Published online Jun 21, 2007. doi: 10.3748/wjg.v13.i23.3255
Revised: April 1, 2007
Accepted: April 4, 2007
Published online: June 21, 2007
We report a patient with hyperplastic polyposis who had two asynchronous colon cancers, a combined adenoma-hyperplastic polyp, a serrated adenoma, and tubular adenomas. Hyperplastic polyposis is thought to be a precancerous lesion; and adenocarcinoma arises from hyperplastic polyposis through the hyperplastic polyp-adenoma-carcinoma sequence. Most polyps in patients with hyperplastic polyposis present as bland-looking hyperplastic polyps, which are regarded as non-neoplastic lesions; however, the risk of malignancy should not be underestimated. In patients with multiple hyperplastic polyps, hyperplastic polyposis should be identified and followed up carefully in order to detect malignant transformation in the early stage.
- Citation: Kurobe M, Abe K, Kinoshita N, Anami M, Tokai H, Ryu Y, Wen CY, Kanematsu T, Hayashi T. Hyperplastic polyposis associated with two asynchronous colon cancers. World J Gastroenterol 2007; 13(23): 3255-3258
- URL: https://www.wjgnet.com/1007-9327/full/v13/i23/3255.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i23.3255
Hyperplastic polyposis is a rare syndrome characterized by multiple hyperplastic polyps, primarily in the proximal colon[1,2]. Sporadic hyperplastic polyps are not generally considered to be precancerous lesions[1-3]. On the other hand, hyperplastic polyposis should be considered a precancerous lesion, because patients with hyperplastic polyposis may frequently have conventional adenomas, serrated adenomas, and adenocarcinomas[2].
We report a patient with hyperplastic polyposis who asynchronically developed two colon adenocarcinomas, as well as a combined adenoma-hyperplastic polyp, a serrated adenoma, and tubular adenomas.
The patient was an 82-year-old, hypertensive, Japanese woman with a previous history of cardiac angina and a gallstone.
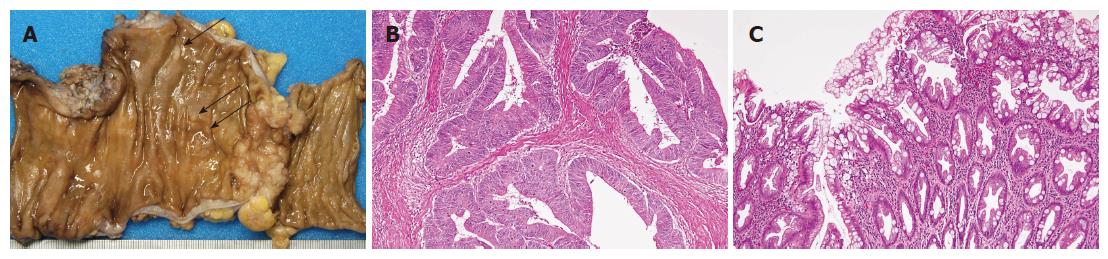
She developed an ileus at the age of 80 years. She was diagnosed as having adenocarcinoma of the sigmoid colon by pathological examination of a biopsy specimen, and underwent sigmoidectomy. The tumor was 4.0 cm × 2.5 cm in size. The adenocarcinoma showed ulcer formation with localized infiltrative growth. There were also multiple small polyps in the resected sigmoid colon (Figure 1A). Histologically, the tumor was a well-differentiated adenocarcinoma with papillary and tubular proliferation invading the subserosal layer (Figure 1B). Mild vascular invasion and a lymph node metastasis were detected. Based on the histology of the small polyps located around the adenocarcinoma, there were 5 hyperplastic polyps in which the proliferating epithelial cells had a serrated appearance (Figure 1C) and a hyperplastic nodule without significant histological changes.
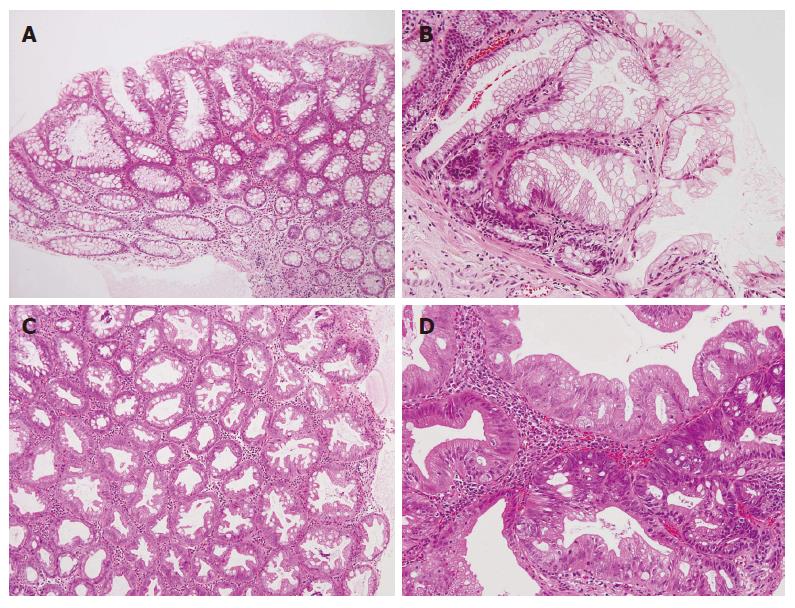
Follow-up colonoscopy seven months later showed two polyps in the transverse colon. Pathologically, one polyp was diagnosed as a tubular adenoma with increased, mildly atypical duct formation and nuclear atypia (Figure 2A), the other polyp as a hyperplastic polyp which had increased serrated ducts without atypical features (Figure 2B).
At the age of 82 years, the patient developed melena. On colonoscopy, some polyps and a diverticulum were seen in the sigmoid colon, and there were some polyps in the ascending colon. One of the polyps in the ascending colon was biopsied and diagnosed as a hyperplastic polyp with serrated structure without atypia (Figure 2C). The other polyp located in the sigmoid colon was a high-grade serrated adenoma having a papillary or serrated structure with atypical cells and an atypical structure, such as fusion of the tubular structure (Figure 2D). There was no evidence of malignancy.
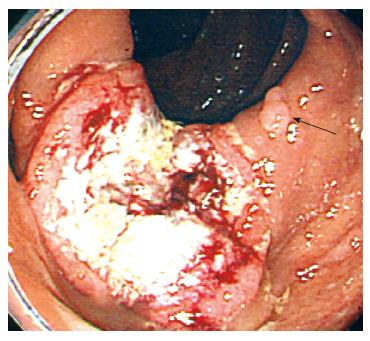
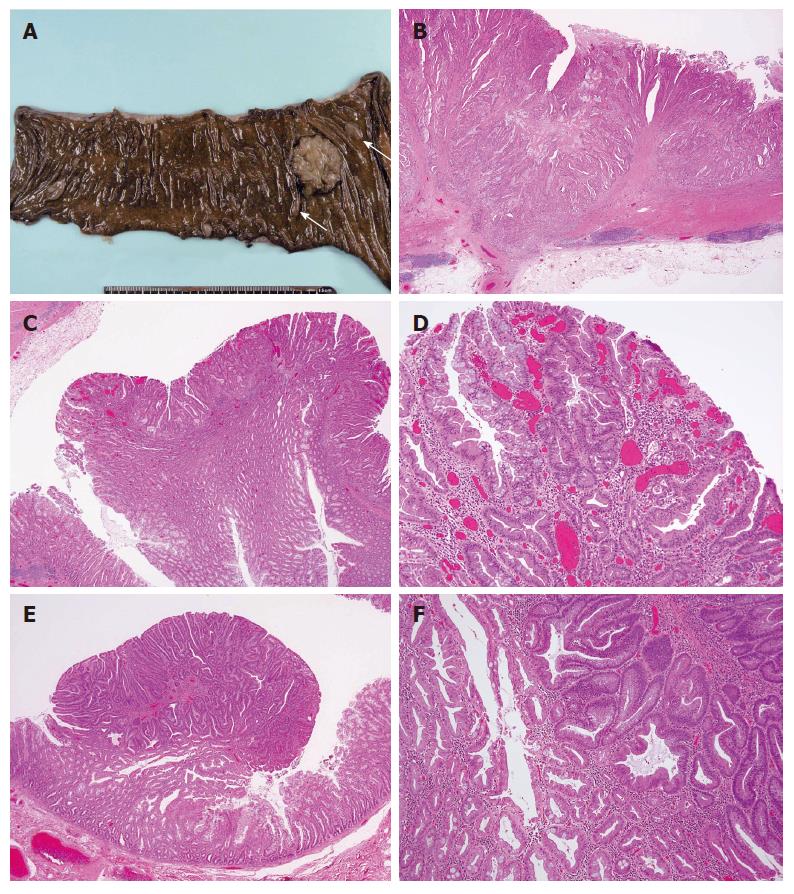
Six months later, the patient complained of constipation and abdominal fullness. On colonoscopy, an ulcerative lesion with localized infiltration was seen in the proximal part of the ascending colon (Figure 3). The patient was diagnosed as having adenocarcinoma pathologically and underwent laparoscopic right colectomy and cholecystectomy. The resected colon was found to have a cancer of 2.8 cm × 3.8 cm in size and multiple small polyps. The two largest polyps were 13 mm × 8 mm and 11 mm × 4 mm in size (Figure 4A). The cancer was a well to moderately differentiated adenocarcinoma with atypical duct formation and a cribriform pattern. The tumor infiltrated the muscular layer and reached the subserosa without serosal exposure (Figure 4B). The adenocarcinoma showed a fusion to a small adenoma-like and a hyperplastic polyp components in the margins (Figure 4C and D). There was no lymph node metastasis.
Eight of the small polyps around the adenocarcinoma were examined; there were four hyperplastic polyps, three tubular adenomas, and one combined adenoma-hyperplastic polyp. The combined adenoma-hyperplastic polyp was 1 cm in size. In the combined adenoma-hyperplastic polyp, the hyperplastic polyp component had a serrated structure with no cell atypism, and the adenoma component consisted of a tubular adenoma with mild cell atypia. At the border of the two components, there was a small amount of serrated structure with mild atypia (Figure 4E and F).
Based on all these pathologic features, this patient was diagnosed as having hyperplastic polyposis with two asynchronous cancers.
The patient was followed up with regular colono-scopies; and one year after the surgery, the patient was tumor-free.
Sporadic hyperplastic polyps are generally thought not to be malignant; and malignant transformation into adenocarcinoma has been rarely reported[1,2,4,5]. However, hyperplastic polyposis is considered to be a precancerous lesion, since conventional adenomas, serrated adenomas, and adenocarcinomas can occur synchronously or asynchronously in the same patient[1,2,4,6]. A case of hyperplastic polyposis was reported with a large hyperplastic polyp (> 1.5 cm) that had an adenomatous lesion within it[3]. Another reported case of hyperplastic polyposis had an adenocarcinoma component within one hyperplastic polyp[7]. There is also a report of a case with hyperplastic polyposis that had two synchronous adenocarcinomas in the transverse colon[2].
The diagnostic criteria for hyperplastic polyposis generally include the presence of[1]: (1) at least five histologically diagnosed hyperplastic polyps proximal to the sigmoid colon, of which two are greater than 10 mm in diameter; or (2) any number of hyperplastic polyps proximal to the sigmoid colon in an individual who has a first-degree relative with hyperplastic polyposis; or (3) more than 30 hyperplastic polyps of any size that are distributed throughout the colon[1]. In the present case, there were five hyperplastic polyps in the sigmoid colon, one hyperplastic polyp in the transverse colon, and five hyperplastic polyps in the ascending colon. Polyps as large as 13 mm × 8 mm and 11 mm × 4 mm were found in the ascending colon. Therefore, our case fulfilled criterion (1) of the above-mentioned criteria.
Hyperplastic polyposis is a precancerous lesion[1]. In the present case, two asynchronous adenocarcinomas were found in the sigmoid colon and the ascending colon, as well as a high-grade serrated adenoma in the sigmoid colon, tubular adenomas, and a combined adenoma-hyperplastic polyp in the ascending colon. Thus, our case supports the notion that hyperplastic polyps in patients with hyperplastic polyposis have a high oncogenicity.
There are two theories about the origin of adenocarci-nomas that develop in hyperplastic polyposis. One theory states that some of the hyperplastic polyps undergo adenomatous changes to become serrated adenomas with atypical cells and then develop into adenocarcinomas. This is called the “serrated pathway”[1-4,6,8,9]. The other theory states that the adenoma component of a combined adenoma-hyperplastic polyp develops into an adenocarcinoma by the loss of the tumor suppressor gene[1,2].
In the present case, a hyperplastic polyp component was detected in the marginal area of the ascending colon adenocarcinoma, and a small serrated adenoma-like component was detected at the border between the adenocarcinoma and the hyperplastic polyp component. Based on this finding, it seems possible that the hyperplastic polyp - serrated adenoma-adenocarcinoma pathway was operative in this case. However, since the adenocarcinoma was large, it was possible that the adenocarcinoma originated from an area distinct from the polyp and then engulfed the combined serrated-hyperplastic polyp. In addition, at the time that the first sigmoid colon cancer was resected, a high-grade serrated adenoma was found in the sigmoid colon. These findings support the notion that this malignancy developed through the “serrated pathway”. However, adenocarcinomas in both the sigmoid and the ascending colon were too large to allow us to definitively determine their origins. Of course, it is still possible that a de novo adenocarcinoma engulfed a hyperplastic polyp and an adenoma.
Combined adenoma-hyperplastic polyps have both hyperplastic lesions and adenoma lesions. The polyps are thought to occur as a result of: (1) engulfment of pre-existing hyperplastic polyps by a growing adenoma; or (2) the induction of mucosal hyperplasia at the advancing edge of an adenoma; or (3) the development of an adenoma within a hyperplastic polyp[4]. In the present case, a small (1 cm), combined adenoma-hyperplastic polyp was detected, which gave evidence of the tumorigenicity of hyperplastic polyps.
Among the cases reported dealing with hyperplastic polyposis, there are reports, including our case, of patients with multiple adenocarcinomas[2,6]. The asynchronous development of multiple neoplastic polyps in our patient may also explain the presence of asynchronous multiple adenocarcinomas.
When an adenoma arises from a hyperplastic polyp, which has been found to have the following molecular biological changes: k-ras and p53 mutations; loss of heterozygosity; and microsatellite instability[4,10]. Adenoma-tous change is rare in a sporadic hyperplastic polyp, but is common in patients with hyperplastic polyposis due to the large number of hyperplastic polyps; thus, the chance of an adenoma arising from a hyperplastic polyp is increased. Therefore, the risk of cancer may also be increased. Although the polyps in patients with hyperplastic polyposis look similar to sporadic hyperplastic polyps, they might be genetically different[4], and the genetic makeup of the polyps of patients with hyperplastic polyposis is more likely to transform into adenocarcinomas.
Hyperplastic polyposis may sometimes be familial[1].But, patients do not generally have a family history of colon cancer[2]; this feature distinguishes it from familial adenomatous polyposis (FAP)[2]. Notably, our patient did not have a family history of colon cancer.
FAP is a syndrome characterized by the presence of at least 100 polyps located mainly in the distal colon; these polyps often appear in the second and third decade of life[4]. Hyperplastic polyposis is usually diagnosed in older adults, and the polyps are predominantly in the proximal colon[1]. Compared to patients with FAP, patients with hyperplastic polyposis do not develop adenocarcinomas at such a young age; thus, a radical cure, such as total colectomy, is not necessary for patients with hyperplastic polyposis. Nevertheless, since in these patients the risk of cancer increases after the fifth decade[2], regular colonoscopic surveillance is suggested in patients with hyperplastic polyposis[5]. However, since most polyps in hyperplastic polyposis present as bland-looking hyperplastic polyps that tend to be regarded as non-neoplastic lesions, the risk of malignancy may be underestimated. Patients with multiple hyperplastic polyps should be assessed so as to determine whether they have hyperplastic polyposis, since patients with hyperplastic polyposis require regular follow-ups to detect cancer at an early stage.
S- Editor Liu Y L- Editor Ma JY E- Editor Ma WH
| 1. | Burt RW, Jass JR. Hyperplastic polyposis. World Health Organisation classification of tumours. Pathology and genetics. Tumours of the digestive system. Berlin: Springer-Verlag 2000; 135-136. |
| 2. | Abeyasundara H, Hampshire P. Hyperplastic polyposis associated with synchronous adenocarcinomas of the transverse colon. ANZ J Surg. 2001;71:686-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Sumner HW, Wasserman NF, McClain CJ. Giant hyperplastic polyposis of the colon. Dig Dis Sci. 1981;26:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 31] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Ponz de Leon M, Di Gregorio C. Pathology of colorectal cancer. Dig Liver Dis. 2001;33:372-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Goswami RS, Minoo P, Baker K, Chong G, Foulkes WD, Jass JR. Hyperplastic polyposis and cancer of the colon with gastrinoma of the duodenum. Nat Clin Pract Oncol. 2006;3:281-284; quiz 285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Jass JR, Iino H, Ruszkiewicz A, Painter D, Solomon MJ, Koorey DJ, Cohn D, Furlong KL, Walsh MD, Palazzo J. Neoplastic progression occurs through mutator pathways in hyperplastic polyposis of the colorectum. Gut. 2000;47:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 176] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Cooper HS, Patchefsky AS, Marks G. Adenomatous and carcinomatous changes within hyperplastic colonic epithelium. Dis Colon Rectum. 1979;22:152-156. [RCA] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 75] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Snover DC, Jass JR, Fenoglio-Preiser C, Batts KP. Serrated polyps of the large intestine: a morphologic and molecular review of an evolving concept. Am J Clin Pathol. 2005;124:380-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 266] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 9. | Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol. 2003;27:65-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 429] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 10. | Rashid A, Houlihan PS, Booker S, Petersen GM, Giardiello FM, Hamilton SR. Phenotypic and molecular characteristics of hyperplastic polyposis. Gastroenterology. 2000;119:323-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 144] [Article Influence: 5.8] [Reference Citation Analysis (0)] |