Published online Jul 7, 2007. doi: 10.3748/wjg.v13.i25.3430
Revised: February 11, 2007
Accepted: March 12, 2007
Published online: July 7, 2007
Nausea and/or vomiting are aversive gastrointestinal (GI) symptoms. Nausea and vomiting manifest unconditionally after a nauseogenic experience. However, there is correlative, quasiexperimental and experimental evidence that nausea and vomiting can also be learned via classical (Pavlovian) conditioning and might occur in anticipation of the nauseogenic event. Classical conditioning of nausea can develop with chemotherapy in cancer patients. Initially, nausea and vomiting occur during and after the administration of cytotoxic drugs (post-treatment nausea and vomiting) as unconditioned responses (UR). In addition, 20%-30% of cancer patients receiving chemotherapy report these side effects, despite antiemetic medication, when being re-exposed to the stimuli that usually signal the chemotherapy session and its drug infusion. These symptoms are called anticipatory nausea (AN) and/or anticipatory vomiting (ANV) and are explained by classical conditioning. Moreover, there is recent evidence for the assumption that post-chemotherapy nausea is at least partly influenced by learning. After summarizing the relevant assumptions of the conditioning model, revealing that a context can become a conditioned stimulus (CS), the present paper summarizes data that nausea and/or vomiting is acquired by classical conditioning and, consequently, may be alleviated by conditioning techniques. Our own research has focussed on two aspects and is emphasized here. First, a conditioned nausea model was established in healthy humans using body rotation as the nausea-inducing treatment. The validity of this motion-sickness model to examine conditioning mechanisms in the acquisition and alleviation of conditioned nausea and associated endocrine and immunological responses is summarized. Results from the rotation-induced motion sickness model showed that gender is an important moderator variable to be considered in further studies. This paper concludes with a review of the application of the demonstrated conditioning principles as interventions to ameliorate distressing AN/ANV in cancer patients undergoing chemotherapy, which is the second focus of our work.
- Citation: Stockhorst U, Enck P, Klosterhalfen S. Role of classical conditioning in learning gastrointestinal symptoms. World J Gastroenterol 2007; 13(25): 3430-3437
- URL: https://www.wjgnet.com/1007-9327/full/v13/i25/3430.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i25.3430
Nausea and/or vomiting are aversive symptoms of the gastrointestinal (GI) system. In the present paper, we review the empirical evidence that nausea and vomiting can be acquired by classical (Pavlovian) conditioning and consequently can be prevented or reduced by means of classical conditioning. With regard to basic research, we will summarize data showing that a context contingently paired with the experience of nausea will become a conditioned stimulus (CS) for inducing nausea. The clinical perspective is on how symptoms such as nausea during motion sickness and anticipatory nausea in cancer patients are acquired by classical conditioning and consequently prevented via conditioning techniques.
Nausea is a "subjective, unpleasant feeling that may signal imminent vomiting. Nausea is accompanied by changes in autonomic nervous system activity, particularly parasympathetic activity, diminished gastric tone, reduced peristalsis and retrograde peristalsis, and retrograde duodenal peristalsis." Vomiting is the "forceful emptying of the gastric contents through the sustained action of abdominal muscles and the opening of the gastric cardia[1]."
Nausea and vomiting occur under a variety of conditions, such as in cancer chemotherapy, after vestibulary stimulation (motion sickness), or during pregnancy. In this paper, we will address two types of nausea: (a) nausea as a symptom of motion sickness, and mainly (b) nausea and vomiting in cancer patients undergoing cytotoxic drug treatment, which are regarded by patients as the most aversive side-effects of cancer treatment. Experiencing these side-effects might result in treatment drop-outs[2]. Referring to recent studies, Bovbjerg[3] pointed out that in the absence of prophylactic antiemetic therapy, the percentage of patients experiencing vomiting after their first chemotherapy would reach 90%. Despite modern antiemetic treatment, about 25% to 30% of chemotherapy patients report these side-effects even prior to a subsequent infusion upon being re-exposed to the stimuli that usually signal the drugs' infusion[4-6]. These symptoms are called anticipatory nausea (AN) and/or anticipatory vomiting (ANV).
The aim of the present paper is to address the question of whether AN, ANV and related symptoms, as well as associated endocrine and immunological responses, are acquired by classical conditioning. In addition, we studied how conditioning techniques might be used to alleviate AN and ANV responses in healthy subjects that are exposed to an experimental nausea model with rotation as the nausea-inducing stimulus and we considered how to extend the effective methods to patients receiving chemotherapy. An aspect of the conditioning model for chemotherapy that has been considered recently is whether post-chemotherapy nausea is augmented by learning processes, which then add to the unconditioned nausea response[3,7]. This question is also addressed in the present paper.
Classical conditioning is a basic associative learning paradigm originally described by I.Pavlov[8]: An organism learns to associate two stimuli, an initially neutral stimulus (the conditioned stimulus, or CS) and a biologically relevant stimulus (the unconditioned stimulus, or US). By pairing a CS with the US in the acquisition phase, the CS comes to evoke a conditioned response (CR), which is commonly similar to the response elicited by the US and is nausea and vomiting in the present case. This association is assumed to take place within the central nervous system (CNS)[9]. There are conditions where even a single pairing ("trial") of CS→US can induce learning, i.e., in conditioned taste aversion.
