Published online Jun 28, 2007. doi: 10.3748/wjg.v13.i24.3384
Revised: March 25, 2007
Accepted: March 31, 2007
Published online: June 28, 2007
The coincidence of a gastrointestinal stromal tumor (GIST) and a neuroendocrine tumor (NET) in neurofibromatosis type 1 (NF1) is described only five times within the literature. We report on a 63 year old Caucasian female with the rare condition of neurofibromatosis type 1 coinciding with recurrent gastrointestinal stromal tumor plus bilateral pheochromocytoma (PCC). After a history of palpitations and dizziness that lasted for years, a left adrenal mass was detected by CT. Laparotomy revealed a pheochromocytoma of the left adrenal gland while an ileoterminal GIST was found incidentally intraoperatively. After six months contralateral PCC and multiple recurrent GIST were resected again. After four years the patient is doing well without any signs of further recurrent tumors. Discussion includes review of the literature.
- Citation: Kramer K, Hasel C, Aschoff AJ, Henne-Bruns D, Wuerl P. Multiple gastrointestinal stromal tumors and bilateral pheochromocytoma in neurofibromatosis. World J Gastroenterol 2007; 13(24): 3384-3387
- URL: https://www.wjgnet.com/1007-9327/full/v13/i24/3384.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i24.3384
Incidence of neuroendocrine tumors (NET) and gastrointestinal stromal tumors (GIST) is 0.5 and 1-2 respectively in 100 000 persons per year. Prevalence of neurofibromatosis type 1 (NF1) is 1 in 3000 live births in Western Countries[1-3]. We report on a case with the rare condition of neurofibromatosis Type1 (von Recklinghausen) coinciding with GIST plus bilaterally pheochromocytoma (PCC).
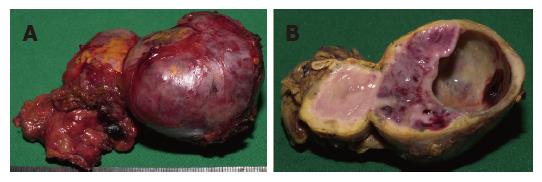
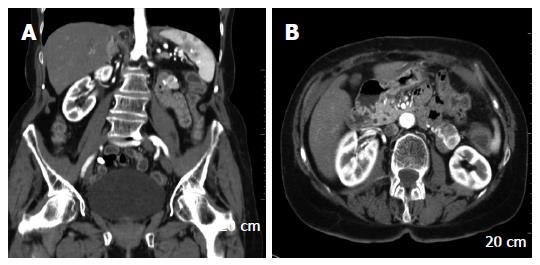
A 63-year-old Caucasian female with NF1-was presenting with a history of recurrent palpitation and hypertension over 13 years associated with nausea and headache. There was no family history of NF1. Recurrent diagnostic check ups-(including specific cardiac and neurological consultation)-did not explain the symptoms. Finally a CT scan confirmed a tumor of the left adrenal gland, 5 cm in diameter. Hormone analyses were nonspecific, with only VMA slightly increased. Surgical resection of the tumor including the left adrenal gland was performed (Figure 1) via transversal laparotomy. In addition, a gastrointestinal stromal tumor of 6.5 cm in diameter, incidentally found within the ileoterminal mesenterial region (Figure 2) was also completely resected. During preparation of the left adrenal gland a hypertensive crisis occurred resulting in hypotension after adrenalectomy. Both could be resolved by anesthesiological intensive care support. After the operation the patient was referred to the intensive care unit where she stayed for two more days.
Left sided adrenal gland, 85 g in weight, 4.5 cm in diameter with a grey-tan colored cut surface including cystic changes with hemorrhage compared to the yellow cortex surrounding it and a small remnant of remaining adrenal. In addition a 8.5 cm segment of the ileum with a solid grayish tumor of 6.5 cm in diameter, which did not relate to the mucosal surface, was removed. Immunohistochemistry confirmed pheochromocytoma of the left adrenal gland with expression of the S100 protein; GIST of the ileoterminal mesenterial region was verified by expression of c-kit and CD34 but not S100 or sm-actin. MIB-1 staining showed a proliferative activity of 2% in tumor cells.
