INTRODUCTION
Neoplastic growth in the small intestine is relatively infrequent. Its incidence is usually one-tenth that of a similar lesion of the colon[1]. According to prospective endoscopic studies on elevated lesions of the duodenum, the incidence of duodenal adenoma in all patients referred to diagnostic endoscopy was only 0.4%[2]. Moreover, duodenal tubulovillous adenomas are rare tumors comprising less than 1% of all duodenal neoplasm[3]. However, there has been no report on tubulovillous adenoma in duodenum which has an inverted cystic growth pattern.
Duodenal sporadic adenoma could present the gastric metaplasia on one or more foci[4]. Although the histogenesis of gastric metaplasia of duodenum is not fully understood, Brunner’s glands has been suggested as a precursor for gastric metaplasia[5-7]. However, there has been no report for benign tubulovillous duodenal adenoma arising from Brunner’ glands through gastric metaplasia, except some malignant lesions with possible associations[6,8]. Herein we present a case of duodenal tubulovillous adenoma with an unusual inverted cystic growth involving Brunner’s glands with gastric metaplasia.
CASE REPORT
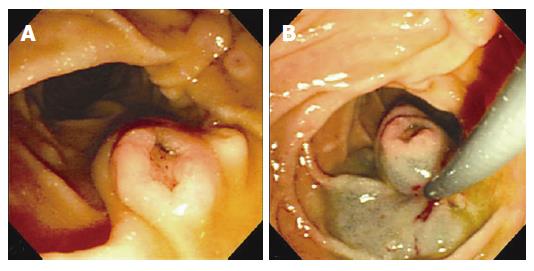
A 72-year-old man presented to the outpatient clinic with epigastric discomfort. His vital signs were normal. Physical examination results and routine laboratory tests were normal. On gastroduodenoscopy, a 1.1 cm sized, centrally depressed, sessile tumor was found in the contralateral portion to the ampulla of Vater. It resembled the normal ampulla of Vater. Multiple biopsy specimens were taken and the tumor was pathologically diagnosed as a tubulovillous adenoma. We decided upon an endoscopic polypectomy to confirm its origin and rule out intrinsic malignancy. We performed snare polypectomy after submucosal injection with a mixture of saline, epineprine and indigocarmine. Snare polypectomy was successfully performed with no complications such as hemorrhage or perforation (Figure 1).
Figure 1 A: Gastroduodenoscopic examination and polypectomy.
1.1 cm sized, centrally depressed, sessile tumor was seen in the contralateral portion to the ampulla of Vater; B: After submucosal injection with a mixture of saline, epineprine and indigocarmine, snare polypectomy was successfully performed.
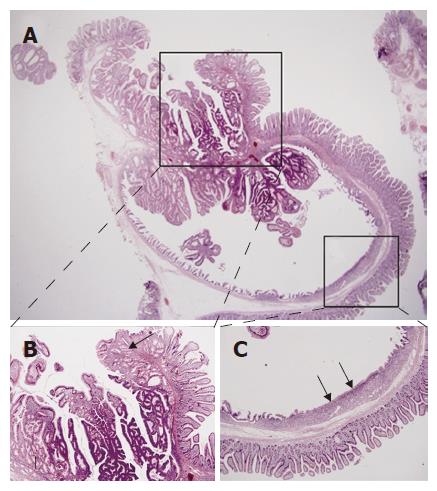
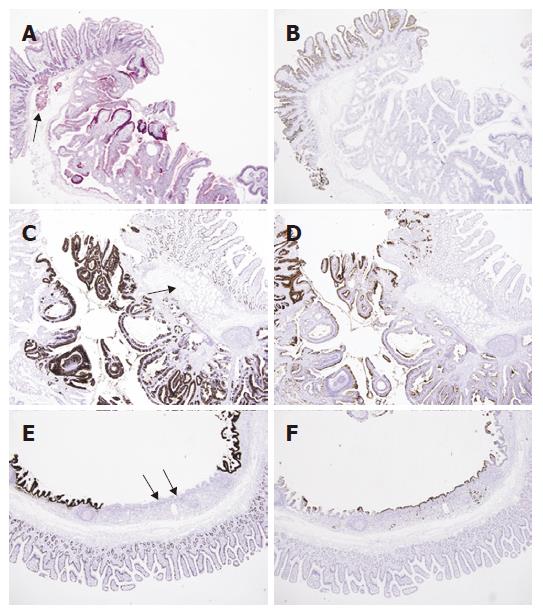
Pathologic examination was done. As a whole, the mucosal surface showed a polypoid mass measuring 1.1 cm × 0.9 cm with central depression. Microscopic examination showed an invagination and cystic change of mucosa with tubulovillous growth along the Brunner’s glands into the deep submucosa. Adjacent surface epithelium was CD10-positive normal duodenal mucosa, and the tubulovillous glands were positive for PAS, MUC2 and MUC5AC. Interestingly, the deep portion of the cyst showed a flat surface epithelium and normal-looking mucus glands, which showed negative staining for MUC2, but positive staining for MUC5AC, representing gastric metaplasia (Figures 2 and 3). There was no pathologic evidence of H pylori infection in stomach and duodenum.
Figure 2 A: Inverted cystic lesion extends along the Brunner gland duct into the submucosa (HE, × 15); B: Normal or hyperplastic Brunner’s glands (arrows) are seen beneath the tubulovillous adenomatous lesion growing into the cyst lumen (HE, × 40); C: The flat surface epithelium and small mucus glands (arrows) are seen among the villiform epithelium (HE, × 40).
Figure 3 A: PAS staining shows positive reaction in the cytoplasm of normal Brunner’s glands (arrow) and tubulovillous glands (PAS, × 40); B: The duodenal surface epithelium is positive for CD10 along the luminal border (arrow), but the Brunner’s glands and adenomatous lesion are negative (Immunohistochemistry CD10, × 40); C: The immunohistochemical stain for MUC2 shows focal positive staining in duodenal mucosa and tubulvillous lesion, but not in Brunner’s glands (arrow) (Immunohistochemistry MUC2, × 40); D: whereas the staining for MUC5AC shows negative staining in duodenal mucosa and Brunner’s glands (arrow), but partly positive in adenomatous lesion (Immunohistochemistry MUC5AC, × 40); E: The area corresponding to the Figure 2B was negative for MUC2 in surface and glandular epithelium (arrows) (Immunohistochemistry MUC2, × 40); F: but positive for MUC5AC (arrows) (Immunohistochemistry MUC5AC, × 40).
DISCUSSION
Benign tumors of the duodenum are very rare with an incidence of 0.008% in a single series of 215 000 autopsies[9]. The prevalence of benign tumors of the duodenum during routine endoscopy was reported to range from 0.3% to 4.6% and the incidence of adenoma was only 0.4% in a prospective endoscopy study[2]. Moreover, duodenal tubulovillous adenomas are uncommon lesions representing less than 1% of all duodenal neoplasm[3,10]. Perry[11] first described villous tumor of the duodenum in 1893 as a broad-based cauliflower-like mass that he referred to as a duodenal papilloma and since that time only 73 cases have been reported by Komorowski et al[1,3] in a 1981 review.
With the advent of diagnostic endoscopy, the prevalence of villous tumors of the duodenum has increased. A review in 1985 reported 161 cases, and more recently, a 15 years search done in 1996 collected 251 cases from the literature[12,13]. Upon microscopic examination, villous adenoma had a frond-like projection of mucosa with branching papillary structure and generally upward growth into the lumen[14]. To our best knowledge, there has been no report on tubulovillous adenoma in duodenum which has inverted cystic growth pattern although inverted hyperplastic polyp of the colon associated with adenoma has been reported[15].
Interestingly, this tubulovillous adenomatous lesion was interrupted by gastric-type mucosa in the deep portion of the cyst with aberrant expression of MUC5AC and closely surrounded by and focally connected with Brunner’s glands. Gastric metaplasia is known to be associated with various diseases and conditions[4,16,17] Although the histogenesis of gastric metaplasia of duodenum is not fully understood, Brunner’s glands has been suggested as a precursor for gastric metaplasia[5,7,18]. And Rubio[4] showed that 59.2% of the duodenal sporadic adenoma, not associated with familial adenomatous polyposis, presented the gastric metaplasia on one or more foci. He suggested that a subset of gastric metaplasia might be present in duodenal mucosa before adenomatous change ensues and might have a similar neoplastic proclivity as the metaplastic epithelium in other gastrointestinal tracts.
Although there are no reports for duodenal adenoma originating from Brunner’s glands, in regard to this un-usual inverted cystic tubulovillous adenoma with gastric metaplasia involving Brunner’ gland and prior literature on adenocarcinoma associated with hyperplasia and gastric metaplasia of Brunner’s glands[6,8], it could be postulated that Brunner’s glands might appear to behave in the manner of adenoma-carcinoma sequence similar to the adenomas of the colon and rectum through the gastric metaplasia.
Although the potential association between H pylori and Brunner’ gland adenoma has been proposed[19], there was no pathologic evidence of H pylori infection in this case.
In this article, we discuss an unusual inverted cystic tubulovillous adenoma involving Brunner’s glands with gastric metaplasia which was successfully diagnosed and treated by endoscopic polypectomy.