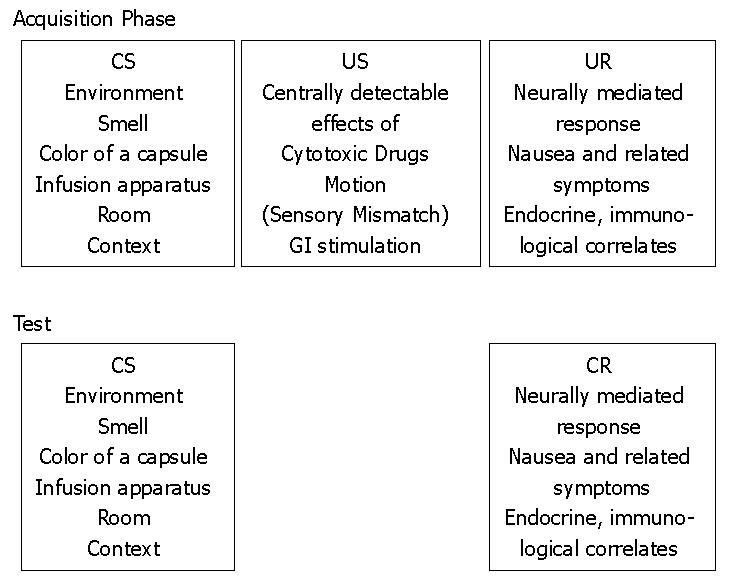
A real-life situation that has features of a classical conditioning trial is the application of a drug in the presence of predictive cues. The drug, or more precisely its centrally detectable effects, constitutes the US. Because the environmental context in which a drug is administered can act like a CS, the environmental stimuli that contingently signal the US may become CSs, so that some degree of illness will come to occur anticipatorily in the presence of these predictive cues[10-12]. Figure 1 describes this learning process for nausea-inducing interventions for the acquisition phase (pairing of CS and US) and the test phase (where the CS elicits the CR).
The first evidence of classical conditioning of nausea and emesis comes from early experiments conducted by Collins and Tatum[13], using morphium sulphate, and from experiments done in the laboratory ofI. Pavlov by his collaborators, using morphine (studies done by Krylov) and apomorphin (studies conducted by Podkopaev)[8]. After animal subjects had experienced the injection of these substances, signals that had reliably predicted drug administration (i.e., entry of the experimenter, drawing of the syringe) resulted in nausea, salivation and emesis.
An often used paradigm showing conditioning effects with nausea-inducing drugs is conditioned taste aversion (CTA). CTA manifests in the avoidance of a taste that was initially preferred but is avoided after this taste had been paired with the administration of an illness-inducing manipulation such as gamma radiation[14], or with illness-inducing drugs such as lithium[15], or cyclophospamide[16]. CTA has some features that make it a unique form of learning in that it can be established after a single CS-US pairing (one trial learning), and CS and US can be separated by long CS-US intervals. Moreover, there is a preparedness for certain qualities of CS and US to become a CS because gustatory and olfactory stimuli are more easily associated with illness-inducing USs than visual and acoustical stimuli.
The CNS mediation of nausea and vomiting is important because the CNS is assumed to be involved in the detection of both the CS and US as well as the formation of the CS-US association. The CNS structures that are involved in nausea and vomiting and the central mechanism regulating nausea are still an area of research.
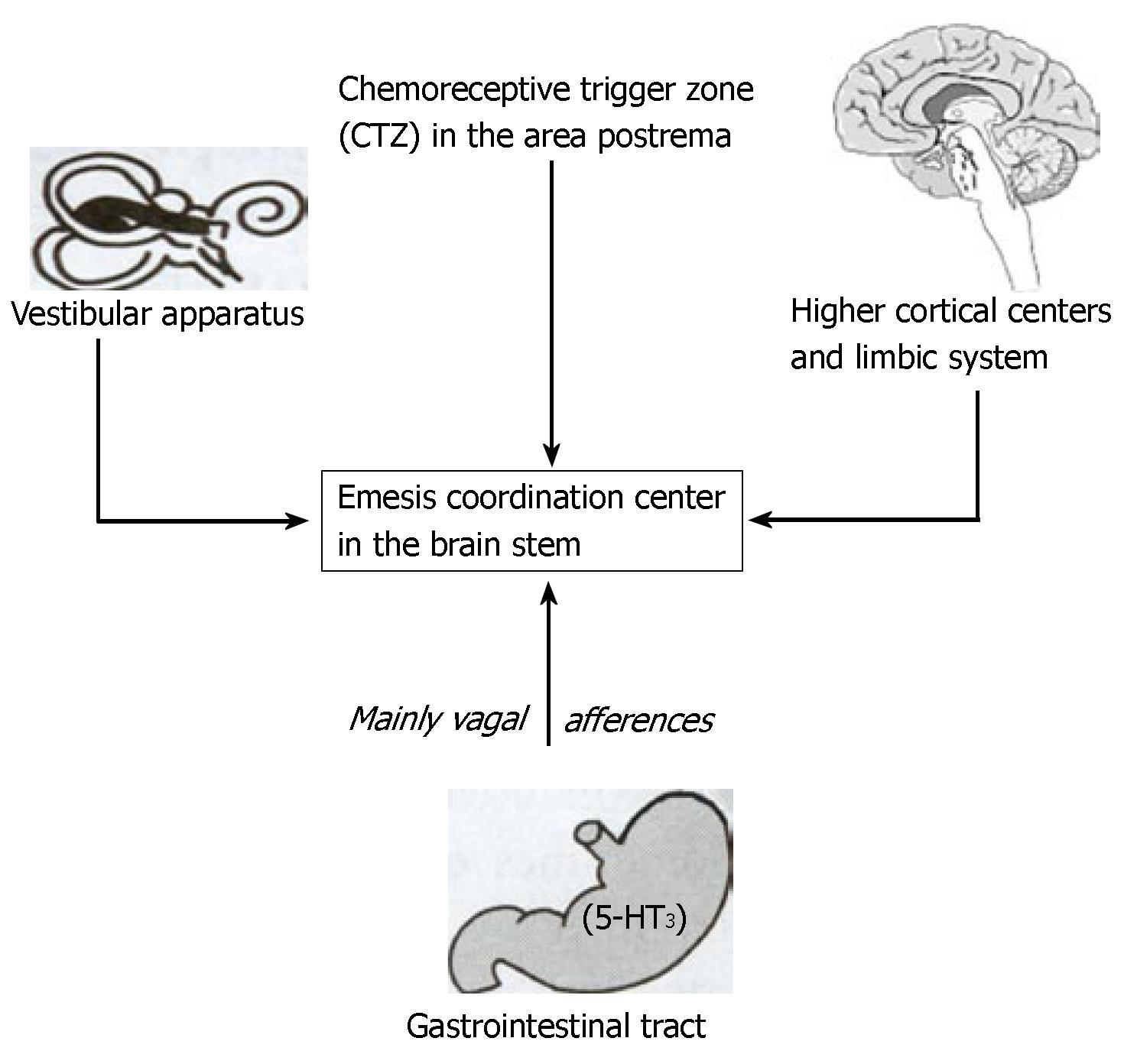
As summarized in Figure 2, the emesis coordination center in the brain stem receives afferents from: (1) the chemoreceptor trigger zone (CTZ) in the area postrema, where drugs, such as cytotoxic substances, apomorphine, and nausea-inducing opioids, are detected via dopaminergic (D2), serotonergic (5-HT), muscarinergic, neurokinin 1 (NK1), opioid, alpha-2 adrenoreceptors and noradrenergic receptors. (2) the vestibular system, where motion or sensory mismatch is detected, and where histaminergic (H1), muscarinergic and 5HT1b receptors are located. (3) mainly vagal afferents from the gastrointestinal tract that are activated by cytotoxic substances (such as cytostatic drugs during chemotherapy), by toxic food or radiotherapy. The receptors that are involved are 5-HT3 and 5-HT4, as well as NK1 receptors in the gastrointestinal tract. (4) higher brain centers and the limbic system.
Accordingly, the USs are the centrally detectable effects of those biologically active stimuli (i.e., the centrally detected effects of cytotoxic drugs, motion or sensory mismatch and/or gastrointestinal stimulation), afferently reaching the emesis center leading to nausea and related symptoms and to the unconditioned endocrine and immunological changes (considered as the unconditioned responses, or URs), which are components that will be evoked by the CS as CRs.
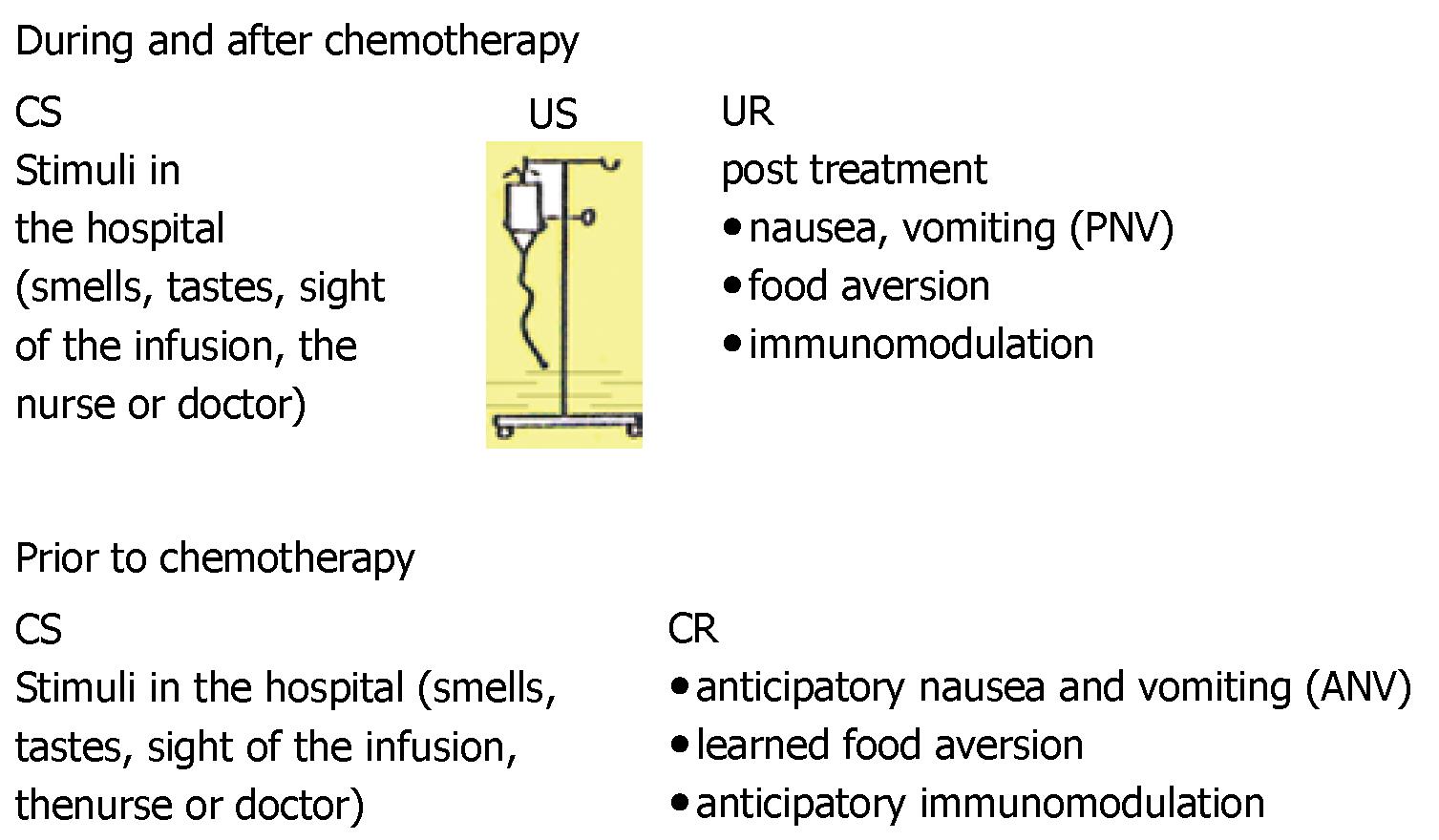
The situation in which a cancer patient receives chemo-therapy has been labelled a natural laboratory[17] to examine Pavlovian conditioning of anticipatory side effects. Figure 3 shows the Pavlovian conditioning model for cancer chemotherapy. The patient will experience the infusion of the cytotoxic drugs in the presence of hospital stimuli, such as smells, tastes, the sight of the infusion apparatus or of the nurse or the doctor. These signals reliably precede the start of the infusion. The drugs of the infusion unconditionally induce nausea, food aversion and immunomodulation after their administration; i.e., in the post-treatment phase as the UR. Finally, the hospital stimuli alone will induce nausea, immunomodulation and food aversion prior to drug administration as anticipatory responses; i.e., AN, ANV, anticipatory immunomodulation and learned food aversion.