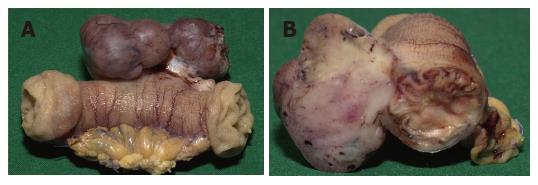
Six months after surgery, a routine control CT scan showed contra-laterally a reemerging tumor of the right adrenal gland and also a mass ventral of the left kidney (Figure 3). Recurrently the patient’s blood pressure was up to 200 mm Hg, associated with headaches and on and off palpitations. Within six weeks the blood pressure was reduced to low normal values by pharmacological alpha blockage. During this time the size of both tumors persisted. Serum and urine levels of catecholamines and VMA remained normal. Finally a transversal relaparotomy was performed with enucleation of the tumor mass in the right adrenal gland while preserving its cortical part. Approximately 20 cm distal of the duodeno-jejunal ligament a jejunal tumor 3 cm in diameter was removed by resection of the jejunal segment. In addition, seven small jejunal tumors measuring up to 2 mm were also resected. The post operative follow up was inconspicuous without any complications.
subtotal resection of the right adrenal gland, revealing a pheochromocytoma measuring 4.5 cm in diameter as well as a solid, grayish-brown tumor of 2.8 cm in diameter in a segment of the ileum as well as 7 small tumor nodules with no relation to the mucosal surface confirmed as GIST by immunohistochemistry.
During follow ups 40 mo and 48 mo after the second operation, the patient feels well without any sign of neoplastic recurrence (CT) nor of any hyper-adrenergic symptoms.
NF1 is an autosomally inherited neurofibromatosis occurring with an incidence of about 1 per 3000 births, equally involving males and females. Genetically it is caused by a mutation at the NF1 gene located on chromosome 17q11.2[2,3]. Its gene product-neurofibromin-acts as a tumor suppressor. Functionally neurofibromin reduces cell proliferation by accelerating the inactivation of the proto-oncogene p21-ras, which plays a cardinal role in mitogenic intracellular pathways[4,5]. Although genetic mutations have been described and the responsible gene product-neurofibromin-has been fully characterized, no frequently recurring mutation has been identified, and diagnosis is still based on established clinical criteria. The “classical triad” of symptoms are “café au lait” spots (CALs), cutaneous neurofibroma, and neoplasms of the peripheral or central nervous system. Malignancies are found in 3% to 15% of patients[2]. The frequencies of gastrointestinal complications in patients with NF1 disease are varying from 12% to 60% of cases and arise during midlife, later than cutaneous lesion[6-8]. They usually occur in three principal forms: hyperplasia of submucosal or myenteric nerve plexus, GIST and periampullary neuroendocrine tumors, sometimes associated with pheochromocytoma[6]. The clinical onset of these lesions, which mostly remain asymptomatic is in general characterized by abdominal pain, obstruction or by bleeding[2].
Epidemiology of GIST and NET in NF1: Only five similar cases with the coincidence of GIST and NET in NF1 have been published[9-13]. World wide in summary there are now six cases of neuroendocrine tumors associated with gastrointestinal stromal tumors and neurofibromatosis-as listed in Table 1[9-13]. It was not clear yet whether these coincidences are just accidental or if there might be a deep underlying relationship of these diseases (see below).
| Patientage, sex | GISTLocation /Diameter | NETLocation + Histology/Diameter | Surgery | Follow up /outcome | Reference [no]/year of publication |
| 36, m | Jejunum 3, 5 cm | Papilla of vater n.i. 1, 2 cm | Duodeno-pancreatectomy | Two years/ well, no recurrence | Karatzas et al[10] 2000 |
| 60, m | Duodenojejunal n.i. | Pheochromocytoma (bilateral) n.i. | n.i. (Article in Italian) | n.i. | Rizzo et al[9] 2001 |
| 64, f | Duodenum, Jejeunum, Stomach Numerous: 0.5-2 cm | Papilla of vater Somatostatinoma n.i. | Local resection | n.i. | Usui et al[11] 2002 |
| 43, f | Jejunum 3, 5 cm | Duodenum neuroendocrine carcinoma 2, 7 cm | Duodeno-pancreatectomy | 54 mo/ well | Kramer et al[12] 2005 |
| 60, m | Small bowel numerous: cm | Pheochromocytoma (bilateral) | n.i. | n.i. | Lisewski et al[13] 2006 |
| 63, f | Ileum 6, 5 cm Jejunum (prerenal) 3 cm | Pheochromocytoma (metachron, bilateral) 5 cm, left adrenal gland right adrenal gland 3 cm | Adrenalectomy intestinal segment resection tumor resection jejunal resection | 48 mo/ well, no recurrence | as presented here |
Epidemiology of GIST in NF1: Patients with NF1 have an increased risk of developing gastrointestinal tumors including rare types such as GIST[7] and GISTs are increasingly being recognized in association with NF1[14]. The incidence of GIST among NF1 patients is varying from 3.9% to 25%, while the overall ratio of NF1 in GIST patients counts up to 6% (10/167)[15,16].
Clinical characteristics of GIST in NF1: Initial clinical manifestation varies: unspecific abdominal pain, palpable abdominal mass, bowel obstruction and/or perforation with a high rate of incidental or emergency cases. Initial manifestation with gastrointestinal bleeding is common[7]. Depending on localization diagnostic procedures include upper and lower endoscopy, CT and MRI scan[17]. GIST in NF1 predominantly involving the small intestine including the duodenum, while in two thirds of the patients - like in the presented case - the tumors often occur in multiples[15,16].
Histology of GIST in NF1: The cross sectional imaging appearance of GIST tumors that occur in patients with NF1 is similar to that of gastrointestinal stromal tumors that occur in the general population[15]. The majority of these tumors are small and mitotically inactive; associated Cajal cell hyperplasia is common[16].
Pathogenesis of GIST in NF1: Ligand independent activation of c-kit (90%-95%) or alternatively of PGGFR-A (5%) is supposed to be the underlying pathogenetic mechanism of sporadic GIST. Different studies have shown that KIT and PDGFRA mutations are rarely found in GISTs in patients with NF1 suggesting that the pathogenesis of GIST in NF1 patients is different from that in non-NF1 patients[14-16]. Some authors presume that Kit germline mutation might be implicated in the pathogenesis of GIST at least in some NF1 patients[18]. Some authors discuss that in the pathogenesis of GIST in NF1 the loss of heterozygosity on the long arm of chromosome 22 (22q)-(allelic losses at 22q)-might be relevant because in 84% of these patients they are associated with high mitotic activity[9,10].
First published in 2004 by Kinoshita et al[19] and more recently confirmed by Maertens et al 2006[14] it is described that mutations in the NF1-gene might be involved in the pathogenesis of GIST in NF1 patients. In summary their demonstrated data suggest that (1) the NF1-related GISTs do not have KIT or PDGFRA mutations, (2) the molecular event underlying GIST development in this patient group is a somatic inactivation of the wild-type NF1 allele in the tumor and (3) inactivation of neurofibromin (NF1 gene) is an alternate mechanism to (hyper) activate the MAP-kinase pathway, while the JAK-STAT3 and PI3K-AKT pathways are less activated in NF1-related GIST compared with sporadic GISTs. Maertens et al[14] conclude, that was based on this molecular pathogenesis of GISTs in NF1 individuals that this type of tumor clearly belongs to the spectrum of clinical symptoms in NF1.
Predisposition of tumorgenesis in NF1 might be explained by overexpression of p21-ras a tumor-suppressor-gene called neurofibromin. Nevertheless the described concurrence of neuroendocrine tumor and gastrointestinal stromal tumor in NF1 is very rare (Table 2). In patients with von Recklinghausen neurofibromatosis the appearance of gastrointestinal symptoms should raise interest to search for gastrointestinal tumors because these patients are at risk for gastrointestinal neoplasms from which symptomatic patients are likely to experience significant morbidity. In accordance with other authors we also recommend that NF-1 patients with gastrointestinal symptoms receive further survey to rule out GISTs[14-16]. Further studies including molecular analysis to clarify the relationship between gastrointestinal tumors-in particular GIST-and neurofibromatosis are needed.
