 expression in vascular smooth muscle cells. A link to endotoxic shock. J Biol Chem. 1997;272:4295-4301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 144] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 47. | Singh S, Shackleton G, Ah-Sing E, Chakraborty J, Bailey ME. Antioxidant defenses in the bile duct-ligated rat. Gastroenterology. 1992;103:1625-1629. [PubMed] |
| 48. | Shibahara S. Regulation of heme oxygenase gene expression. Semin Hematol. 1988;25:370-376. [PubMed] |
| 49. | Chu CJ, Lee FY, Wang SS, Lu RH, Tsai YT, Lin HC, Hou MC, Chan CC, Lee SD. Hyperdynamic circulation of cirrhotic rats with ascites: role of endotoxin, tumour necrosis factor-alpha and nitric oxide. Clin Sci (Lond). 1997;93:219-225. [PubMed] |
| 50. | Carter EP, Hartsfield CL, Miyazono M, Jakkula M, Morris KG, McMurtry IF. Regulation of heme oxygenase-1 by nitric oxide during hepatopulmonary syndrome. Am J Physiol Lung Cell Mol Physiol. 2002;283:L346-L353. [PubMed] |
| 51. | Flores O, Criado M, Sánchez-Rodríguez A, Hidalgo F, Collía F, López-Novoa JM, Esteller A. Relationships between NOS2 and HO-1 in liver of rats with chronic bile duct ligation. Hepatol Res. 2005;32:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Soares MP, Lin Y, Anrather J, Csizmadia E, Takigami K, Sato K, Grey ST, Colvin RB, Choi AM, Poss KD. Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nat Med. 1998;4:1073-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 508] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 53. | Ferris CD, Jaffrey SR, Sawa A, Takahashi M, Brady SD, Barrow RK, Tysoe SA, Wolosker H, Barañano DE, Doré S. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat Cell Biol. 1999;1:152-157. [PubMed] |
| 54. | Choi BM, Pae HO, Chung HT. Nitric oxide priming protects nitric oxide-mediated apoptosis via heme oxygenase-1 induction. Free Radic Biol Med. 2003;34:1136-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Lee KS, Buck M, Houglum K, Chojkier M. Activation of hepatic stellate cells by TGF alpha and collagen type I is mediated by oxidative stress through c-myb expression. J Clin Invest. 1995;96:2461-2468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 406] [Article Influence: 13.5] [Reference Citation Analysis (1)] |
| 56. | Kim KY, Choi I, Kim SS. Progression of hepatic stellate cell activation is associated with the level of oxidative stress rather than cytokines during CCl4-induced fibrogenesis. Mol Cells. 2000;10:289-300. [PubMed] |
| 57. | Tsui TY, Lau CK, Ma J, Wu X, Wang YQ, Farkas S, Xu R, Schlitt HJ, Fan ST. rAAV-mediated stable expression of heme oxygenase-1 in stellate cells: a new approach to attenuate liver fibrosis in rats. Hepatology. 2005;42:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 58. | Li L, Grenard P, Nhieu JT, Julien B, Mallat A, Habib A, Lotersztajn S. Heme oxygenase-1 is an antifibrogenic protein in human hepatic myofibroblasts. Gastroenterology. 2003;125:460-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 59. | Hellerbrand C, Stefanovic B, Giordano F, Burchardt ER, Brenner DA. The role of TGFbeta1 in initiating hepatic stellate cell activation in vivo. J Hepatol. 1999;30:77-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 317] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 60. | Friedman SL. Cytokines and fibrogenesis. Semin Liver Dis. 1999;19:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 262] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 61. | Leonarduzzi G, Scavazza A, Biasi F, Chiarpotto E, Camandola S, Vogel S, Dargel R, Poli G. The lipid peroxidation end product 4-hydroxy-2,3-nonenal up-regulates transforming growth factor beta1 expression in the macrophage lineage: a link between oxidative injury and fibrosclerosis. FASEB J. 1997;11:851-857. [PubMed] |
| 62. | Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol. 1996;10:1077-1083. [PubMed] |
| 63. | Griendling KK, Sorescu D, Lassègue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 2000;20:2175-2183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 626] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 64. | Makino N, Suematsu M, Sugiura Y, Morikawa H, Shiomi S, Goda N, Sano T, Nimura Y, Sugimachi K, Ishimura Y. Altered expression of heme oxygenase-1 in the livers of patients with portal hypertensive diseases. Hepatology. 2001;33:32-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 65. | Bauer I, Wanner GA, Rensing H, Alte C, Miescher EA, Wolf B, Pannen BH, Clemens MG, Bauer M. Expression pattern of heme oxygenase isoenzymes 1 and 2 in normal and stress-exposed rat liver. Hepatology. 1998;27:829-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 139] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 66. | Katori M, Buelow R, Ke B, Ma J, Coito AJ, Iyer S, Southard D, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 overexpression protects rat hearts from cold ischemia/reperfusion injury via an antiapoptotic pathway. Transplantation. 2002;73:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 67. | Maines MD, Raju VS, Panahian N. Spin trap (N-t-butyl-alpha-phenylnitrone)-mediated suprainduction of heme oxygenase-1 in kidney ischemia/reperfusion model: role of the oxygenase in protection against oxidative injury. J Pharmacol Exp Ther. 1999;291:911-919. [PubMed] |
| 68. | Rivera CA, Bradford BU, Hunt KJ, Adachi Y, Schrum LW, Koop DR, Burchardt ER, Rippe RA, Thurman RG. Attenuation of CCl(4)-induced hepatic fibrosis by GdCl(3) treatment or dietary glycine. Am J Physiol Gastrointest Liver Physiol. 2001;281:G200-G207. [PubMed] |
| 69. | Roggin KK, Papa EF, Kurkchubasche AG, Tracy TF. Kupffer cell inactivation delays repair in a rat model of reversible biliary obstruction. J Surg Res. 2000;90:166-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 70. | Alcolado R, Arthur MJ, Iredale JP. Pathogenesis of liver fibrosis. Clin Sci (Lond). 1997;92:103-112. [PubMed] |
| 71. | Bemelmans MH, Gouma DJ, Greve JW, Buurman WA. Cytokines tumor necrosis factor and interleukin-6 in experimental biliary obstruction in mice. Hepatology. 1992;15:1132-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 146] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 72. | Fox ES, Kim JC, Tracy TF. NF-kappaB activation and modulation in hepatic macrophages during cholestatic injury. J Surg Res. 1997;72:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 73. | Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 856] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 74. | Sass G, Soares MC, Yamashita K, Seyfried S, Zimmermann WH, Eschenhagen T, Kaczmarek E, Ritter T, Volk HD, Tiegs G. Heme oxygenase-1 and its reaction product, carbon monoxide, prevent inflammation-related apoptotic liver damage in mice. Hepatology. 2003;38:909-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 75. | Sass G, Seyfried S, Parreira Soares M, Yamashita K, Kaczmarek E, Neuhuber WL, Tiegs G. Cooperative effect of biliverdin and carbon monoxide on survival of mice in immune-mediated liver injury. Hepatology. 2004;40:1128-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |