Published online Jun 28, 2007. doi: 10.3748/wjg.v13.i24.3301
Revised: December 13, 2006
Accepted: December 20, 2006
Published online: June 28, 2007
This review is part one of three, which will present an update on diagnostic procedures for gastrointestinal (GI) submucosal tumors (SMTs). Part two identifies the classification and part three the therapeutic methods regarding GI SMTs. Submucosal tumors are typically asymptomatic and therefore encountered incidentally. Advances in diagnostic tools for gastrointestinal submucosal tumors have emerged over the past decade. The aim of this paper is to provide the readers with guidelines for the use of diagnostic procedures, when a submucosal tumor is suspected. Literature searches were performed to find information on diagnostics for gastrointestinal submucosal tumors. Based on the searches, the optimal diagnostic procedures and specific features of the submucosal tumors could be outlined. Standard endoscppy, capsule endoscopy and push-and-pull enteroscopy (PPE) together with barium contrast X-ray do not alone provide sufficient information, when examining submucosal tumors. Endoscopic ultrasound (EUS), computed tomography (CT), magnetic resonance imaging (MRI) and fluorodeoxyglucose-labeled positron emission tomography (FDG-PET) are recommended as supplementary tools.
- Citation: Ponsaing LG, Kiss K, Loft A, Jensen LI, Hansen MB. Diagnostic procedures for submucosal tumors in the gastrointestinal tract. World J Gastroenterol 2007; 13(24): 3301-3310
- URL: https://www.wjgnet.com/1007-9327/full/v13/i24/3301.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i24.3301
A submucosal tumor (SMT) is defined as any intramural growth underneath the mucosa, where etiology cannot readily be determined by luminal diagnostic endoscopy or barium radiography[1].
The incidence of SMTs in the entire gastrointestinal (GI) tract is not known. However, gastric SMTs occur with an incidence of about 0.4% in diagnostic endoscopy[2]. Following the introduction of new diagnostic procedures, e.g. capsule endoscopy, a more accurate incidence may be found within the next years. Final diagnosis is made with immunohistochemistry and electron microscopy as described in part two of this series of reviews.
SMTs are usually asymptomatic and therefore most often discovered as accidental findings during surgery, autopsy or diagnostic procedures. If symptoms do occur, they are unspecific such as abdominal pain, obstruction, hemorrhage and intussusception[1,3-5]. Like other malignancies, malignant SMTs may present with systemic symptoms, especially weight loss[1,4,6].
The aim of this paper is to update the reader on diagnostic procedures, when investigating a lesion suspected to be a SMT in the GI tract.
Due to their lack of overt symptoms, SMTs are generally discovered accidentally during standard endoscopic examination. A lumen diminishing process with or without ulcerations is typically seen, but extramural pathology must be considered as a differential diagnosis[2]. Since standard endoscopy is not sufficient for diagnosing SMTs, suspicion of such requires further examination by means of diagnostic procedures mentioned below[7].
With capsule endoscopy parts of the small intestine inaccessible to standard endoscopy can be viewed. Its main indication is obscure hemorrhage with negative upper and lower standard endoscopic findings. A period of 8-12 h of fasting prior to the examination is required[8]. The capsule provides approximately 8 h of continuous endoscopic video imaging of the esophagus, stomach, small intestine and right colon. The capsule is wireless, equipped with white light-emitting diodes and has a size of approximately 1 cm × 2.5 cm. It is disposable, propelled by peristalsis and excreted after 24-48 h. There is no need for air inflation of the gut lumen. Data are transmitted employing radiotelemetry to aerials attached to the body. A study typically takes 30-60 min to review. The procedure is safe, painless, does not require sedation, can be performed ambulatory and does not have the risk of perforation as does standard endoscopy[8,9].
With capsule endoscopy, a villus-based view is generated as opposed to the lumen-based view in standard endoscopy. Therefore, tumors may have a different appearance in these two procedures[8]. The capsule cannot wash an area, and it is not possible to re-examine a possible abnormality, take biopsies or deliver therapy as it is with standard endoscopy[8] and PPE[10]. Furthermore, a recent study found a tendency towards poor interobserver agreement for abnormalities in relief (tumors and ulcers), but good for red-colored abnormalities (bleeding and angiodysplasia). However interobserver agreement was significantly better among experienced endoscopists than among less experienced[9].
Occasionally, the capsule is caught in a stricture or diverticulum. A plain abdominal X-ray can be performed to determine whether the capsule is retained or not. However, this often happens at the site of pathology, where surgery is required anyway. Removal of an impacted capsule may be performed endoscopically[8].
PPE is an alternative to capsule endoscopy. With PPE the small intestine can be examined using a double-balloon technique with an oral and/or anal approach. Indications include GI bleeding, abdominal pain and surveillance of known disease. The advantage of PPE is that it is relatively safe, has a high diagnostic yield and both biopsy and endoscopic therapy can be performed[10].
Disadvantages include the risk of perforation, the need for conscious sedation and the related complications, and the fact that the latex balloons used create a potential risk of anaphylactic shock in patients with latex allergy[10,11].
Side effects are usually mild, such as abdominal pain for 1-2 d, brief fever, reddening of the mucosa, slight intramucosal hemorrhage in the small-bowel tissue and vomiting after the procedures. Aspiration pneumonia after an epileptic attack induced by the propofol anesthesia was found as the only complication in a recent prospective study of 100 patients[10].
The tool of first choice for examining SMTs in the upper GI tract is endoscopic ultrasonography (EUS). It is the most accurate procedure for detecting and diagnosing SMTs, due to its high sensitivity and specificity[12-16]. EUS is performed as the second intervention following standard endoscopy[16].
The most important application of EUS is staging of GI malignancies, since this dictates the management and predicts survival of patients[15,17,18]. EUS features suggestive of malignancy are irregular borders, abnormal lymph nodes, ulcer, and a shape that is not oval or round[19]. Heterogeneous echopattern is a feature of controversy[20].
EUS is useful in differentiating between intramural tumors, intramural vascular lesions and extraluminal impressions with or without the use of Doppler-EUS[13,14,21]. EUS can provide information concerning origin, size, borders, homogeneity and foci with echogenic or anechoic features (Table 1)[12,22-24]. In addition, EUS can indicate whether endoscopic resection is appropriate[13,14,25].
| Endo-scopical | Size | Distinctborders | Ulcer | Layer | Form | Echogenecity | Number | Consistency | ||
| Leiomyoma[3,21,22,29,32,63,101-105] | Umbilicated | < 5 cm | Yes | Central or normal mucosa | 4th (2nd) | Smooth | Homo | Hypo | - | Firm |
| Granular cell tumor[4,22,28,60,62,63,106-108] | Yellow | < 2 cm | Mostly no | No | 2nd, 3rd, 4th | Sessile polyps, nodules or plaques | Mosaic | Hypo | S (M) | Very firm |
| Ectopic pancreas[4,13,21,22,31,64,66] | Duct opening | 1-4 cm | Yes (no) | No | 3rd,4th (2nd, 5th) | Sessile, hemispherical | Perhaps hetero | Hyper | S | Firm |
| Schwannoma [19,22,28,58,95,109] | Spherical (multi- nodular) | 3 cm (0.5-10 cm) | Yes (sometimes fibrous capsule) | No | 4th (3rd) | Round/oval (multinodular) | (Homo) | Hypo, bull's eye5 | S (M) | - |
| Lipoma[4,13,21,22,29,32,67,110,111] | Yellow | - | Yes, pseudocapsule | Most often intact mucosa, but ulcers do occur | 3rd (4th, 5th) | Polypoid, discrete, round | Homo | Hyper | S | Soft, compressible |
| 3Neurofibroma [28,50,68,74,112] | Some times long segments of nodular thickening | Few mm. up to a meter | Yes, often macroscopically | No | May involve all layers | Fusiform, diffuse, "ropelike" or "bag of worms" | Hetero | - | M | Rubbery or firm |
| Vascular[4,13,21,28,29,73,113] | Lymph- angiomas: yellow | Depending on type | Yes | No | 2nd, 3rd; cavernous may involve all layers | Round/ oval/ wavy | Homo | An-/hyper1 | M/S | Liquid/soft |
| Leiomyosarcoma [21,22,32,43,72,78] | Exophytic | > 3 cm | Irregular | Deep ulcer (> 5 mm) | 2nd, 4th | Nodular, polypous | Hetero | An- areas2 | S | Softer than leiomyomas |
| Kaposi’s sarcoma [4,43,63,85] | Red-purple | Varying (see text) | - | Often ulceration and bleeding | 1st, 2nd, 3rd | Maculopapular/ nodular/ polypous | - | - | M/S | - |
| Metastases[6,22,86,114] | Endo-/ exophytic | - | No | Yes/No | All layers | Volcanolesions, nodules, polyps, linitis plastica | Depends on the primary tumor | - | M/S | - |
| GIST[4,13,19,22,50,93] | Varying | > 2 cm | Yes/no | Occasionally | 4th (2nd, 3rd, 5th) | Elliptical, multilobular/ pedunculated; smooth/nodular | Homo/ hetero4 | Hypo, bull's eye5 | S3 | Friable |
In tumors smaller than 0.5 cm, high-frequency transducers can obtain information that is not available even with highly sophisticated CT, magnetic resonance imaging (MRI), transabdominal ultrasound[26] or positron emission tomography (PET)[27]. Intramural abnormalities can be investigated with frequencies of 12 MHz, whereas 7.5 MHz reveals the extramural structures[21]. Its high resolution and the close proximity of the ultrasound probe to the site of the SMT makes EUS valuable in determining the layer of origin of a SMT and the possible invasion of other layers[21].
However, benign SMTs, malignancies and nonneoplastic lesions, such as inflammation, can not be distinguished enosonographically[21,22]. Nevertheless, as EUS is a valuable tool in assessing local lymph node involvement[28], this finding supports the differentiation. A study concerning EUS evaluation of leiomyomas concludes that EUS is quite observer-dependent because the interobserver agreement had a kappa value of only 0.53[29]. Optimally, the same examiner should perform all of the EUS examinations concerning the same patient in order to determine tumor progression versus regression.
Some important tasks of EUS in SMTs are shown in Table 1. EUS criteria for malignancy are outlined in Table 2, to which rapid growth rate found on follow-up can be added[13]. It must however be emphasized that only microscopic examination can determine the final diagnosis and whether the SMT is benign or malignant[19].
| Reference | Tumor type | Criteria fordetermining theSMT as malignantor borderline | Size | Irregularborders | Abnormalregionallymphnodes | Heterogeneouscho pattern | Shape notoval/round | Ulcer | |
| Cysticspaces | Echo-genicfoci | ||||||||
| Ando et al 2002[30] | GIST | Size > 5 cm and at least 1 of the 2 other features: | > 5 cm | Yes | - | Yes | - | - | - |
| Nickl et al 2002[20] | Hypo- echoic SMTs | 1 or more of the features: | > 3 cm | Yes | Yes | No | No | Yes | Yes |
| Brand et al 2002[24] | SMTs in general | 2 or more of the features or 1 and clinical symptoms (pain, dysphagia, weight loss, hemorrhage) | > 3 cm | Yes | - | Heterogeneous echo pattern | - | - | |
| Rösch et al 2002[12] | SMTs in general | 2 or more of the features: | > 3 cm | Yes | Yes | Heterogeneous echo pattern | - | - | |
| Palazzo et al 2000[23] | GIST | 2 or more of the features: | - | Yes | Yes | Yes | - | - | - |
| Chak et al 1997[115] | GIST | 2 or more of the features: | > 4 cm | Yes | - | > 4 mm | > 3 mm | - | - |
Endosonographically, the wall of the GI tract consists of 5 layers of alternating echogenicity (Figure 1). The 1st layer is hyperechoic and represents the superficial layer of the mucosa. The 2nd layer is hypoechoic and constitutes of the deep layer of the mucosa, including the muscularis mucosae. The 3rd, hyperechoic layer is the submucosa, the 4th hypoechoic the muscularis propria and the 5th hyperechoic is the serosa/adventitia[21,22]. As an example, a myogenic SMT can be diagnosed with confidence, if there is continuity between a hypoechoic SMT and the 4th, hypoechoic, layer of the adjacent normal GI tract wall[30].
Catheter probe-endoscopic ultrasonography (CP-EUS) can probably be used instead of EUS for the evaluation of small SMTs[31]. The concept of CP-EUS is that an ultrasound catheter probe can be inserted through the accessory channel of a conventional endoscope. Thereby both endoscopy and EUS can be performed during the same intervention[16,31]. The clinician should bear in mind that CP-EUS images tend to be more hypoechoic than EUS images[16].
Due to the small diameter of the CP-EUS probe and the absence of a balloon at its tip, compression of the inner layers is avoided and thus blurring[16]. CP-EUS identifies the layer of origin of myogenic SMTs with great precision and is better than EUS at distinguishing between the two layers of the muscularis propria[16].
In a recent study, CP-EUS diagnosed more than 95% correctly in large intestinal SMTs, confirmed by biopsy or surgical resection[32]. Another study of 25 SMTs, showed that CP-EUS and EUS equally visualized all SMTs, with image quality and determination of tumor diameters and margins being comparable[16]. On the contrary, Chak et al[31] found that CP-EUS, but not EUS, staged submucosal lesions correctly in all cases, confirmed by histology. Staging of regional cancer was concordant between EUS and CP-EUS in 80% of the cases. However, these results may be influenced by a selection bias, as only smaller SMTs were chosen for CP-EUS examination.
A shortcoming of CP-EUS is the risk of neglecting other SMTs, since examination occurs only directly at the region of interest[16]. Due to its smaller diameter it could be suspected that CP-EUS would have an advantage in stenosing SMTs that cannot be traversed by an EUS-endoscope. However, stenosing tumors tend to be bulky, and since CP-EUS has limited depth penetration compared to EUS, CP-EUS may fail to visualize the extraluminal margin and assess adjacent lymph nodes[31]. If the SMT is larger than 5 cm in diameter, EUS or CT may be the preferable imaging techniques[16].
In the esophagus, the acoustic coupling needed for EUS is impaired by the lack of a water-filled lumen. Therefore a method has been developed specifically for this situation: small-diameter CP-EUS with an attached latex condom (condom-CP-EUS) that can be filled with water[33].
A limitation of condom-CP-EUS, when using large echoendoscopes, is compression of small esophageal tumors and thus distortion of the image[33]. The wall layers are also often compressed, and therefore only 3 layers of the esophageal wall are seen, compared to the 5 layer pattern with 7.5-12 MHz probes[21].
Other shortcomings are limited depth penetration and poor acoustic coupling, resulting in low quality images and impeded evaluation of lymph nodes and bulky SMTs. Moreover, a large volume of water is needed for adequate acoustic coupling, which may leak and cause aspiration. Large SMTs may create air artifacts between the condom and the esophageal wall[33]. Additionally, there is a potential risk of anaphylactic shock, due to latex allergy, which has a prevalence of less than 1% in the normal population[11].
The need for three-dimensional (3D) EUS has arisen as a consequence of the difficulty less experienced endosonographers witness interpreting two-dimensional (2D) EUS images[34]. Indeed, 3D-EUS, compared to 2D-EUS, is relatively easy to use and the examination time will not be extended, as it is possible to view the whole lesion and perform a new scan immediately after a poor scan result[34].
However, some criterions are to be fulfilled in order to create a good image. The probe must be parallel and close to the mucosal surface. This is difficult in the stomach, but relatively easy in the esophagus, though probe wobbling can be caused by the peristalsis, respiratory movement or cardiac impulse[34-36]. Furthermore, the time factor is critical, as the risk of probe wobbling increases with the time needed for completing a scan. In an investigation it took 3-4 s to complete a scan[34] as opposed to 3-5 min in another investigation[37]. More recent publications show promising results concerning the reduction of the time needed for processing the scans[35,36]. Finally, the size of the SMT is crucial. Due to the limited depth penetration in these probes, the results of 3D-EUS are better when applied to small SMTs, although the size of small SMTs (< 1 cm) tends to be overestimated by 3D-EUS[35,36].
3D-EUS data on GI SMTs are however sparse[36]. Thus, data on mucosal cancer is used in this paper to give an impression of 3D-EUS in practice. In a study of 43 upper GI lesions, depth staging was correct in 80% of the cases of esophageal cancer and almost 70% of the cases of gastric cancer, histologically confirmed. However, only 37% of the 3D-EUS images were of an acceptable quality, which meant that several images had to be made for each lesion[34].
Since it is impossible to differentiate definitely between benign and malignant SMTs by means of any imaging technique, histological or cytological confirmation is a necessity[16,30,38-40]. A study shows that only in 35% of cases was an acceptable submucosal representation achieved with forceps biopsy during standard endoscopy, even though the endoscopist intended to obtain submucosal tissue[2]. On the contrary, endosonographically performed fine needle aspiration (EUS-FNA) is a good method for obtaining cytological samples[30,39].
In EUS-FNA the aspiration needle can be inserted more precisely into the SMT than in percutaneous FNA[39]. Moreover, the incidence of malignant seeding is relatively low[15,39]. This may, however, be the result of selection bias: more biopsies are performed percutaneously and therefore more cases of cutaneous seeding than mucosal are seen. These advantages may be outweighed, though, by the risks of conscious sedation in endoscopy[39].
EUS-FNA contributes to solving therapeutic dilemmas. A study showed that due to the result of EUS-FNA the decision to abandon surgery was directly affected in 26% of patients with primary malignancies. The reason for this was severe malignancy, such as distant nodal metastasis[39].
The sensitivity of cytological samples achieved through EUS-FNA has been reported to be 88%-91% and the specificity close to a 100% for the diagnosis of malignant lesions confirmed by the surgical findings or long-term clinical follow-up[15,22,39,41]. However, as some investigators point out, in order to obtain an adequate cytological sample, the optimal situation is that a cytologist is present during the procedure[39]. Furthermore, there are different ways of handling the cytological samples obtained by EUS-FNA, such as performing smears and cell-blocks. It must be emphasized that neither mitotic counts nor immunohistochemistry can be performed on smears. Therefore the optimal situation is when cell blocks are made from the cytological sample. If the number of cells is too small to count mitotic figures per 50 high power fields, immunohistochemical staining with MIB-1 (a proliferation marker) can provide information of the cellular activity[30,42].
Sometimes examination of the whole SMT is needed in order to differentiate between benign and malignant, and a pitfall is the aspiration of normal smooth-muscle cells[22]. If possible, cells should be obtained from different parts of the SMT using a large needle (18-20G)[30].
Complications to EUS-FNA appear to be rare, as two investigations have shown a complication rate of 0%-2%[39,41]. However, careful Doppler-EUS examination must always be performed prior to EUS-FNA in order to prevent rupture of a possible varice[16].
Barium studies can reveal several pathological conditions, such as submucosal infiltration, ulceration, mass presence and lumen stenosis, which may all be present in SMTs. Barium studies are also valuable in assessing the extent and multiplicity of SMTs[43]. The typical appearance of a SMT is an intramural mass with intact or ulcerated overlying mucosa[44]. The tumor is seen as a smoothly circumscribed mass, when seen en face and the margins as obtuse or right angles, when viewed in profile[45]. However, barium studies are limited to exophytic masses[44], and in staging and detection of early or subtle SMTs this method is of little value[43].
Recent advances in CT have drawn attention to the use of CT for evaluating the GI tract[40]. Cross-sectional CT has the primary role in staging GI tumors[43]. New multi-slice CT has some advantages compared to single-slice spiral scanners, such as elimination of motion artifacts and acquisition of thinner sections. This improves the quality of 3D data, but the thin collimation involves an increase in the radiation dose to the patient[40]. CT has an advantage compared with EUS, namely the possibility of delineating the full extension of the tumor[44]. The forces of CT are demonstration of a tumor, its size, relation to adjacent organs and revelation of metastasis[46], and therefore are the tasks of CT primarily staging, surgical planning[47] and follow-up[47,48].
CT cannot classify SMTs as demonstrated in a recent study, where CT was inconclusive in more than 50% of GI stromal tumors (GISTs)[46]. CT cannot either differentiate between malignant and benign SMTs, unless obvious local invasion or metastases are present. CT, especially CT angiography, is valuable for the detection of gastric varices. In large and exophytic gastric stromal tumors, 3D-CT can be helpful in better characterizing the mass and determining its origin[40].
Traditional oral contrast agents of high attenuation have some disadvantages when evaluating the GI tract. An example is when the contrast does not mix uniformly with gastric contents, resulting in the creation of pseudotumors. Therefore water, which is of low attenuation, is preferred as an oral contrast agent. Simultaneously non-ionic contrast material is given intravenously, which enhances the GI walls. Furthermore, adequate distension of the stomach is important for proper imaging. Failure in the latter may result in overlooked disease or the collapsed gastric wall mimicking disease[40].
Like CT, MRI is valuable in diagnosis and evaluating the extent of the tumor, including staging[49,50]. However, due to variable and non-specific appearances, MRI offers no additional information compared to CT concerning the internal features of, at least, GISTs[44].
Concerning limitations in both CT and MRI, it can be difficult to determine the organ of origin from cross sectional imaging alone in the presence of a significantly exophytic tumor[51]. However, MRI is a helpful adjunct to CT, especially concerning large SMTs, where the multiplanar capability of magnetic resonance can aid the determination of organ of origin, the relationship to other organs and delineate the major blood vessels. The new multi-channel CT-scanners have the same capability and may become the method of choice. In GISTs, the solid parts of the tumor are typically of low signal-intensity on T1-weighted images, but high signal-intensity on T2-weighted images. However, the degree of necrosis and hemorrhage greatly affect the signal-intensity pattern. Depending on the age of the hemorrhage, the signal-intensity will vary from high to low on both T1- and T2-weighted images. Due to gadolinium enhancement in viable tumor tissue, areas of necrosis can be outlined[45].
In the recent years PET has shown to have great value primarily in the early assessment of response in GISTs to treatment with imatinib[48,52-54]. The reason for its effectiveness lies in the radiolabeled surrogate marker for glucose metabolism, fluorodeoxyglucose (FDG). FDG highlights areas of the body with enhanced metabolism, such as malignant SMTs[48]. Metabolic changes occur prior to morphological changes, which explains why several investigations conclude that PET is superior to CT and MRI in predicting early response to therapy[44,48,55]. A recent investigation on GISTs concludes that FDG-PET can separate imatinib-responders from -non-responders as early as 1 wk after initiation of treatment[54]. Furthermore, PET is indicated in cases, where equivocal CT- or MRI-images suspect metastases[47]. The risk of misinterpretation is minimized with the new combined PET/CT scanners uniting functional and morphologic imaging[27].
With PET, not only is the evaluation of response to therapy facilitated, but also the determination of the diagnosis, recurrence, staging and extent of disease[48,54,56].
To the disadvantages of PET count the fact that the acquisition time is 3-5 min per bed position. Due to respiratory motion, very small SMTs (< 5 mm) may be blurred and therefore missed[27].
As FDG is not a specific cancer tracer, uptake is seen in cicatrices following surgery due to benign inflammation, and therefore PET scans should not be performed until 3-4 wk after surgery to avoid these artifacts mimicking tumors. Other situations with increased uptake are tense muscles, catheters, tubes, stomas, the bone marrow in patients treated with chemotherapy and excretion of FDG to the urinary tract[57]. Physiological excretion of FDG can also be seen in the bowel, which can be difficult to differentiate from SMTs. Furthermore, it must be taken into consideration that slowly growing tumors, such as benign SMTs, only rarely absorb FDG. Moreover, hyperglycemia and administration of insulin may alter the distribution of FDG. Therefore at least 5 h of fasting and measurement of the blood glucose level prior to the scan is recommended[57].
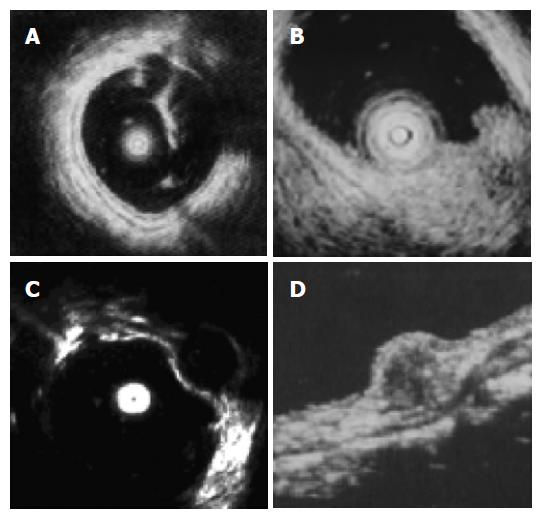
For all of the SMTs mentioned below, the endoscopic, EUS and macroscopic features can be seen in Table 1, and an EUS image of a leiomyoma and a lipoma is shown in the Figure 1.
Leiomyomas are the commonest mesenchymal tumors in the esophagus[58] as opposed to the rest of the GI tract, where GISTs are the most frequent[44]. Leiomyomas are found in the esophagus, colon and rectum, but are very rare in the stomach and small intestine[58].
Differential diagnoses to leiomyomas are preoperatively mostly leiomyosarcomas and, in the esophagus, carcinomas[59].
GI Schwannomas are rare. Their ratio to GISTs, the most frequent GI SMTs, is approximately 1:50-100[58]. They are mostly found in the stomach.
In the GI tract granular cell tumor mostly involves the middle to distal parts of the esophagus, with 1/3 of all the GI cases occurring at this site[4,60-63]. They are solitary in 80%-90% of all cases[62,63].
Heterotopic pancreatic tissue is mostly located within 3-4 cm on both sides of the pylorus, but may occur in Meckel's diverticulum and rarely in the small intestine. Heterotopic pancreas is a nonneoplastic[22], congenital tumor thought to be a result of separation of fragments from the main pancreatic mass due to the rotation of the foregut[1,4,64]. An investigation found heterotopic pancreas in 0.25% of all explorative laparotomies[65].
A distinctive feature of heterotopic pancreatic tissue may be the presence of an opening, visible as a dimple on the surface[4,66], from which fluid may trickle on pressure[64]. Concerning differential diagnoses, both carcinoid tumors and heterotopic pancreatic tissue appear hypoechoic and irregular endosonographically[21].
GI lipomas occur throughout the GI tract, but are undoubtedly most frequent in the colon as a solitary, slowly growing, benign tumor, originating within the submucosa and protruding into the lumen[4,5,28].
CT findings are a well-circumscribed, submucosal lesion with uniform fat attenuation and, occasionally, a fibrous capsule[5]. X-ray criteria for lipomas are changing size and shape during the course of examination, reflecting their soft consistency[67].
Differential diagnoses from the endoscopic appearance are leiomyoma, neurofibroma, adenomatous polyp and villose adenoma. Differentiation is based on consistency, polypoid features and surface pattern of the different tumors[67].
Solitary neurofibromas are rare. Therefore, neuro-fibromatosis should be suspected when neurofibromas are encountered[4]. Neurofibromatosis type 1 (NF1, von Recklinghausen Disease) is relatively common with a prevalence of 1/3000 births in Western countries[68]. The neurofibroma is derived from perineural cells on peripheral nerves[69]. GI involvement is common in NF1[28,50,70]. These SMTs have a predilection for the duodenum, especially the ampulla of Vater[71].
NF1 is associated with gliomas, meningeomas, phaeo-chromocytomas, hemangiomas and GISTs[28,50,72,73]. In the latter, the incidence of GISTs may be 200 times the incidence in an unaffected population[28,50,74]. The GISTs in patients with NF1 tend to be multiple[50]. Furthermore, it should be kept in mind that NF1 is also associated with carcinoid tumors that tend to occur at the ampulla of Vater, like the neurofibromas. The explanation for this co-location may be a transformation of an endo-ectodermal complex located near the ampulla of Vater in NF1-patients[75].
Hemangiomas: Multiple hemangiomas may be found, as in the blue rubber-bleb nevi syndrome that mostly affects the skin and GI tract[76]. Approaches to diagnosing vascular lesions are typically Doppler-EUS and CT-angiography[40], but a 99mTc-labeled redcell scan may also be performed to reveal hemangiomas or other transiently or mildly bleeding lesions[77], but endoscopy is regarded as the first choice. Logically, hemorrhage is a typical complication to hemangiomas[4]. One should keep in mind the differential diagnosis of esophageal and gastric varices[21].
Lymphangiomas: Lymphangiomas are rare, probably hamartomatous, anomalies that occur solitarily, mostly in the duodenum. Endoscopically, yellow-tan lesions are seen, occasionally with satellite lesions. When biopsy is performed, exudation of yellow chylous liquid is seen[4].
Leiomyosarcomas are mostly found in the small intestine[78], where they constitute more than 10% of all malignant lesions[79], and mostly behave in a highly malignant fashion[63]. A palpable abdominal mass may be encountered in almost 50% of cases of leiomyosarcomas in the small intestine[78].
Endoscopically, leiomyosarcomas are as a rule single and have a predominantly exophytic component[43,78].
Radiologically, excavated leiomyosarcomas may be confused with lymphomas and metastatic melanomas[43]. Leiomyosarcomas are expected to have a higher glucose metabolism than leiomyomas, and thus PET or PET/CT could aid the differentiation[54,80,81]. In addition, the EUS criteria mentioned in Table 1 may be helpful.
The causative viral agent of Kaposi’s sarcoma is human herpes virus 8[4,28,82-84]. Kaposi’s sarcoma is considerably more frequent in men than women and is mostly coursed by immunosuppression, especially HIV[63,84].
Endoscopically, Kaposi’s sarcomas may be mucosal, but are usually submucosal and either isolated or extensively involve the bowel wall. All parts of the GI tract are at risk[4,63,85].
In the esophagus, acquired immunodeficiency syndrome-related lymphoma should be considered as a differential diagnosis, when viewed radiologically[43].
The most frequent primary tumors that result in GI metastases are breast cancer, melanoma and lung cancer[22]. The occurrence of metastases to the stomach from fatal breast cancer has been reported to be 8%[86]. Metastases may be brought about by hematogenic or lymphatic spread or seeding through the peritoneum[6,87].
Endoscopic findings are mainly sorted under three morphological features: nonulcerative SMTs, SMTs with elevation and ulceration at the apex (volcano lesions), and multiple nodules of varying sizes with tip ulceration[6]. EUS is valuable in evaluating the mode of spread, site of origin and the pathology[6].
GISTs are the commonest mesenchymal tumors in the GI tract[88-90]. The annual incidence is estimated to be at least 10 to 20 cases per million[81,91]. Their origin is supposedly multipotential mesenchymal stem cells, and therefore both myogenic and neurogenic features may be present[1,46,92-96]. GI autonomic nerve tumors (GANTs), are now categorized under GIST owing to their great immunohistochemical and ultrastructural resemblance[97].
65% of GISTs occur in the stomach, 30%-35% in the small intestine and 5%-10% in the colon[98]. Colonic GISTs have a high proportion of malignancy[4,28].
Endosonographically, large size, lobulation, irregular borders and echogenic foci indicate malignancy (Tables 1 and 2)[42]. On CT, the signs that indicate a highly malignant tumor are calcification, ulceration, necrosis, cystic areas, fistula, metastasis, ascites and infiltration[46].
Endoscopic differential diagnoses are gastric lymphoma[99] and an inflammatory fibroid polyp[100]. There are quite a few differential diagnoses, when using CT, but if lymph node enlargement is seen, adenocarcinoma or lymphoma should be considered[44]. Differentiation is made with immunohistochemistry or electron microscopy.
Standard endoscopy, capsule endoscopy, push-and-pull enteroscopy, barium contrast X-ray and forceps biopsies can not differentiate between extraluminal compression and SMTs. Therefore, there is a need for EUS or whole body imaging procedures as well as in the diagnosis of SMTs. EUS with biopsy is the first choice of diagnostic tool, but if depth penetration is improved in CP-EUS, this may be preferred due to the reduced number of intubations and examination time. So far 3D-EUS has not shown acceptable results, but it is expected to facilitate the assessment of borders, extent and size of SMTs in the future. Still, EUS is rather subjective and therefore the reproducibility of the results is reduced.
Biopsies should only be obtained, if the outcome could lead to a cancellation of a planned operation, due to the risk of malignant seeding in any malignant SMT[46] and due to the risk of hemorrhage if biopsies are taken from GISTs because of their brittleness[47].
Even in GISTs responsive to imatinib therapy, tumor size may decrease over months or not at all[47,48]. Therefore, with CT it may take months to reach conclusions regarding GIST responsiveness, whereas FDG-PET determines this within days to weeks after commenced treatment[48]. However, unless short-term follow-up is needed, CT is a sufficient way of monitoring. The quality of multi-slice CT is now comparable to MRI, but MRI has the advantage of disclosing necrosis, due to the enhancement of gadolinium in viable tumor tissue. MRI is especially an option when assessing liver metastases, while FDG-PET detects even small, malignant SMTs that may be overlooked by other diagnostic methods. Thus PET or PET/CT are recommendable for SMTs larger than 5 mm whereas (CP-) EUS is preferred for SMTs smaller than 5 mm.
We thank PH Zhou for his endosonographic image.
S- Editor Liu Y L- Editor Alpini GD E- Editor Lu W
| 1. | Chak A. EUS in submucosal tumors. Gastrointest Endosc. 2002;56:S43-S48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc. 1991;5:20-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 207] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Gill SS, Heuman DM, Mihas AA. Small intestinal neoplasms. J Clin Gastroenterol. 2001;33:267-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 146] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Day D, Jass J, Price AB, Shepherd NA, Sloan JM, Talbot IC, Warren BF, Williams GT. Morson & Dawson's Gastrointestinal Pathology. Massachusetts: Blackwell Science Ltd 2003; . [DOI] [Full Text] |
| 5. | Mouës CM, Steenvoorde P, Viersma JH, van Groningen K, de Bruïne JF. Jejunal intussusception of a gastric lipoma: a review of literature. Dig Surg. 2002;19:418-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Hsu CC, Chen JJ, Changchien CS. Endoscopic features of metastatic tumors in the upper gastrointestinal tract. Endoscopy. 1996;28:249-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Knoop M, St Friedrichs K, Dierschke J. Surgical management of gastrointestinal stromal tumors of the stomach. Langenbecks Arch Surg. 2000;385:194-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Swain P, Adler D, Enns R. Capsule endoscopy in obscure intestinal bleeding. Endoscopy. 2005;37:655-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | De Leusse A, Landi B, Edery J, Burtin P, Lecomte T, Seksik P, Bloch F, Jian R, Cellier C. Video capsule endoscopy for investigation of obscure gastrointestinal bleeding: feasibility, results, and interobserver agreement. Endoscopy. 2005;37:617-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Ell C, May A, Nachbar L, Cellier C, Landi B, di Caro S, Gasbarrini A. Push-and-pull enteroscopy in the small bowel using the double-balloon technique: results of a prospective European multicenter study. Endoscopy. 2005;37:613-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | Turjanmaa K, Mäkinen-Kiljunen S. Latex allergy: prevalence, risk factors, and cross-reactivity. Methods. 2002;27:10-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Rösch T, Kapfer B, Will U, Baronius W, Strobel M, Lorenz R, Ulm K. Accuracy of endoscopic ultrasonography in upper gastrointestinal submucosal lesions: a prospective multicenter study. Scand J Gastroenterol. 2002;37:856-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 13. | Shim CS, Jung IS. Endoscopic removal of submucosal tumors: preprocedure diagnosis, technical options, and results. Endoscopy. 2005;37:646-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Hizawa K, Matsumoto T, Kouzuki T, Suekane H, Esaki M, Fujishima M. Cystic submucosal tumors in the gastrointestinal tract: endosonographic findings and endoscopic removal. Endoscopy. 2000;32:712-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Hünerbein M, Dohmoto M, Haensch W, Schlag PM. Endosonography-guided biopsy of mediastinal and pancreatic tumors. Endoscopy. 1998;30:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Buscarini E, Stasi MD, Rossi S, Silva M, Giangregorio F, Adriano Z, Buscarini L. Endosonographic diagnosis of submucosal upper gastrointestinal tract lesions and large fold gastropathies by catheter ultrasound probe. Gastrointest Endosc. 1999;49:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Nakazawa S. Recent advances in endoscopic ultrasonography. J Gastroenterol. 2000;35:257-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Nakazawa S, Inui K. Endosonography and endoscopic magnetic resonance imaging. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:21-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Polkowski M. Endoscopic ultrasound and endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy. 2005;37:635-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Nickl N, Gress F, McClave S, Fockens P, Chak A, Savides T, Catalano M, Behling C, Odegaard S, Chang K. Hypoechoic intramural tumor study: final report. Gastrointest Endosc. 2002;55:AB98. |
| 21. | Fockens P. Current endosonographic possibilities in the upper gastrointestinal tract. Baillieres Clin Gastroenterol. 1994;8:603-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Wiech T, Walch A, Werner M. Histopathological classification of nonneoplastic and neoplastic gastrointestinal submucosal lesions. Endoscopy. 2005;37:630-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Palazzo L, Landi B, Cellier C, Cuillerier E, Roseau G, Barbier JP. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut. 2000;46:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 220] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 24. | Brand B, Oesterhelweg L, Binmoeller KF, Sriram PV, Bohnacker S, Seewald S, De Weerth A, Soehendra N. Impact of endoscopic ultrasound for evaluation of submucosal lesions in gastrointestinal tract. Dig Liver Dis. 2002;34:290-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Waxman I, Saitoh Y, Raju GS, Watari J, Yokota K, Reeves AL, Kohgo Y. High-frequency probe EUS-assisted endoscopic mucosal resection: a therapeutic strategy for submucosal tumors of the GI tract. Gastrointest Endosc. 2002;55:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Liu JB, Goldberg BB. 2-D and 3-D endoluminal ultrasound: vascular and nonvascular applications. Ultrasound Med Biol. 1999;25:159-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Antoch G, Kanja J, Bauer S, Kuehl H, Renzing-Koehler K, Schuette J, Bockisch A, Debatin JF, Freudenberg LS. Comparison of PET, CT, and dual-modality PET/CT imaging for monitoring of imatinib (STI571) therapy in patients with gastrointestinal stromal tumors. J Nucl Med. 2004;45:357-365. [PubMed] |
| 28. | Miettinen M, Blay JY, Sobin LH, Kindblom LG. World Health Organization classification of tumors. Pathology and genetics of tumor of digestive system. Lyon: IARC Press 2000; 103-143. |
| 29. | Gress F, Schmitt C, Savides T, Faigel DO, Catalano M, Wassef W, Roubein L, Nickl N, Ciaccia D, Bhutani M. Interobserver agreement for EUS in the evaluation and diagnosis of submucosal masses. Gastrointest Endosc. 2001;53:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Ando N, Goto H, Niwa Y, Hirooka Y, Ohmiya N, Nagasaka T, Hayakawa T. The diagnosis of GI stromal tumors with EUS-guided fine needle aspiration with immunohistochemical analysis. Gastrointest Endosc. 2002;55:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 231] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 31. | Chak A, Soweid A, Hoffman B, Stevens P, Hawes RH, Lightdale CJ, Cooper GS, Canto MI, Sivak MV. Clinical implications of endoluminal ultrasonography using through-the-scope catheter probes. Gastrointest Endosc. 1998;48:485-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Zhou PH, Yao LQ, Zhong YS, He GJ, Xu MD, Qin XY. Role of endoscopic miniprobe ultrasonography in diagnosis of submucosal tumor of large intestine. World J Gastroenterol. 2004;10:2444-2446. [PubMed] |
| 33. | Wallace MB, Hoffman BJ, Sahai AS, Inoue H, Van Velse A, Hawes RH. Imaging of esophageal tumors with a water-filled condom and a catheter US probe. Gastrointest Endosc. 2000;51:597-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Tokiyama H, Yanai H, Nakamura H, Takeo Y, Yoshida T, Okita K. Three-dimensional endoscopic ultrasonography of lesions of the upper gastrointestinal tract using a radial-linear switchable thin ultrasound probe. J Gastroenterol Hepatol. 1999;14:1212-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Watanabe M, Kida M, Yamada Y, Saigenji K. Measuring tumor volume with three-dimensional endoscopic ultrasonography: an experimental and clinical study (including video). Endoscopy. 2004;36:976-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Sumiyama K, Suzuki N, Kakutani H, Hino S, Tajiri H, Suzuki H, Aoki T. A novel 3-dimensional EUS technique for real-time visualization of the volume data reconstruction process. Gastrointest Endosc. 2002;55:723-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Nishimura K, Niwa Y, Goto H, Hase S, Arisawa T, Hayakawa T. Three-dimensional endoscopic ultrasonography of gastrointestinal lesions using an ultrasound probe. Scand J Gastroenterol. 1997;32:862-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Kojima T, Takahashi H, Parra-Blanco A, Kohsen K, Fujita R. Diagnosis of submucosal tumor of the upper GI tract by endoscopic resection. Gastrointest Endosc. 1999;50:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 39. | Chang KJ, Katz KD, Durbin TE, Erickson RA, Butler JA, Lin F, Wuerker RB. Endoscopic ultrasound-guided fine-needle aspiration. Gastrointest Endosc. 1994;40:694-699. [PubMed] |
| 40. | Horton KM, Fishman EK. Current role of CT in imaging of the stomach. Radiographics. 2003;23:75-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Gress FG, Hawes RH, Savides TJ, Ikenberry SO, Lehman GA. Endoscopic ultrasound-guided fine-needle aspiration biopsy using linear array and radial scanning endosonography. Gastrointest Endosc. 1997;45:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 262] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 42. | Okubo K, Yamao K, Nakamura T, Tajika M, Sawaki A, Hara K, Kawai H, Yamamura Y, Mochizuki Y, Koshikawa T. Endoscopic ultrasound-guided fine-needle aspiration biopsy for the diagnosis of gastrointestinal stromal tumors in the stomach. J Gastroenterol. 2004;39:747-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. | Gourtsoyiannis N, Grammatikakis J, Prassopoulos P. Role of conventional radiology in the diagnosis and staging of gastrointestinal tract neoplasms. Semin Surg Oncol. 2001;20:91-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Lau S, Tam KF, Kam CK, Lui CY, Siu CW, Lam HS, Mak KL. Imaging of gastrointestinal stromal tumour (GIST). Clin Radiol. 2004;59:487-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 45. | Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics. 2003;23:283-304, 456; quiz 532. [PubMed] |
| 46. | El-Zohairy M, Khalil el-SA, Fakhr I, El-Shahawy M, Gouda I. Gastrointestinal stromal tumor (GIST)'s surgical treatment, NCI experience. J Egypt Natl Canc Inst. 2005;17:56-66. [PubMed] |
| 47. | Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, Emile JF, Gronchi A, Hogendoorn PC, Joensuu H. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol. 2005;16:566-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 486] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 48. | Stroobants S, Goeminne J, Seegers M, Dimitrijevic S, Dupont P, Nuyts J, Martens M, van den Borne B, Cole P, Sciot R. 18FDG-Positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec). Eur J Cancer. 2003;39:2012-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 352] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 49. | Campos FG, Leite AF, Araújo SE, Atuí FC, Seid V, Habr-Gama A, Kiss DR, Gama-Rodrigues J. Anorectal leiomyomas: report of two cases with different anatomical patterns and literature review. Rev Hosp Clin Fac Med Sao Paulo. 2004;59:296-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 50. | Giuly JA, Picand R, Giuly D, Monges B, Nguyen-Cat R. Von Recklinghausen disease and gastrointestinal stromal tumors. Am J Surg. 2003;185:86-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Anorectal gastrointestinal stromal tumors: CT and MR imaging features with clinical and pathologic correlation. AJR Am J Roentgenol. 2003;180:1607-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 52. | Blasberg RG, Tjuvajev JG. Molecular-genetic imaging: current and future perspectives. J Clin Invest. 2003;111:1620-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 53. | Reddy MP, Reddy P, Lilien DL. F-18 FDG PET imaging in gastrointestinal stromal tumor. Clin Nucl Med. 2003;28:677-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Jager PL, Gietema JA, van der Graaf WT. Imatinib mesylate for the treatment of gastrointestinal stromal tumours: best monitored with FDG PET. Nucl Med Commun. 2004;25:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 55. | Gayed I, Vu T, Iyer R, Johnson M, Macapinlac H, Swanston N, Podoloff D. The role of 18F-FDG PET in staging and early prediction of response to therapy of recurrent gastrointestinal stromal tumors. J Nucl Med. 2004;45:17-21. [PubMed] |
| 56. | Wilkinson MD, Fulham MJ. FDG PET imaging of metastatic gastrointestinal stromal tumor. Clin Nucl Med. 2003;28:780-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Bhargava P, Zhuang H, Kumar R, Charron M, Alavi A. Iatrogenic artifacts on whole-body F-18 FDG PET imaging. Clin Nucl Med. 2004;29:429-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 58. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1177] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 59. | Hatch GF, Wertheimer-Hatch L, Hatch KF, Davis GB, Blanchard DK, Foster RS, Skandalakis JE. Tumors of the esophagus. World J Surg. 2000;24:401-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 60. | Palazzo L, Landi B, Cellier C, Roseau G, Chaussade S, Couturier D, Barbier J. Endosonographic features of esophageal granular cell tumors. Endoscopy. 1997;29:850-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 61. | Norberto L, Urso E, Angriman I, Ranzato R, Erroi F, Marino S, Tosato S, Ruffolo C, D'Amico DF. Yttrium-aluminum-garnet laser therapy of esophageal granular cell tumor. Surg Endosc. 2002;16:361-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 62. | Nakachi A, Miyazato H, Oshiro T, Shimoji H, Shiraishi M, Muto Y. Granular cell tumor of the rectum: a case report and review of the literature. J Gastroenterol. 2000;35:631-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 63. | Odze RD, Antonioli DA, Wallace MB, Thomas Jr CR, Keohan ML, Hibshoosh H, Antman KH. Gastrointestinal Cancers-A comparison to Sleisenger and Fordtran's Gastrointestinal and Liver Disease. Spain: Elsevier Science Limited 2003; 265-266, 671, 724. |
| 64. | Nickels J, Laasonen EM. Pancreatic heterotopia. Scand J Gastroenterol. 1970;5:639-640. [PubMed] |
| 65. | Tanaka K, Tsunoda T, Eto T, Yamada M, Tajima Y, Shimogama H, Yamaguchi T, Matsuo S, Izawa K. Diagnosis and management of heterotopic pancreas. Int Surg. 1993;78:32-35. [PubMed] |
| 66. | Sloots CE, de Brauw LM, Bot FJ, Greve JW. False-positive cytology in diagnostic laparoscopy due to ectopic pancreas. Dig Surg. 1999;16:434-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 67. | Fernandez MJ, Davis RP, Nora PF. Gastrointestinal lipomas. Arch Surg. 1983;118:1081-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 68. | Crowe F, Schull W, Neel J. A Clinical, Pathological and Genetic Study of Multiple Neurofibromatosis. Springfield, IL, Charles C: Thomas 1956; . |
| 69. | Stevens A, Lowe J, Young B. Wheater's Basic Histopathology-a colour atlas and text. Edinburgh, London, New York, Philadelphia, St. Louis, Sydney, Toronto: Churchill Livingstone 2002; . |
| 70. | Levy AD, Patel N, Dow N, Abbott RM, Miettinen M, Sobin LH. From the archives of the AFIP: abdominal neoplasms in patients with neurofibromatosis type 1: radiologic-pathologic correlation. Radiographics. 2005;25:455-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 71. | Hirsch NP, Murphy A, Radcliffe JJ. Neurofibromatosis: clinical presentations and anaesthetic implications. Br J Anaesth. 2001;86:555-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 70] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 72. | Rubin E. Essential Pathology. Philadelphia: Lippincott Williams & Wilkins 2001; . |
| 73. | Schroeder TV, Sillesen H, Paulson OB. Medicinsk Kompendium Compendium of Medicine. Copenhagen: Nyt Nordisk Forlag Arnold Busck 2004; . |
| 74. | Rubin B, Demetri G. Gastrointestinal Oncology-principles and practice. Philadelphia: Lippincott Williams & Wilkins 2002; . |
| 75. | Buck L, Perry WB, Richards ML. Periampullary carcinoid tumor in a woman with neurofibromatosis. Curr Surg. 2006;63:252-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 76. | Dobru D, Seuchea N, Dorin M, Careianu V. Blue rubber bleb nevus syndrome: case report and literature review. Rom J Gastroenterol. 2004;13:237-240. [PubMed] |
| 77. | Chan AO, Lai KC. A patient with long-standing iron-deficient anemia. Nat Clin Pract Gastroenterol Hepatol. 2006;3:112-116; quiz 117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 78. | Gourtsoyiannis N, Makó E. Imaging of primary small intestinal tumours by enteroclysis and CT with pathological correlation. Eur Radiol. 1997;7:625-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 79. | Lee YT. Leiomyosarcoma of the gastro-intestinal tract: general pattern of metastasis and recurrence. Cancer Treat Rev. 1983;10:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 80. | Jadvar H, Fischman AJ. Evaluation of Rare Tumors with [F-18]Fluorodeoxyglucose Positron Emission Tomography. Clin Positron Imaging. 1999;2:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 81. | Saund MS, Demetri GD, Ashley SW. Gastrointestinal stromal tumors (GISTs). Curr Opin Gastroenterol. 2004;20:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 82. | Stedman's Medical Dictionary. Maryland: Lippincott Williams & Wilkins 2000; . |
| 83. | Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4341] [Cited by in RCA: 4123] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 84. | Fitzpatrick TB, Johnson RA, Wolff K, Polano MK, Suurmond D. Atlas und Synopsis der klinischen Dermatologie -- H�ufige und bedrohliche Krankheiten. Color Atlas and Synopsis of Clinical Dermatology. Common and Serious Diseases. London: McGraw-Hill 1998; . |
| 85. | Dezube BJ. Acquired immunodeficiency syndrome-related Kaposi's sarcoma: clinical features, staging, and treatment. Semin Oncol. 2000;27:424-430. [PubMed] |
| 86. | Choi SH, Sheehan FR, Pickren JW. Metastatic involvement of the stomach by breast cancer. Cancer. 1964;17:791-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 87. | Sheth S, Horton KM, Garland MR, Fishman EK. Mesenteric neoplasms: CT appearances of primary and secondary tumors and differential diagnosis. Radiographics. 2003;23:457-473; quiz 535-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 88. | Yokoi K, Tanaka N, Shoji K, Ishikawa N, Seya T, Horiba K, Kanazawa Y, Yamashita K, Ohaki Y, Tajiri T. A study of histopathological assessment criteria for assessing malignancy of gastrointestinal stromal tumor, from a clinical standpoint. J Gastroenterol. 2005;40:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 89. | Nilsson B, Bümming P, Meis-Kindblom JM, Odén A, Dortok A, Gustavsson B, Sablinska K, Kindblom LG. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer. 2005;103:821-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 875] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 90. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3215] [Cited by in RCA: 3113] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 91. | Miettinen M, Hirota S, Nishida T, Kitamura Y, Shirao K, Yamao K, Koseki M, Okamura T, Ohtsu A, Sugiyama T. Gastrointestinal stromal tumor (GIST): from pathology to molecular target therapy. Tokyo: Japan Scientific Societies Press 2004; 6, 35, 155, 156. |
| 92. | Naitoh I, Okayama Y, Hirai M, Kitajima Y, Hayashi K, Okamoto T, Akita S, Gotoh K, Mizusima M, Sano H. Exophytic pedunculated gastrointestinal stromal tumor with remarkable cystic change. J Gastroenterol. 2003;38:1181-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 93. | Pidhorecky I, Cheney RT, Kraybill WG, Gibbs JF. Gastrointestinal stromal tumors: current diagnosis, biologic behavior, and management. Ann Surg Oncol. 2000;7:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 316] [Article Influence: 12.6] [Reference Citation Analysis (1)] |
| 94. | Bucher P, Taylor S, Villiger P, Morel P, Brundler MA. Are there any prognostic factors for small intestinal stromal tumors? Am J Surg. 2004;187:761-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 95. | Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum Pathol. 1999;30:1213-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 522] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 96. | Breiner JA, Meis-Kindblom J, Kindblom LG, McComb E, Liu J, Nelson M, Bridge JA. Loss of 14q and 22q in gastrointestinal stromal tumors (pacemaker cell tumors). Cancer Genet Cytogenet. 2000;120:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 97. | Joensuu H, Fletcher C, Dimitrijevic S, Silberman S, Roberts P, Demetri G. Management of malignant gastrointestinal stromal tumours. Lancet Oncol. 2002;3:655-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 395] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 98. | Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumours. Ann Chir Gynaecol. 1998;87:278-281. [PubMed] |
| 99. | Lehnert T. Gastrointestinal sarcoma (GIST)--a review of surgical management. Ann Chir Gynaecol. 1998;87:297-305. [PubMed] |
| 100. | Zinkiewicz K, Zgodzinski W, Dabrowski A, Szumilo J, Cwik G, Wallner G. Recurrent inflammatory fibroid polyp of cardia: a case report. World J Gastroenterol. 2004;10:767-768. [PubMed] |
| 101. | Davis GB, Blanchard DK, Hatch GF, Wertheimer-Hatch L, Hatch KF, Foster RS, Skandalakis JE. Tumors of the stomach. World J Surg. 2000;24:412-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 102. | Rice DC, Bakaeen F, Farley DR, Unni KK, van Heerden JA. Surgical management of duodenal leiomyomas. World J Surg. 2001;25:562-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 103. | Hatch KF, Blanchard DK, Hatch GF, Wertheimer-Hatch L, Davis GB, Foster RS, Skandalakis JE. Tumors of the rectum and anal canal. World J Surg. 2000;24:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 104. | Blanchard DK, Budde JM, Hatch GF, Wertheimer-Hatch L, Hatch KF, Davis GB, Foster RS, Skandalakis JE. Tumors of the small intestine. World J Surg. 2000;24:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 105. | Smith LE, Hill M.C. Gastrointestinal Oncology. Pensylvania: JB Lippincott Company 1992; . |
| 106. | David O, Jakate S. Multifocal granular cell tumor of the esophagus and proximal stomach with infiltrative pattern: a case report and review of the literature. Arch Pathol Lab Med. 1999;123:967-973. [PubMed] |
| 107. | Rossi GB, de Bellis M, Marone P, De Chiara A, Losito S, Tempesta A. Granular cell tumors of the colon: report of a case and review of the literature. J Clin Gastroenterol. 2000;30:197-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 108. | Domagk D, Seidel M, Ullerich H, August C, Menzel J, Domschke W. [Abrikossoff's tumor--a rare differential diagnosis in neoplastic lesions of the esophagus]. Z Gastroenterol. 1999;37:1101-1104. [PubMed] |
| 109. | Inagawa S, Hori M, Shimazaki J, Matsumoto S, Ishii H, Itabashi M, Adachi S, Kawamoto T, Fukao K. Solitary schwannoma of the colon: report of two cases. Surg Today. 2001;31:833-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 110. | Agha FP, Dent TL, Fiddian-Green RG, Braunstein AH, Nostrant TT. Bleeding lipomas of the upper gastrointestinal tract. A diagnostic challenge. Am Surg. 1985;51:279-285. [PubMed] |
| 111. | Maderal F, Hunter F, Fuselier G, Gonzales-Rogue P, Torres O. Gastric lipomas--an update of clinical presentation, diagnosis, and treatment. Am J Gastroenterol. 1984;79:964-967. [PubMed] |
| 112. | Leslie A, Virjee JP, Moorghen M. Plexiform neurofibroma of the small bowel infiltrated with metastatic adenocarcinoma. Br J Radiol. 1999;72:604-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 113. | Wei SC, Wong JM, Shieh MJ, Sun CT, Wang CY, Wang TH. Endoscopic resection of gastrointestinal submucosal tumors. Hepatogastroenterology. 1998;45:114-118. [PubMed] |
| 114. | Gupta RK, Naran S, Lallu S, Fauck R. Cytodiagnosis of neoplasms of the central nervous system in cerebrospinal fluid samples with an application of selective immunostains in differentiation. Cytopathology. 2004;15:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 115. | Chak A, Canto MI, Rösch T, Dittler HJ, Hawes RH, Tio TL, Lightdale CJ, Boyce HW, Scheiman J, Carpenter SL. Endosonographic differentiation of benign and malignant stromal cell tumors. Gastrointest Endosc. 1997;45:468-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 206] [Article Influence: 7.4] [Reference Citation Analysis (0)] |