| Time | Tumor (size) | Localization | Treatment | |
| 0 | PCC (3 cm) GIST (6.5 cm) | Left adrenal gland ileo-terminal | Adrenalectomy segment resection | |
| 9 m | PCC (3 cm) GIST (3 cm) 7 x GIST (2 mm) | Right adrenal gland proximo-jejunal | Tumor-resection segment resection tumor resection | |
| 45 m | No tumor | Corticoid substitution | ||
S- Editor Liu Y L- Editor Alpini GD E- Editor Wang HF
| 1. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2231] [Cited by in RCA: 2149] [Article Influence: 93.4] [Reference Citation Analysis (1)] |
| 2. | Costi R, Caruana P, Sarli L, Violi V, Roncoroni L, Bordi C. Ampullary adenocarcinoma in neurofibromatosis type 1. Case report and literature review. Mod Pathol. 2001;14:1169-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Baker D, Wright E, Nguyen K, Cannon L, Fain P, Goldgar D et al Gene for von Recklinghausen neurofibromatosis is the pericentromeric region of chromosome 17. Science. 1987;236:1100-1102. [RCA] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 428] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 4. | Rasmussen SA, Friedman JM. NF1 gene and neurofibromatosis 1. Am J Epidemiol. 2000;151:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 261] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 5. | Xu GF, O'Connell P, Viskochil D, Cawthon R, Robertson M, Culver M, Dunn D, Stevens J, Gesteland R, White R. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell. 1990;62:599-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 806] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 6. | Fuller CE, Williams GT. Gastrointestinal manifestations of type 1 neurofibromatosis (von Recklinghausen's disease). Histopathology. 1991;19:1-11. [RCA] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 153] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Giuly JA, Picand R, Giuly D, Monges B, Nguyen-Cat R. Von Recklinghausen disease and gastrointestinal stromal tumors. Am J Surg. 2003;185:86-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Mullan MH, Gauger PG, Thompson NW. Endocrine tumours of the pancreas: review and recent advances. ANZ J Surg. 2001;71:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Rizzo S, Bonomo S, Moser A, Bottura D, Castellini C, Mazzola F, Lauro E, Vicenzi L, Betresini B, Angeli G. Bilateral pheochromocytoma associated with duodeno-jejunal GIST in patient with von Recklinghausen disease: report of a clinical case. Chir Ital. 2001;53:243-246. [PubMed] |
| 10. | Karatzas G, Kouraklis G, Karayiannakis A, Patapis P, Givalos N, Kaperonis E. Ampullary carcinoid and jejunal stromal tumour associated with von Recklinghausen's disease presenting as gastrointestinal bleeding and jaundice. Eur J Surg Oncol. 2000;26:428-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Usui M, Matsuda S, Suzuki H, Hirata K, Ogura Y, Shiraishi T. Somatostatinoma of the papilla of Vater with multiple gastrointestinal stromal tumors in a patient with von Recklinghausen's disease. J Gastroenterol. 2002;37:947-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Kramer K, Siech M, Sträter J, Aschoff AJ, Henne-Bruns D. [GI hemorrhage with fulminant shock induced by jejunal gastrointestinal stromal tumor (GIST) coincident with duodenal neuroendocrine carcinoma (NET) + neurofibromatosis (NF) -- case report and review of the literature]. Z Gastroenterol. 2005;43:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Lisewski D, Ryan S, Lim EM, Frost F, Nguyen H. Concomitant compostite adrenal phoechromocytoma, multipte gastric stromal tumours and pseudohermaphrodism in a patient with von Recklinghausen's disease. Int Semin Surg Oncol. 2006;3:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Maertens O, Prenen H, Debiec-Rychter M, Wozniak A, Sciot R, Pauwels P, De Wever I, Vermeesch JR, de Raedt T, De Paepe A. Molecular pathogenesis of multiple gastrointestinal stromal tumors in NF1 patients. Hum Mol Genet. 2006;15:1015-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | Miettinen M, Fetsch JF, Sobin LH, Lasota J. Gastrointestinal stromal tumors in patients with neurofibromatosis 1: a clinicopathologic and molecular genetic study of 45 cases. Am J Surg Pathol. 2006;30:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 321] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 16. | Andersson J, Sihto H, Meis-Kindblom JM, Joensuu H, Nupponen N, Kindblom LG. NF1-associated gastrointestinal stromal tumors have unique clinical, phenotypic, and genotypic characteristics. Am J Surg Pathol. 2005;29:1170-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 193] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 17. | Kimura N, Watanabe T, Fukase M, Wakita A, Noshiro T, Kimura I. Neurofibromin and NF1 gene analysis in composite pheochromocytoma and tumors associated with von Recklinghausen's disease. Mod Pathol. 2002;15:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Yantiss RK, Rosenberg AE, Sarran L, Besmer P, Antonescu CR. Multiple gastrointestinal stromal tumors in type I neurofibromatosis: a pathologic and molecular study. Mod Pathol. 2005;18:475-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Kinoshita K, Hirota S, Isozaki K, Ohashi A, Nishida T, Kitamura Y, Shinomura Y, Matsuzawa Y. Absence of c-kit gene mutations in gastrointestinal stromal tumours from neurofibromatosis type 1 patients. J Pathol. 2004;202:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |