Published online Jun 28, 2007. doi: 10.3748/wjg.v13.i24.3359
Revised: April 10, 2007
Accepted: April 16, 2007
Published online: June 28, 2007
AIM: To evaluate safety and feasibility of autologous bone marrow-enriched CD34+ hematopoietic stem cell Tx through the hepatic artery in patients with decompensated cirrhosis.
METHODS: Four patients with decompensated cirrhosis were included. Approximately 200 mL of the bone marrow of the patients was aspirated, and CD34+ stem cells were selected. Between 3 to 10 million CD34+ cells were isolated. The cells were slowly infused through the hepatic artery of the patients.
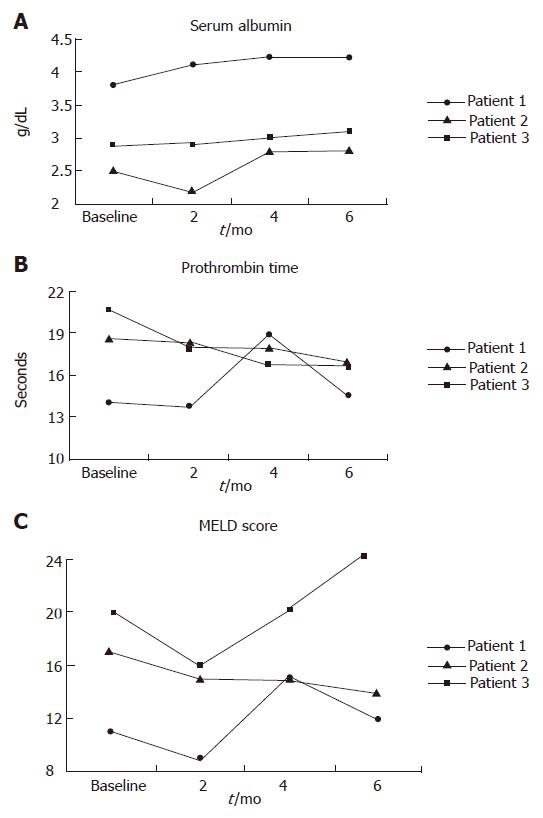
RESULTS: Patient 1 showed marginal improvement in serum albumin and no significant changes in other test results. In patient 2 prothrombin time was decreased; however, her total bilirubin, serum creatinine, and Model of End-Stage Liver Disease (MELD) score worsened at the end of follow up. In patient 3 there was improvement in serum albumin, porthrombin time (PT), and MELD score. Patient 4 developed radiocontrast nephropathy after the procedure, and progressed to type 1 hepatorenal syndrome and died of liver failure a few days later. Because of the major side effects seen in the last patient, the trial was prematurely stopped.
CONCLUSION: Infusion of CD34+ stem cells through the hepatic artery is not safe in decompensated cirrhosis. Radiocontrast nephropathy and hepatorenal syndrome could be major side effects. However, this study does not preclude infusion of CD34+ stem cells through other routes.
- Citation: Mohamadnejad M, Namiri M, Bagheri M, Hashemi SM, Ghanaati H, Zare Mehrjardi N, Kazemi Ashtiani S, Malekzadeh R, Baharvand H. Phase 1 human trial of autologous bone marrow-hematopoietic stem cell transplantation in patients with decompensated cirrhosis. World J Gastroenterol 2007; 13(24): 3359-3363
- URL: https://www.wjgnet.com/1007-9327/full/v13/i24/3359.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i24.3359
Cirrhosis represents a late stage of progressive hepatic fibrosis characterized by distortion of the hepatic architecture and the formation of regenerative nodules. Orthotopic liver transplantation (OLT) is the standard treatment modality in patients with decompensated cirrhosis. However, it has several limitations such as shortage of organ donors, high cost, and several complications.
For example, in Iran the minimum number of patients who need liver transplant each year is around 1000[1], but the maximum number of OLT is only 100 per year[2]; therefore, the majority of patients with end stage liver disease in Iran are presently dying at the end of their natural history of liver disease. Living donor liver transplantation provides one means to expand organ availability. However, there is a real need for an alternative therapies for end stage liver disease.
Preliminary experience with clinical hepatocyte transplantation during the past decade has provided proof of concept that cell therapy can be effective for the treatment of some liver diseases. Recent progress in cell biology resulting in the isolation and characterization of bone marrow stem cells and progenitor cells further increases the expectation for a new approach to the treatment of genetic and chronic liver disease[3].