In our studies on ANV, we use the following procedure to measure the unconditioned as well as the conditioned subjective side-effects of cytotoxic drugs in chemotherapy. Subjects are asked to indicate the occurrence and intensity of nausea, vomiting, dizziness, hot flashes, heart beat, weakness, headache and, additionally, loss of appetite. These symptoms are recorded using diary-like symptom lists 48 h prior to (to measure ANV) as well as 48 h after (to measure PNV) a chemotherapy cycle. Additionally, food aversion (i.e., the reduction in the hedonic quality of food that had been consumed prior to the infusion), cortisol and immune parameters were measured in one of our studies[18].
A successful way to induce nausea and related symptoms in healthy humans is body rotation. In this model, the afferent signals from the vestibular system constitute the US. Body rotation results in a number of symptoms summarized as motion sickness, defined as a "malady characterized by the combination of signs and symptoms that accompany movement or perceived movement in the environment"[19]. A common feature of the diverse situations capable of inducing motion sickness is that they are all characterized by a condition of sensory rearrangement in which motion information signaled by the vestibular receptors, the eyes, and non-vestibular proprioceptors are contradictory to common past experience, i.e.,[19], a sensory conflict occurs. The most prominent symptoms of motion sickness in humans are nausea and vomiting, pallor, and cold sweating[19].
In our group ([20-22], Stockhorst et al, under revision), we exposed subjects to body rotation in the following way. Subjects were rotated around their vertical axis at 120 degrees/sec while seated in a rotation chair. During the rotations, they were instructed to move their heads up and down every 6 s with their eyes closed. Each rotation trial lasted 60 s, followed by a 1-min break. In the studies conducted so far, between two and five rotations were provided. To measure the subjective correlates of motion-sickness (and thus the UR and CR) in our experiments, subjects indicate the intensity of 7 symptoms (nausea, urge to vomit, dizziness, headache, tiredness, sweating, and general discomfort) and these symptoms were measured prior to and at several time-points after the end of rotation.
Predictions about the putative immunological correlates of nausea and related symptoms can be derived from data obtained in studies on the cytokine-induced "sickness behaviour"[23]. Cytokine-induced sickness behaviour is defined as the constellation of non-specific symptoms (such as weakness, malaise, listlessness and inability to concentrate) as well as depression, lethargy, and loss of appetite and drinking that occur during the course of an infection[24]. These symptoms are assumed to be mediated by proinflammatory cytokines (mainly interleukin [IL-1β] and tumor-necrosis factor [TNF-α]) on brain cell targets[24], and are assumed to be activated and/or transmitted to the brain via both neural and humoral communication pathways. This model is supported by studies showing that the central administration of IL-1β induces food aversion and anorexia[25,26], i.e., the avoidance of food that has been paired with a sickness-inducing drug (e.g., IL-1β) with subsequent weight reduction. Sickness behaviour also shares features in common with the nonspecific symptoms of cancer and cancer treatment side-effects[24,27,28]. Accordingly, a recent hypothesis is that activation of cytokines in the brain (via cytokines released by cancer cells or by stress from chemotherapy or radiotherapy) is responsible for the nonspecific symptoms of cancer including pain, cachexia, fatigue, sleep disorders and cognitive alterations. Consequently, these cytokines should be assessed during cancer chemotherapy. We can assess these in our stressful rotation-induced sickness model as well. In our studies, TNF-α was measured in all rotation studies and in one study with cancer patients[18].
Cortisol was measured in all of the rotation experiments and in one of our cancer chemotherapy studies[18]. The rationale to explore cortisol was based on two ideas. First, since rotation is an effective stressor, cortisol can index acute rotation-induced stress[20-22]. Secondly, there are data that indicate that endogenous (habitual) cortisol might protect against chemotherapy-induced nausea: It has been shown that patients with a high overnight level of urinary cortisol preceding the onset of a chemotherapy infusion show less nausea during and after chemotherapy[29]. Also, dexamethasone, as a synthetic member of the glucocorticoid class of hormones, is used as an antiemetic drug and is assumed to augment the antiemetic effect of 5-HT3 receptor antagonists. Similarly, glucocorticoid treatment via prednisone was effective in reducing acute mountain sickness (characterized by headache, nausea, excessive fatigue, loss of appetite, irritability, and insomina)[30]. Thus, cortisol is of interest as a potential state (i.e., acute) and trait (chronic) measure for nausea susceptibility.
In order to explain the development of anticipatory nausea to contextual hospital stimuli or to the rotation environment by means of classical conditioning, a conditioning paradigm is necessary that shows an association has developed between the context, as the CS, and the nausea-inducing drug, as the US. This model was elegantly established by Hall and collaborators[11,12,31,32]. The authors first demonstrated that the experience of a nausea-inducing agent (i.e., lithium chloride [LiCl]) in conjunction with exposure to a novel context will render the context aversive. In order to demonstrate this context aversion, rats received an injection of LiCl while being exposed to a novel context (i.e., a new cage different from the home cage in a number of features). In order to validate the nauseogenic nature of the context, one of the following test procedures, either the consumption test or the blocking test[31], was used.
The consumption test demonstrates that the contextual cues are capable of inducing, as the CR, some aspect of the state induced by the US itself. Subjects are first exposed to two different contexts (e.g., cage A and B). The exposure to only one of these contexts is preceded by an injection of LiCl, and the other is preceded by the injection of saline. Rats are then exposed to one of the contexts where they can consume sucrose solution. The result is the consumption of sucrose is only suppressed in the context that had been experienced with LiCl.
The blocking test is another way to demonstrate the acquisition of conditioned nausea. In the first phase, subjects experience the differential context exposure where only one of two contexts is associated with the illness-inducing drug (LiCl). After the injection, subjects are placed in one of two contexts, either A or B. Both groups received a compound CS, consisting of the context plus the flavor. As expected, the context blocks the acquisition of the conditioned aversion to the flavor only if that specific context had been paired with the injection of LiCl. Moreover, Hall and collaborators provided evidence that the context truly induces nausea (and not only taste avoidance).
Using these indicators of context aversion conditioning with nausea-inducing drugs, Hall and collaborators demonstrated both latent inhibition[31] as well as over-shadowing[31,32]. Overshadowing and latent inhibition (LI) are both techniques to reduce the association between the putative CS and the US. Overshadowing was originally described by Pavlov[8]. In a typical overshadowing experiment, a compound CS is paired with the US, where one of the CS compound elements is more salient than the other. This procedure results in only a weak association between the less salient CS and the US. Latent inhibition (LI)[33] consists of pre-exposing the event to be used as the CS prior to the first administration of the US, and this pre-exposure reduces the amount of classical conditioning that subsequently occurs.
The concrete procedures are illustrated in experiments carried out by our group aiming at reducing conditioned nausea in the rotation paradigm with healthy humans and in our overshadowing experiment with cancer patients, as described in the following subchapters of this paper.
In pilot studies (25 female and 25 male university students), screening for rotation susceptible subjects, TNF-α and cortisol (in saliva) were measured prior to rotation (= conditioned anticipatory symptoms) and immediately, 15 min and 30 min after the end of the last of a series of maximally five rotations (unconditioned post-rotation symptoms)[34]. Rotation resulted in an immediate increase in post-rotation symptoms of nausea (P < 0.001), and a tendency for an increase in TNF-α (P < 0.10). The cortisol increase peaked 15 min after the end of rotation (P < 0.001), corresponding to the temporal dynamics of the cortisol response seen after psychosocial stressors.
Another indicator for the validity of the rotation paradigm in conditioning research is the development of CTA, which was also demonstrated in the rotation paradigm. CTA developed to a novel taste (elderberry) if it was presented as the CS immediately prior to rotation onset (US) in the experimental group, whereas a familiar taste (water) immediately prior to rotation (US) in the control group, or the novel taste in a 1-h distance from the US did not yield CTA[20].
As already outlined for animal studies, conditioning techniques that are well known to attenuate the CS-US contingency would be expected to reduce conditioned nausea in the rotation paradigm[34]. Two experiments were conducted in our group using latent inhibition[21] and overshadowing (Stockhorst et al, under revision).
For inducing LI in the rotation paradigm, the CS (the rotation context) was presented without the US (rotation); i.e., subjects were exposed to the rotation context prior to their first rotation. Subjects were assigned to one of three groups, differing in the number of pre-exposures (0, 1 or 3). Subjects receiving 0 pre-exposures (Group LI0) were placed in a neutral environment on the three days preceding the first rotation day, while those receiving one pre-exposure (LI1) were placed in the neutral environment two days before and exposed to the rotation environment. Subjects receiving three pre-exposures (LI3) were seated in the rotation environment without rotation for three days. All subjects were given a maximum of five rotations on d 3 and 4, and on d 5 (test) they were all seated in the rotation environment without rotation. Significantly reduced anticipatory nausea-related symptoms were found in Groups LI3 and LI1 compared to LI0[21]. In general, females tended to show higher baseline scores in the symptom rating; i.e., anticipatory nausea at d 5, compared to males. However, they also showed a larger symptom reduction as a result of CS-preexposure relative to the untreated control group, LI0, mainly after one pre-exposure (LI1 vs LI0), but also after three pre-exposures (LI3 vs LI0)[21].
The context pre-exposure LI treatments did not affect post-rotation nausea, acute TNF-α or cortisol levels. Cortisol levels increased acutely in all groups after rotation, but decreased over repeated rotation exposures, more so in the female subjects. TNF-α levels decreased after rotation, but did not decrease from one to the next rotation session.
To induce overshadowing in the rotation paradigm, healthy, rotation-susceptible subjects were assigned to one of two groups. During the acquisition phase, subjects of the experimental group were presented 100 mL of salient tasting beverages with the taste changing on each day (elderberry, haw sallow-thorn, sloe) prior to rotation onset on three consecutive days. Subjects in the control group drank water. To control for the different taste experiences per se, subjects consumed the counterbalanced drink (i.e., water in the experimental group, and the salient beverage in the control group) 12 h later in their home environment. On the 4th day, subjects of both groups consumed water prior to rotation in the rotation environment. As with the cancer patients[7], subjects that drank the salient beverages showed reduced (anticipatory) conditioned symptoms, as well as attenuated post rotation symptoms (Stockhorst et al, under revision).
In our rotation experiments, we found gender differences in the unconditioned nausea response to rotation and in the hormonal and the immunological response patterns (see also[34]).
With regard to the unconditioned responses to motion-sickness inducing treatment specified below, data from other groups agree that women report more symptoms than men[35] or report a greater incidence in their history of previous motion sickness[36,37], but do not show differences from males in their symptoms after acute rotation[36,37]. They also do not differ in their physiological responses such as gastric tachyarrhythmia during exposure to an optokinetic drum[35], to viewing a rotating optokinetic drum[36], or when exposed to coriolis cross-coupling stimulation[37]; i.e., the unusual stimulation of the semicircular canals induced by off-axis head-movements (e.g., forward head movements) during rotation. In one of our experimental studies, we found a higher baseline (i.e., anticipatory) nausea response as measured by self-report in women than men[21].
Our results on the endocrine and immunological responses reveal a gender-specific effect. Gender-specific effects became evident in a study where healthy male and female subjects were exposed to repeated nauseogenic body rotation in a rotation drum[22] on four consecutive days (with a maximum of five 1-min rotations per day). The free cortisol level increased after rotation, but these rotation-induced cortisol increases lowered from d 2 to 4 in females, but not in males. This means, habituation of the cortisol response after repeated rotation was restricted to women, whereas men still showed cortisol reagibility after repetitive rotation experience. These results are supported by data in the latent inhibition study: Women showed lower cortisol increases after rotation than men[21]. There also is evidence for a gender-specific response pattern in immune parameters. In the habituation study[22], the in vitro production of the proinflammatory cytokine production did not habituate in men, but it did so in women. Moreover, there was evidence for a higher responsiveness in women compared to men both for conditioning of anticipatory nausea and for its modification by both overshadowing and latent inhibition[21]. A questionnaire study by Fessler and Arguello[38] is of interest here. In females, self-reported motion-sickness susceptibility was positively correlated with the number of conditioned food aversions, whereas no such correlation appeared in male participants.
To this point we have shown with the rotation-induced motion sickness model that known principles of conditioning do apply. Specifically, we showed that conditioning procedures, such as overshadowing and latent inhibition, can work to ameliorate or block conditioning of nausea. So it is natural to explore parallels in cancer patients undergoing chemotherapy.
There are two ways of testing the conditioning model in cancer chemotherapy, both of which we have used. First, by a correlative and quasi-experimental (and thus descriptive) approach, examining whether the occurrence of ANV corresponds to the conditioning model (correlative) and whether ANV differs in a relatively CS-free vs CS-containing environment (quasi-experimental). Secondly, by using an experimental approach where patients who were assigned to a conditioning intervention to prevent ANV are compared with patients receiving a control treatment.
In terms of classical conditioning, a CR is a direct function of the intensity of the US (and thus also of the intensity of the UR), the intensity of the CS[39], and the temporal contiguity between the CS and US[40]. In the chemotherapy situation, the emetogenity of the cytotoxic drug constitutes the US, the duration and intensity of post-treatment nausea and vomiting (PNV) indicate the duration and intensity of the UR, and the temporal distance between CS exposure and infusion onset (US) indicates the CS-US contiguity[34].
In the first correlative study[41], 55 ambulatory adult cancer patients [mean age: 50.78 ± (SEM) 2.03 years] with a mean preexperience of 9.8 ± 0.96 infusions, were asked to record nine symptoms (nausea, vomiting, dizziness, sweating, hot flashes, heartburn, headache, weakness, and heartbeat) after an infusion to assess post treatment symptoms and prior to a subsequent infusion (to assess anticipatory symptoms). Each measurement period (post-treatment and anticipatory) covered a maximum of 48 h, divided into four 12-h periods of days (6 am to 6 pm) and nights (6 pm to 6 am). AN was reported by 18.1% of the patients and, in correspondence with the conditioning predictions, there was a statistically significant association between the occurrence of post-treatment nausea (PN) and AN, and post-treatment vomiting (PV) and AN (i.e., occurrence of the UR and the CR), and between AN and the degree of emetogenity of the drug previously experienced (US-intensity). Furthermore, the duration of reported AN increased as temporal proximity to scheduled infusion onset increased[41].
Similar results[18] were obtained in a study with pediatric cancer patients (M = 10.1 ± 0.9 years), with a preexperience of 6.1 ± 0.5 previous cycles of chemotherapy. Patients were observed over two consecutive cycles of chemotherapy (cycle A and B), assessing symptoms during 48 h prior to the onset and 48 h after the end of the infusions. As predicted, the occurrence of ANV was positively associated with drug emetogenity, and this was demonstrated in the more emetogenic cycle A. Duration of AN tended to be associated with PN and AN increased as infusion onset time approached.
To assess the development of food aversions, patients had to rate the hedonic quality (liking) of those food items that they had consumed prior to the chemotherapy cycle. These food items were then repeatedly rated on a five-point graphically anchored scale (as liking the food very much, much, neither liking nor disliking, not liking, not at all) over 96 h surrounding a chemotherapy infusion. Compared to the initial rating during consumption, the hedonic quality of the food items decreased in the anticipatory and the post-treatment interval, especially in those patients that had developed ANV[34].
Bovbjerg[3] recently reviewed and summarized the variables that affect ANV in cancer patients. In addition to the variables that were found in our studies (i.e., intensity of the US [emetogenity of the chemotherapy protocol] and intensity of the UR [intensity of post-treatment nausea]), the following results are in correspondence with a classical conditioning interpretation of ANV: The likelihood of ANV increases across repeated treatment infusions; i.e., with a higher number of conditioning trials[42] and with the proportion of infusions followed by nausea (% reinforcement)[43]. Using an experimental design, a gustatory cue that had been explicitly paired with chemotherapy infusion caused conditioned nausea as a CR outside the hospital when patients were reexposed to that CS[44].
Bovbjerg[3] also reported interesting data showing that post-treatment nausea is partly influenced by AN; i.e., the amount of AN developed during earlier cycles of chemotherapy might add to post-treatment nausea. He analyzed 40 early stage breast cancer patients who developed AN in the clinic prior to their treatment infusion and their subsequent post-treatment nausea after infusion. A stepwise regression analysis revealed that conditioned nausea affects the severity of subsequent post-treatment nausea. Post-treatment nausea was primarily affected by the nausea response elicited by the infusion preceding the target infusion, and then secondly by the conditioned nausea response in the clinic prior to the target infusion. Variables, that turned out to be significantly associated with ANV in bivariate correlation analyses (i.e., age, Karnofsky index, life history of nausea) turned out to no longer contribute in the stepwise regression analysis.
Based on the assumption that AN develops when a cancer patient associates the context (CS) with the emetogenic and nausea-inducing cytotoxic drugs (US), one therapeutic aim is to restrict the development of the CS-US contingency and thus prevent the context CS becoming associated with the drug US.
Using the overshadowing intervention technique developed above[7], 16 cancer patients (most of them receiving their first chemotherapy infusion treatment for malignant systemic diseases; i.e., Non-Hodgkin and Hodgkin-lymphoma) were assigned to one of two groups and either received an overshadowing intervention or a control treatment prior to all infusions of two consecutive chemotherapy cycles (A and B). The overshadowing stimuli were beverages that had been pre-tested as being salient (haw, elderberry; red fruit mix; mixed drink of bitter lemon, apple, orange juice; woodruff, guave; grapefruit; herbal soda; orange sallow-thorn; tropical fruit) and the overshadowed stimulus was the context. Patients were instructed to drink 250 mL of a beverage in the interval from 10 min prior to 10 min after the start of each infusion; a different beverage was used prior to each infusion of the two chemotherapy cycles (A, B). Patients in the control group drank an equal volume of water at the same time-points. Prior to cycle C, all subjects were given water. As predicted, AN did not occur in the experimental group, whereas two of eight patients in the control group (25%) showed AN in cycle C. The intervention effects were not restricted to AN. The manipulation tended to attenuate the adverse UR as well. The overshadowing stimuli led to a shorter duration post-treatment nausea and a longer latency between infusion onset and nausea onset. This gives rise to the following interpretation[7]. The response to the US may be also influenced by the subjects' response to the CS, which persists into the measurement phase of the UR (in our case post-treatment nausea). Consequently, overshadowing might also lead to a reduction of the UR (not only the CR). This can be related to the phenomenon of conditioned diminution of responding to the US[45], and is also in correspondence with the data by Bovbjerg[3] who showed that AN is affected by post-treatment nausea. Consequently, conditioning techniques that reduce AN should reduce post-treatment nausea as well.
Although proinflammatory cytokines are regarded as correlates of CTA[25,26] and of sickness behaviour[23,24], there were no data correlating cytokine levels and food aversion. Consequently, these immunological parameters were measured in our study with pediatric cancer patients[18]. Prior to one of the observed chemotherapy cycles (cycle B), blood was sampled in the home (day-2) and hospital (day 0) environment to measure natural killer cell activity (NKCA) and a number of cytokines (IL-1β, IL-2, IL-10, interferon [IFN]-γ, TNF-α). NKCA as well as the IFN-γ level increased from home to hospital, indicating a CS-related response also in immune parameters. Based on TNF's relation to food aversion in animal studies[25], TNF-α level was assessed for a correlation with anticipatory food aversion and post-treatment loss of appetite. We found some positive correlation for TNF-α, measured in the hospital prior to the start of an infusion, with anticipatory food aversion as well as with post-treatment loss of appetite[34].
We have shown important effects of classical conditioning in establishing AN and in preventing (or at least reducing) it. Male and female subjects differ in susceptibility to both conditioned and unconditioned nausea, and this merits physicians' attention to gender in planning chemotherapy protocols for cancer.
Future studies should further evaluate the conditioning view of nausea, especially in chemotherapy patients. Here it is now also interesting and important to address post-chemotherapy nausea as well, and find out whether it is modified by classical conditioning. Consequently, post-treatment nausea should be also treated by conditioning techniques, as already indicated in our overshadowing experiment[7]. The potential for alleviating patient distress when undergoing a series of chemotherapy treatments is an important goal in itself and is an adjunct to patients accepting to continue chemotherapy. Cytokines can be regarded as a useful marker of both conditioning etiology and the effectiveness of the conditioning-based interventions, which we have outlined in this paper.
S- Editor Liu Y L- Editor Lutze M E- Editor Wang HF
| 1. | Morrow GR, Hickok JT, Rosenthal SN. Progress in reducing nausea and emesis. Comparisons of ondansetron (Zofran), granisetron (Kytril), and tropisetron (Navoban). Cancer. 1995;76:343-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Miller M, Kearney N. Chemotherapy-related nausea and vomiting - past reflections, present practice and future management. Eur J Cancer Care (Engl). 2004;13:71-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Bovbjerg DH. The continuing problem of post chemotherapy nausea and vomiting: contributions of classical conditioning. Auton Neurosci. 2006;129:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Aapro MS, Kirchner V, Terrey JP. The incidence of anticipatory nausea and vomiting after repeat cycle chemotherapy: the effect of granisetron. Br J Cancer. 1994;69:957-960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Aapro MS, Molassiotis A, Olver I. Anticipatory nausea and vomiting. Support Care Cancer. 2005;13:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Morrow GR, Roscoe JA, Kirshner JJ, Hynes HE, Rosenbluth RJ. Anticipatory nausea and vomiting in the era of 5-HT3 antiemetics. Support Care Cancer. 1998;6:244-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Stockhorst U, Wiener JA, Klosterhalfen S, Klosterhalfen W, Aul C, Steingrüber HJ. Effects of overshadowing on conditioned nausea in cancer patients: an experimental study. Physiol Behav. 1998;64:743-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Pavlov IP. Conditioned reflexes. New York: Dover 1960; . |
| 9. | Ramsay DS, Woods SC. Biological consequences of drug administration: implications for acute and chronic tolerance. Psychol Rev. 1997;104:170-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 102] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Rodriguez M, Lopez M, Symonds M, Hall G. Lithium-induced context aversion in rats as a model of anticipatory nausea in humans. Physiol Behav. 2000;71:571-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Symonds M, Hall G. Contextual conditioning with an illness US is attenuated by the antiemetic ondansetron. Psychobiology. 2000;28:360-366. |
| 12. | Symonds M, Hall G. Postinjection suppression of drinking is modified by the presence of conditioned contextual cues: implications for both anticipatory and posttreatment nausea in humans. Anim Learn Behav. 2002;30:355-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Collins KH, Tatum AL. A conditioned salivary reflex established by chronic morphine poisoning. Amer J Physiol. 1925;74:14-15. |
| 14. | Garcia J, Kimeldorf DJ, Koelling RA. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science. 1955;122:157-158. [PubMed] |
| 15. | Garcia J, Koelling RA. Relation of cue to consequence in avoidance learning. Psychonom Soc. 1966;4:123-124. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1192] [Cited by in RCA: 939] [Article Influence: 85.4] [Reference Citation Analysis (0)] |
| 16. | Ader R, Cohen N. Behaviorally conditioned immunosuppression. Psychosom Med. 1975;37:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 475] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 17. | Andrykowski MA, Otis ML. Development of learned food aversions in humans: investigation in a "natural laboratory" of cancer chemotherapy. Appetite. 1990;14:145-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Stockhorst U, Spennes-Saleh S, Körholz D, Göbel U, Schneider ME, Steingrüber HJ, Klosterhalfen S. Anticipatory symptoms and anticipatory immune responses in pediatric cancer patients receiving chemotherapy: features of a classically conditioned response? Brain Behav Immun. 2000;14:198-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Yates BJ, Miller AD, Lucot JB. Physiological basis and pharmacology of motion sickness: an update. Brain Res Bull. 1998;47:395-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 142] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Klosterhalfen S, Rüttgers A, Krumrey E, Otto B, Stockhorst U, Riepl RL, Probst T, Enck P. Pavlovian conditioning of taste aversion using a motion sickness paradigm. Psychosom Med. 2000;62:671-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Klosterhalfen S, Kellermann S, Stockhorst U, Wolf J, Kirschbaum C, Hall G, Enck P. Latent inhibition of rotation chair-induced nausea in healthy male and female volunteers. Psychosom Med. 2005;67:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Rohleder N, Otto B, Wolf JM, Klose J, Kirschbaum C, Enck P, Klosterhalfen S. Sex-specific adaptation of endocrine and inflammatory responses to repeated nauseogenic body rotation. Psychoneuroendocrinology. 2006;31:226-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci. 2001;933:222-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 610] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 24. | Dantzer R. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol. 2004;500:399-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 519] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 25. | Bernstein IL. Neutral mediation of food aversions and anorexia induced by tumor necrosis factor and tumors. Neurosci Biobehav Rev. 1996;20:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Plata-Salamán CR. 1998 Curt P. Richter Award. Brain mechanisms in cytokine-induced anorexia. Psychoneuroendocrinology. 1999;24:25-41. [PubMed] |
| 27. | Cleeland CS, Bennett GJ, Dantzer R, Dougherty PM, Dunn AJ, Meyers CA, Miller AH, Payne R, Reuben JM, Wang XS. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97:2919-2925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 360] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 28. | Lee BN, Dantzer R, Langley KE, Bennett GJ, Dougherty PM, Dunn AJ, Meyers CA, Miller AH, Payne R, Reuben JM. A cytokine-based neuroimmunologic mechanism of cancer-related symptoms. Neuroimmunomodulation. 2004;11:279-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 201] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 29. | Fredrikson M, Hursti T, Fürst CJ, Steineck G, Börjeson S, Wikblom M, Peterson C. Nausea in cancer chemotherapy is inversely related to urinary cortisol excretion. Br J Cancer. 1992;65:779-780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Basu M, Sawhney RC, Kumar S, Pal K, Prasad R, Selvamurthy W. Glucocorticoids as prophylaxis against acute mountain sickness. Clin Endocrinol (Oxf). 2002;57:761-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Hall G, Symonds M. Overshadowing and latent inhibition of context aversion conditioning in the rat. Auton Neurosci. 2006;129:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Symonds M, Hall G. Overshadowing not potentiation of illness- based contextual conditioning by a novel taste. Anim Learn Behav. 1999;27:379-390. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Lubow RE, Moore AU. Latent inhibition: the effect of nonreinforced pre-exposure to the conditional stimulus. J Comp Physiol Psychol. 1959;52:415-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 505] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 34. | Stockhorst U, Steingrueber HJ, Enck P, Klosterhalfen S. Pavlovian conditioning of nausea and vomiting. Auton Neurosci. 2006;129:50-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Jokerst MD, Gatto M, Fazio R, Gianaros PJ, Stern RM, Koch KL. Effects of gender of subjects and experimenter on susceptibility to motion sickness. Aviat Space Environ Med. 1999;70:962-965. [PubMed] |
| 36. | Park AH, Hu S. Gender differences in motion sickness history and susceptibility to optokinetic rotation-induced motion sickness. Aviat Space Environ Med. 1999;70:1077-1080. [PubMed] |
| 37. | Cheung B, Hofer K. Lack of gender difference in motion sickness induced by vestibular Coriolis cross-coupling. J Vestib Res. 2002;12:191-200. [PubMed] |
| 38. | Fessler DM, Arguello AP. The relationship between susceptibility to nausea and vomiting and the possession of conditioned food aversions. Appetite. 2004;43:331-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Mazur JE. Learning and Behavior. New Jersey: Prentice Hall 2002; . |
| 40. | Flaherty CF. Animal Learning and Cognition. New York: Alfred A Knopf Inc 1985; . |
| 41. | Stockhorst U, Klosterhalfen S, Klosterhalfen W, Winkelmann M, Steingrueber HJ. Anticipatory nausea in cancer patients receiving chemotherapy: classical conditioning etiology and therapeutical implications. Integr Physiol Behav Sci. 1993;28:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Montgomery GH, Bovbjerg DH. The development of anticipatory nausea in patients receiving adjuvant chemotherapy for breast cancer. Physiol Behav. 1997;61:737-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Tomoyasu N, Bovbjerg DH, Jacobsen PB. Conditioned reactions to cancer chemotherapy: percent reinforcement predicts anticipatory nausea. Physiol Behav. 1996;59:273-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Bovbjerg DH, Redd WH, Jacobsen PB, Manne SL, Taylor KL, Surbone A, Crown JP, Norton L, Gilewski TA, Hudis CA. An experimental analysis of classically conditioned nausea during cancer chemotherapy. Psychosom Med. 1992;54:623-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 45. | Donegan NH. Priming-produced facilitation or diminution of responding to a Pavlovian unconditioned stimulus. J Exp Psychol Anim Behav Process. 1981;7:295-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 0.7] [Reference Citation Analysis (0)] |