There are at least two types of stem cells in the human bone marrow; mesenchymal stem cells, and hematopoietic stem cells (HSCs). HSCs are CD34+ and CD133+ and they can give rise to all lineages of blood cell differentiation. Recently, intracoronary infusion of bone marrow stem cells was reported to be safe and effective in patients with acute myocardial infarction[4].
Furthermore, in vivo trans-differentiation of human HSCs to functional hepatocytes has been demonstrated[5]. Also, it has been shown that infusion of bone marrow stem cells to animal models of liver cirrhosis can lead to regression of liver fibrosis[6]. Recently, am Esch et al[7] reported that portal administration of autologous CD133+ HSCs accelerated liver regeneration. We hypothesized that infusion of HSCs may help to reverse liver failure in patients with decompensated cirrhosis. Thus, we conducted a phase 1 human trial to evaluate safety and feasibility of autologous bone marrow-enriched CD34+ HSC transplantation in patients with decompensated cirrhosis.
One day before stem cell infusion, a total of 200 mL of bone marrow was aspirated from four different sites of the iliac crest in the right and left side (50 mL at each site) of the patients in a standard fashion. The harvested bone marrow was placed in sterile tubes containing 1500 U/50 mL of heparin sulfate to avoid platelet clumping. The procedure of stem cell isolation was performed in a clean room (FS 209 E & ISO 14 644). To reduce the volume of red blood cells, hydroxyethyl starch was used.
Mononuclear cells were separated by Ficoll-Hypaque (Lymphodex, inno TRAin, H9L6114) and then these cells were diluted in cliniMACS buffer. The bone marrow LD-MNCs were incubated for 45 m at 4°C with the CD34 monoclonal antibody (mAb) directly labeled to microbeads (MACS, Miltenyi Biotec GmbH, 171-01, Bergisch Gladbach, Germany), washed with cliniMACS buffer and placed on a column in the miniMACS cell separator (Miltenyi Biotec). The labeled cells were separated using a high-gradient magnetic field, and eluted from the column after their removal from the magnet. The positive fraction was then placed on a new column and the magnetic separation step repeated. At the end of the separation, the cells were counted and assessed for viability using Trypan Blue dye exclusion; their purity was determined using a FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA, USA). Enriched CD34+ cells were stored at 4°C in 2% human serum albumin (Human Albumin 20%, USP, Bayer, 683-20) in a sterile tube until the stem cell infusion the next day.
After local anesthesia, puncture of right femoral artery was performed, and 5 French sheaths were inserted. Simon III catheter advanced to the descending aorta, and catheterization of celiac axis and then hepatic artery was performed. The mean duration of catheterization was 9.5 m (range: 5-15 m). Nonionic low osmolal radiocontrast agent was used to visualize the hepatic artery. Then CD34+ stem cells were selectively applied to the hepatic artery as equal aliquots of 10 mL, taking an average time of 10 m. After that, the catheter was flushed with 10 cc of normal saline and the procedure was finished. After the stem cell infusion the catheter and the sheath were removed.
The proposal was designed to include 6 patients with decompensated cirrhosis. The project was approved by the Ethics Committee and the research council of digestive disease research center, Tehran University of medical sciences. The written informed consent was assigned by the patients. Inclusion criteria were age 18-60 years; chronic liver failure, ultrasonographic evidences of cirrhosis and portal hypertension, abnormal serum albumin, and/or bilirubin and/or prothrombin time (PT); Child-Pough score of 7 or more. Exclusion criteria were history of moderate to severe hepatic encephalopathy or variceal bleeding during the last 2 mo before enrolment; serum Cr ≥ 2 mg/dL, or GFR < 40 mL/min; serum sodium < 129 meq/L; serum AST or ALT more than 3 times normal; lines of evidence of active autoimmune liver disease (serum gammaglobulin > twice normal; serum transaminases > 120 U/L); human immunodeficiency virus or hepatitis C virus seropositivity; serum hepatitis B virus DNA of more than 10 000 copies/mL in patients with positive hepatitis B surface antigen; lines of evidence of extrahepatic biliary diseases (e.g. presence of primary sclerosing cholangitis, or dilated common bile duct on ultrasonography; presence of active untreated infectious disease; presence of hepatic, portal, or splenic vein thromboses on Doppler ultrasonography; presence of severe comorbid diseases (e.g. severe renal, respiratory, or cardiac disease), or presence of any types of malignancy; history of alcohol use, or use of hepatotoxic drugs within the last 6 mo before enrolment; active substance abuse; lack of a supportive family; and unwilling to assign the inform consent. All patients were on the waiting list of liver transplantation.
Patients were admitted and observed in Shariati hospital (Tehran, Iran) for 7 d. The following tests were performed at d 0, 1, 3, 5, and 7, wk 2, 3, and 4, mo 2, 3, and 6 post-transplantation: complete blood counts, PT, and international normalized ratio (INR), serum albumin, urea, creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum alkaline phosphatase, serum total and direct bilirubin, alfa-fetoprotein. Also, 10 mL of the patients’ serum samples were collected and stored frozen at -70°C at each visit. Liver volume of the patients was measured at baseline and at the end of follow up by multislice spiral CT scan without administration of intravenous contrast. The SF-36 questionnaire which was previously validated in Persian language[8] was completed by the patients at 1 d before the stem cells infusion, and was repeated at the end of follow up.
Primary aim of the study was to assess safety and feasibility of the study. Secondary end points were to assess changes in MELD score, liver volume, and quality of life of the patients at the end of follow up.
Patients’ safeties were evaluated at each visit according to the above mentioned schedule. Clinical, laboratory, and safety-related data were prospectively collected. Procedural complications were defined as any hemodynamic instability during the cell infusion. Major side effects were defined as development of any of the following complications during the follow up: acute renal failure, worsening hepatic decompensation that requires urgent liver transplantation, progressive elevation in serum AFP, or development of liver mass on follow up CT scans.
The mean volume of bone marrow aspirated from the ilium of the patients was 215.8 mL. The mean number of mononuclear cells achieved from the patients’ bone marrow was 3.13 × 108 cells. The mean number of CD34+ cells achieved after isolation was 5.25 × 106 (range: 2.5-8 × 106). The mean rate of viability of the cells was 90.75%. The mean purity of CD34+ cells was 90.5%.
The study was designed to enroll 6 patients. Four patients (2 male, 2 female) with the mean age of 47.8 years (range: 40-53) were enrolled and underwent the procedure (Table 1).
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
| Age (yr) | 40 | 53 | 45 | 53 |
| Gender | Male | Female | Male | Female |
| Etiology of cirrhosis | Hepatitis B | PBC | Cryptogenic | AIH |
| Medications | Lamivudine, Spironolactone, Furosmide | UDCA, Spironolactone, Furosmide | Spironolactone, Furosmide | Spironolactone, Furosmide |
CD34+ stem cells were slowly infused through the hepatic artery. Vital signs of the patients remained stable during the stem cell infusion. The results of the study are shown in Table 2 and Figure 1.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |||||
| Baseline | mo 6 | Baseline | mo 6 | Baseline | mo 6 | Baseline | mo 61 | |
| Edema | 2+ | None | 2+ | 2+ | 1+ | 1+ | 2+ | – |
| Ascites | Moderate | None | Moderate | Moderate | Moderate | Moderate | Severe | – |
| Serum albumin (g/dL) | 3.8 | 4.2 | 2.9 | 3.1 | 2.5 | 2.8 | 2.6 | – |
| PT (seconds) | 14 | 14.6 | 20.7 | 17 | 18.7 | 16.7 | 19.5 | – |
| INR | 1.2 | 1.3 | 2.2 | 1.8 | 1.9 | 1.6 | 2 | – |
| Cr (mg/dL) | 1.2 | 0.83 | 0.95 | 1.8 | 0.72 | 0.8 | 1.37 | – |
| Total bilirubin (mg/dL) | 1.2 | 1.87 | 3.31 | 5 | 2.37 | 2.02 | 19.85 | – |
| Direct bilirubin (mg/dL) | 0.4 | 0.43 | 2.03 | 2.8 | 0.66 | 0.82 | 13.82 | – |
| AST (IU/mL) | 43 | 52 | 52 | 75 | 62 | 37 | 2202 | – |
| ALT (IU/mL) | 31 | 34 | 23 | 30 | 30 | 15 | 2163 | – |
| AFP (mcg/L) | 4.7 | 3.7 | 3.48 | 5.5 | 1.7 | 3.8 | – | |
| MELD score | 11 | 12 | 20 | 25 | 17 | 14 | 29 | – |
| Liver volume (cm3) | 1205 | 1180 | 960 | NA | 896 | 474 | 1159 | – |
There were no any adverse effects in patients 1, and 3 during the 6 mo of follow up. Patient 2 did not experience any side effects until mo 5. However, after that she developed some degree of renal failure. The correlation between the procedure and renal failure in this patient is unclear. Patient 4 developed progressive renal failure, and went on type 1 hepatorenal syndrome and died of liver failure prior to urgent liver transplantation. The trigger factor for acute renal failure was most probably radio-contrast nephropathy. Afterwards, the project was prematurely stopped.
Cell based therapies are increasingly studied in various types of human diseases[4,9,10]. However, safety issues should be carefully considered in these novel treatment approaches. Recently, Gaia et al[11] mobilized CD34+ cells and observed bone marrow-derived cells may represent an easy immature cell source potentially useful for novel approaches for liver regeneration. Gordon et al[12], reported that HSCs infusion through the portal vein or hepatic artery was safe and may be effective in decompensated cirrhosis.
However, in our study, one patient developed some degrees of renal failure 5 mo after undergoing the procedure. Most importantly, another patient developed radiocontrast nephropathy and rapidly progressed to type 1 hepatorenal syndrome, and died of liver failure before embarking on urgent liver transplantation. After that, we prematurely stopped the project. That patient had tense ascites and serum creatinine of 1.37 g/dL before undergoing the procedure. She probably had some degree of type 2 hepatorenal syndrome at baseline, and radiocontrast nephropathy was a triggering factor to progress to type 1 hepatorenal syndrome. We used nonionic low osmolal radiocontrast agents which reduce the risk of contrast nephropathy in those who are at increased risk for this condition[13,14]; although such adverse effects could not be prevented in our patient. We suggest that other routes of cell infusion (e.g. other than hepatic artery) be considered in the future studies. An alternative method would be to use carbon dioxide to visualize hepatic artery[15].
Recently, Gasbarini et al[16] reported successful portal infusion of CD34+ stem cells in a case of drug-induced acute liver failure. Also, Gordon et al[12] injected autologous CD34+ stem cells through hepatic artery or portal vein in 5 patients with cirrhosis. In that study, the procedure was safe, and 3 of 5 patients showed improvement in serum bilirubin, and 4 of 5 in serum albumin after two months follow up. Accordingly, in our study, we observed some improvements in the serum albumin and PT in 2 of 3 patients at 6 mo of follow up. However, we are not sure if such improvement was related to HSCs infusion or simply related to the natural course of the disease in the patients.
Although transplanted HSCs can generate hepatocytes, it seems that it is a rare event[17]. There are controversies in the mechanisms by which HSCs generate hepatoytes. While, Wang et al[18] reported that cell fusion is the principle source of bone-marrow-derived hepatocytes; Jang, et al[19] reported HSCs convert into liver cells within days without fusion. The exact therapeutic role of HSCs in liver cirrhosis should be evaluated in further controlled trials.
In our study, there was significant improvement in the quality of life of all three patients at the end of follow up. Such improvement may be related to the improvement of liver function, or may be due to a placebo effect.
We have performed another phase 1 study of mesenchymal stem cell (MSC) transplantation in cirrhosis (Mohamadnejad et al 2006; Submitted for publication). The results of our MSC transplantation were more promising than this study of hematopoietic stem cell transplantation. However, the efficacy of each type of bone marrow stem cells in the improvement of liver function and quality of life of the patients should be evaluated in further controlled trials.
One of the limitations of our work was the fact that we did not track the infused HSCs in the patients’ bodies. Although tracing of infused stem cell in the body seems to be a complicated issue and the interpretation of tracing studies has recently created a lot of controversies[20,21], it is very important to understand the way stem cells act to improve liver function. We are now planning to trace the stem cells in our future studies.
In conclusion, we found that infusion of HSCs through the hepatic artery in decompensated cirrhosis may marginally improve liver functions in some patients; however, it may cause major side effects in the patients. In fusion of HSCs through the hepatic artery may best be avoided in further trials. Although, this study does not preclude infusion of CD34+ stem cells through other routes.
This project was supported by SBDC of Royan institute and industrial development and renovation organization of Iran. We kindly thank Dr. Ali Montazeri from the Iranian institute for health sciences research for his letting us to use the Iranian version of SF-36 questionnaire and calculating SF-36 scores.
S- Editor Liu Y L- Editor Rippe RA E- Editor Wang HF
| 1. | Ganji A, Safavi M, Nouraie SM, Nasseri-Moghadam S, Merat Sh, Vahedi H, Malekzadeh R. Digestive and liver diseases statistics in several referral centers in Tehran, 2000-2004. Govaresh. 2006;11:33-38. |
| 2. | Malek-Hosseini SA, Mehdizadeh AR, Salahi H, Saberi-Firouzi M, Bagheri-Lankarani K, Bahador A, Imanieh MH, Nik-Eghbalian S, Lahsaee M, Khosravi MB. Results of liver transplantation: analysis of 140 cases at a single center. Transplant Proc. 2005;37:3157-3158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Sakaida I, Terai S, Nishina H, Okita K. Development of cell therapy using autologous bone marrow cells for liver cirrhosis. Med Mol Morphol. 2005;38:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, Yu J, Corti R, Mathey DG, Hamm CW. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1481] [Cited by in RCA: 1368] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 5. | Almeida-Porada G, Porada CD, Chamberlain J, Torabi A, Zanjani ED. Formation of human hepatocytes by human hematopoietic stem cells in sheep. Blood. 2004;104:2582-2590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, Okita K. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 416] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 7. | am Esch JS, Knoefel WT, Klein M, Ghodsizad A, Fuerst G, Poll LW, Piechaczek C, Burchardt ER, Feifel N, Stoldt V. Portal application of autologous CD133+ bone marrow cells to the liver: a novel concept to support hepatic regeneration. Stem Cells. 2005;23:463-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 187] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Montazeri A, Goshtasebi A, Vahdaninia M, Gandek B. The Short Form Health Survey (SF-36): translation and validation study of the Iranian version. Qual Life Res. 2005;14:875-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 566] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 9. | Mohyeddin Bonab M, Yazdanbakhsh S, Alimoghaddam K, Talebian F, Hooshmand F, Lotfi J, Ghavamzadeh A. Mesenchymal stem cell therapy for multiple sclerosis. Int J Hematol Oncol BMT. 2005;2:10-16. |
| 10. | Snyder EY, Daley GQ, Goodell M. Taking stock and planning for the next decade: realistic prospects for stem cell therapies for the nervous system. J Neurosci Res. 2004;76:157-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Gaia S, Smedile A, Omedè P, Olivero A, Sanavio F, Balzola F, Ottobrelli A, Abate ML, Marzano A, Rizzetto M. Feasibility and safety of G-CSF administration to induce bone marrow-derived cells mobilization in patients with end stage liver disease. J Hepatol. 2006;45:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Gordon MY, Levicar N, Pai M, Bachellier P, Dimarakis I, Al-Allaf F, M'Hamdi H, Thalji T, Welsh JP, Marley SB. Characterization and clinical application of human CD34+ stem/progenitor cell populations mobilized into the blood by granulocyte colony-stimulating factor. Stem Cells. 2006;24:1822-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 197] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 13. | Lautin EM, Freeman NJ, Schoenfeld AH, Bakal CW, Haramati N, Friedman AC, Lautin JL, Braha S, Kadish EG. Radiocontrast-associated renal dysfunction: a comparison of lower-osmolality and conventional high-osmolality contrast media. AJR Am J Roentgenol. 1991;157:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 79] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Moore RD, Steinberg EP, Powe NR, Brinker JA, Fishman EK, Graziano S, Gopalan R. Nephrotoxicity of high-osmolality versus low-osmolality contrast media: randomized clinical trial. Radiology. 1992;182:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 130] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Back MR, Caridi JG, Hawkins IF, Seeger JM. Angiography with carbon dioxide (CO2). Surg Clin North Am. 1998;78:575-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Gasbarrini A, Rapaccini GL, Rutella S, Zocco MA, Tittoto P, Leone G, Pola P, Gasbarrini G, Di Campli C. Rescue therapy by portal infusion of autologous stem cells in a case of drug-induced hepatitis. Dig Liver Dis. 2007;39:878-882. [PubMed] |
| 17. | Thorgeirsson SS, Grisham JW. Hematopoietic cells as hepatocyte stem cells: a critical review of the evidence. Hepatology. 2006;43:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 155] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 18. | Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1114] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 19. | Jang YY, Collector MI, Baylin SB, Diehl AM, Sharkis SJ. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol. 2004;6:532-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 415] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 20. | Burns TC, Ortiz-González XR, Gutiérrez-Pérez M, Keene CD, Sharda R, Demorest ZL, Jiang Y, Nelson-Holte M, Soriano M, Nakagawa Y. Thymidine analogs are transferred from prelabeled donor to host cells in the central nervous system after transplantation: a word of caution. Stem Cells. 2006;24:1121-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Pearson H. Stem-cell tagging shows flaws. Nature. 2006;439:519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |