Published online Jun 21, 2007. doi: 10.3748/wjg.v13.i23.3171
Revised: February 1, 2007
Accepted: February 8, 2007
Published online: June 21, 2007
AIM: To investigate the anti-tumor effect and mechanisms of magnetic nanoparticles targeting hepatocellular carcinoma.
METHODS: Human hepatocellular carcinoma was induced in nude mice, and the mice were randomly divided into group A receiving normal saline, group B receiving magnetic nanoparticles containing 5-fluorouracil (5-FU), group C receiving 5-FU, and group D receiving magnetic nanoparticles containing 5-FU with a magnetic field built in tumor tissues. The tumor volume was measured on the day before treatment and 1, 4, 7, 10 and 13 d after treatment. Tumor tissues were isolated for examination of the expression of bcl-2, bax and caspase 3 by immunohistochemical method, reverse transcription polymerase chain reaction and Western blotting.
RESULTS: The tumor volume was markedly lower in groups C and D than in groups A and B (group C or D vs group A or B, P < 0.01). The volume was markedly lower in group D than in group C (P < 0.05). The expression of protein and mRNA of bcl-2 was markedly lower in groups C and D than in groups A and B (group C or D vs group A or B, P < 0.01), and was markedly lower in group D than in group C (P < 0.01). The expression of bax and caspase 3 in groups C and D was significantly increased, compared with that in groups A and B (P < 0.01).
CONCLUSION: The targeted magnetic nanoparticles containing 5-FU can improve the chemotherapeutic effect of 5-FU against hepatocellular carcinoma by decreasing the expression of bcl-2 gene, and increasing the expression of bax and caspase 3 genes.
-
Citation: Wang JM, Xiao BL, Zheng JW, Chen HB, Zou SQ. Effect of targeted magnetic nanoparticles containing 5-FU on expression of
bcl-2 ,bax andcaspase 3 in nude mice with transplanted human liver cancer. World J Gastroenterol 2007; 13(23): 3171-3175 - URL: https://www.wjgnet.com/1007-9327/full/v13/i23/3171.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i23.3171
Targeted magnetic chemotherapy can selectively focus chemotherapeutics on tumor tissue when a magnetic field is used to confine the magnetic drug carrier to the target site[1-3]. Targeted drug delivery is an attractive field in tumor therapy[4]. In our experiment, nanochemotherapy was used in nude mice with transplanted human liver cancer and the anti-tumor effect and mechanism of targeted magnetic nanoparticles were studied by examining the expression of bcl-2, bax and caspase 3 in tumor tissue.
Magnetic nanoparticles containing 5-fluorouracil (5-FU, 10.1% ± 1.2%) were prepared by the Pharmaceutical Department of Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China). Magnetic metal rack for medication was prepared by Department of Material, Wuhan University of Technology (Wuhan, China). Culture media (RPMI-1640) were obtained from Gibco Co., Ltd, USA. Calf serum was purchased from Hangzhou Sijiqing Co., Ltd, China. Rabbit polyclonal antibodies against human bcl-2, bax, caspase 3 and β-actin protein, and goat anti-rabbit polyclonal antibody were purchased from Wuhan Boster Biological Technology Co., Ltd, China.
Cells of HepG2, a primary human hepatocellular carcinoma cell line, were provided by the Surgical Laboratory of Tongji Hospital. All media were supplemented with 100 mL/L, heat-inactivated calf serum, penicillin G (100 IU/mL), and streptomycin (100 μg/mL). The cells were incubated as a monolayer in RPMI 1640 at 37°C in a humidified atmosphere containing 50 mL/L CO2.
Thirty-two BALB/C male nude mice weighing 17-20 g at 3-5 wk of age, provided by the Experimental Animal Center of Tongji Medical College, Huazhong University of Science and Technology, were used in this study. The study protocol was approved by the Animal Ethics Committee of Huazhong University of Science and Technology.
HepG2 cells were grown in monolayer culture, harvested and adjusted to 5 × 107 cells/mL. For subcutaneous tumor formation, 0.2 mL of cell solution was injected subcutaneously into the right back of each nude mouse. After 14 d, when the volume of tumor was about 130 mm3, the mice were randomly divided into group A receiving normal saline, group B receiving magnetic nanoparticles containing 250 mg/kg 5-FU, group C receiving 25 mg/kg 5-FU, group D receiving magnetic nanoparticles containing 250 mg/kg 5-FU, with a magnetic field at 300 gauss built in tumor tissues. Each group was treated with the same volume (0.2 mL) by vena caudalis injection, once a day for 5 d.
Each tumor was measured with a sliding caliper for a maximal diameter (a) and a minimal diameter (b) on the day before treatment and 1, 4, 7, 10 and 13 d after treatment, and calculated using the following formula: volume = a × b × b/2. All animals were sacrificed and underwent complete examination of abdominal cavity 20 d after treatment. The tumor mass was then isolated.
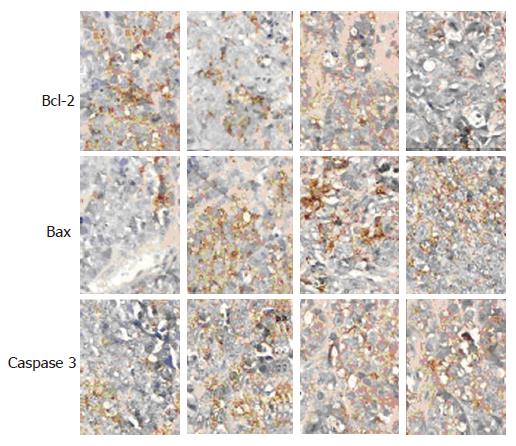
Bcl-2, bax and caspase3 were detected with immuno-histochemical (SABC) method. In brief, tumor specimens were incubated with 3 mL/L hydrogen peroxide in methanol for 30 min to block endogenous peroxidase activity, washed in phosphate buffered saline (PBS) and incubated in 100 mL/L normal goat serum for 20 min to reduce nonspecific antibody binding. Specimens were then incubated with rabbit polyclonal antibodies at a dilution of 1:100 against human bcl-2, bax or caspase 3 overnight at 4°C, followed by three washes with PBS, then incubated with biotinylated goat anti-rabbit polyclonal antibody at a dilution of 1:100 for 30 min followed by 3 washes. Slides were treated with streptavidin peroxidase reagent for 30 min at a dilution of 1:100 and washed 3 times with PBS. Finally, slides were incubated in PBS containing diaminobenzidine and 10 L/L hydrogen peroxide for 10 min, counterstained with hematine and mounted. The rabbit antibody was replaced by PBS as a blank. Five high power fields of each section were selected randomly and input to the HM IAS-2000 analysis system for staining intensity (average absorbance) analysis.
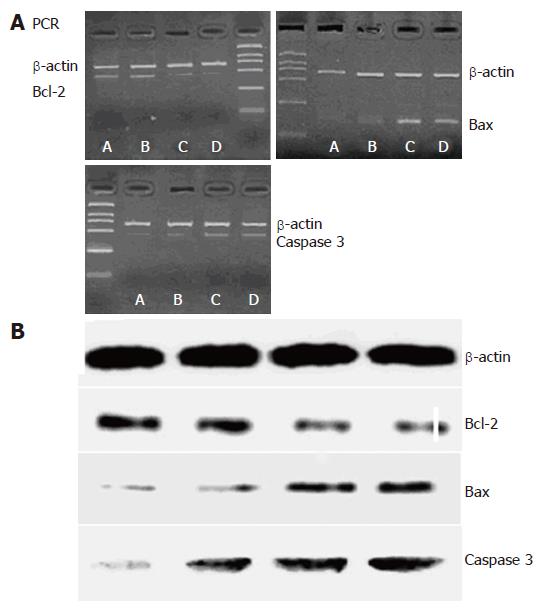
The tumor mass was isolated and total RNA was extracted with TRIzol (GIBCO, Gran Island, NY, USA). The sequence of primers (primer 5.0, Shanghai Sangon Biological Engineering & Technology and Service Co.Ltd, Shanghai, China) used is: bcl-2 (455 bp): sense: 5’-GGCACTGTCTTGACCCAC-3’ and antisense: 5’-TCATAAACCCTGCTTGCTG-3’; bax (121 bp): sense: 5’-AATGCCCGTTCATCTCAG-3’ and antisense: 5’-GGGACATCAGTCGCTTCA-3’; caspase 3 (445 bp): sense: 5’-CACAATAGCACCCATCCG-3’ and antisense: 5’-GGGACATCAGTCGCTTCA-3’; β-actin (550 bp): sense: 5’-GGTGGGGCGCCC CAGGCACCA-3’ and antisense: 5’-GCTCCTTAATGT CACGCACGA-3’. The following PCR conditions were used: initiation at 94°C for 4 min, then 35 cycles of denaturing at 94°C for 45 s, annealing at 48°C (bcl-2, and bax for 45 s, at 50°C (caspase 3) for 45 s or at 52°C (β-actin) for 45 s, extension at 72°C for 1 min followed by a final extension at 72°C for 10 min. The PCR products were separated by electrophoresis on a 1.5% agarose gel and vidualized by ethidium bromide staining using Gel-Pro analyzer. The bands were quantitated by densitometry and the gene expression was represented as the ratio of target mRNA in comparison to that of β-actin.
Western blotting was used for the detection of bcl-2, bax and caspase 3 proteins. On day 20 after treatment, 0.1 g tumor tissue was collected from each mouse and homogenized. Tissues were lysed in a lysis buffer containing 50 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, 0.5% deoxycholic acid, 1% NP40, 0.1% SDS, 1 mmol/L PMSF, and 100 μg/mL leupeptin. Protein concentration was measured using a Bio-Rad colorimetric protein assay kit (Bio-Rad, Hercules, California, USA). A 50 μg sample of protein was separated on SDS-polyacrylamide gels and transferred onto a nitrocellulose membrane. Rabbit anti-bcl-2 antibody (1:500), rabbit anti-bax antibody (1:500), rabbit anti-caspase 3 antibody (1:500) or rabbit anti-β-actin antibody (1:500) was used respectively as the primary antibody. Horseradish peroxidase-conjugated anti-rabbit antibody (1:1000) was used to probe for bcl-2, bax, caspase 3 or β-actin as the secondary antibody. The band was detected using the enhanced chemiluminescence (ECL) detection system. The protein expression was represented as the optical density ratio of the interest protein in comparison to that of β-actin.
Data were presented as mean ± SD. Repetitive analyses of variance (ANOVA) were performed for comparison of tumor volume among treatment groups. One-way ANOVA was performed for comparison of the expression levels of bcl-2, bax and caspase 3 among groups. All data were analyzed by SPSS 12.0, and P < 0.05 was considered statistically significant.
All models of nude mice with transplanted hepatocellular carcinoma were established 14 d after subcutaneous injection, with an average volume of 130 mm3. The rate of subcutaneous tumor formation was 100%. There was no significant difference in the average tumor volume between the groups of A and B. The average tumor volume was smaller in groups C and D than in groups A and B (P < 0.01). Also, the volume was smaller in group D than in group C (P < 0.05) (Table 1).
| Group | n | 0 | 1d | 4d | 7d | 10d | 13d |
| A | 8 | 131.9 ± 4.3 | 261.0 ± 29.7 | 362.0 ± 52.9 | 513.1 ± 67.3 | 643.9 ± 83.4 | 776.1 ± 116.2 |
| B | 8 | 133.7 ± 8.3 | 253.0 ± 27.8 | 341.5 ± 34.6 | 473.4 ± 53.2 | 600.2 ± 72.4 | 764.7 ± 100.6 |
| Ca | 8 | 134.4 ± 4.7 | 228.7 ± 26.7 | 294.8 ± 31.6 | 364.9 ± 34.5 | 416.9 ± 64.4 | 465.0 ± 91.1 |
| Dab | 8 | 135.8 ± 6.8 | 213.0 ± 18.1 | 261.5 ± 22.0 | 315.5 ± 27.6 | 337.2 ± 32.6 | 367.8 ± 44.0 |
The expression of bcl-2, bax and caspase 3 proteins observed under microscope was positive. The distribution of the Buffy particles was diffuse. The expression of bcl-2 protein was markedly decreased, while that of bax and caspase 3 proteins was markedly increased in groups C and D, in comparison with groups A and B (P < 0.01). The expression of bcl-2 protein was markedly decreased whereas that of bax and caspase 3 proteins was markedly increased in group D compared with group C (P < 0.01) (Table 2, Figure 1).
The mRNA expression in bcl-2 was lower in groups C and D than in groups A and B (P < 0.01), and was lower in group D than in group C (P < 0.01). The mRNA expression in bax and caspase 3 was higher in groups C and D than in groups A and B (P < 0.01), and was markedly higher in group D than in group C (P < 0.01, Table 3, Figure 2A).
Western blot was used for examining the expression of bcl-2, bax and caspase 3 proteins. The expression of bcl-2 was lower in groups C and D than in groups A and B (P < 0.01), and was markedly lower in group D than in group C (P < 0.01). The expression of bax and caspase 3 proteins was higher in groups C and D than in groups A and B (P < 0.01), and markedly higher in group D than in group C (P < 0.01, Table 4, Figure 2B).
Hepatocellular carcinoma (HCC) is a serious problem in developing countries, accounting for 81% of the total cases in the world and 54% of the total cases in China[5]. Even 5 years after curative resection of small HCC, the recurrent rate is as high as 40%-60%[6,7]. Furthermore, these tumors are quite resistant to radiotherapy and chemotherapy[8]. No effective postoperative adjuvant chemotherapeutic agent is available so far.
At present, the main chemotherapeutics of HCC are 5-FU, cis-diaminedichloroplatinum (CDDP), adriamycin (ADM), mitomycin (MMC)[9-11]. The newly developed antineoplastic agents, such as capecitabine[12], are expected to increase the therapeutic effect on liver cancer. However, the cost of treatment is expensive and the therapeutic effect is uncertain, indicating that more clinical data and trials are needed. Patients with HCC have complications of liver cirrhosis and chronic hepatitis. Since almost all chemotherapeutics have side effects, the tolerance of patients to chemotherapy is usually poor[13]. Magnetic drug-coated nanoparticles provide a new method for the treatment of cancer[14]. Under the guidance of magnetic fields to the tumor, the target distribution of magnetic nanoparticles containing drugs can increase the therapeutic effect on the tumor by increasing the concentration of drugs, both in tumor tissue and in tumor cell embolism in tumor blood vessels[15,16], and by reducing their side effects[17,18].
In the present study, magnetic nanoparticles containing 5-FU with a magnetic field built in tumor tissues could selectively act on the transplanted liver cancer in nude mice, indicating that targeted therapy with magnetic nanoparticles containing 5-FU inhibits the growth of hepatocellular carcinoma (P < 0.05).
Experimental studies have shown that the antitumor effect of 5-FU, CDDP and MMC is correlated to the induction of apoptotic cells[19]. Apoptosis is regulated by some genes or other factors[20]. Escape from apoptotic signals often accompanies tumor progression. Genes controlling apoptosis can be divided into suppressor gene or trigger gene. Bcl-2, bcl-XL and mcl-1 are the main suppressor genes of apoptosis, and bcl-2 is the most important. Bax, bcl-XS, p53 and c-myc are the main trigger genes of apoptosis[21,22]. Human bcl-2 gene, located on chromosome 18, is a proto-oncogene in the hematopoietic system. Bcl-2 gene suppresses apoptosis induced by many other factors[23,24] and bax gene encodes a 21 kD protein, and has 21% homology with bcl-2 gene. Over-expression of bax gene can inhibit the function of bcl-2 and promote apoptosis[25]. Caspases are crucial mediators for apoptosis. Among them, caspase-3 is a frequently activated death protease, catalyzing the specific cleavage of many key cellular proteins. Caspase-3 may be the best understood mammalian caspases in terms of its specificity and role in apoptosis[26,27].
Our study showed that the targeted therapy with magnetic nanoparticles containing 5-FU could decrease the expression of bcl-2, and increase the expression of bax and caspase 3 (P < 0.01), indicating that this therapy can inhibit the growth of hepatocellular carcinoma and up-regulate apoptosis of liver cancer cells by decreasing the expression of bcl-2 gene and increasing the expression of bax and caspase 3 genes.
The targeted therapy with magnetic nanoparticles containing 5-FU can selectively make 5-FU focus on tumor tissue, with a high concentration in tumor tissues. Also, concentrated nanoparticles can lead to embolism in tumor blood vessels, ischemia and anoxyaemia in tumor tissues, and a higher sensitivity of chemotherapy. Moreover, this therapy could decrease the expression of bcl-2 gene, increase the expression of bax and caspase 3 genes, suggesting that it up-regulates apoptosis of liver cancer cells and inhibits the growth of hepatocellular carcinoma.
In conclusion, targeted magnetic nanoparticles containing 5-FU can improve the chemotherapeutic effect of 5-FU on hepatocellular carcinoma by decreasing the expression of bcl-2 gene and increasing the expression of bax and caspase 3 genes.
S- Editor Wang J L- Editor Wang XL E- Editor Wang HF
| 1. | Widder KJ, Morris RM, Poore GA, Howard DP, Senyei AE. Selective targeting of magnetic albumin microspheres containing low-dose doxorubicin: total remission in Yoshida sarcoma-bearing rats. Eur J Cancer Clin Oncol. 1983;19:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 77] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Babincová M, Altanerová V, Lampert M, Altaner C, Machová E, Srámka M, Babinec P. Site-specific in vivo targeting of magnetoliposomes using externally applied magnetic field. Z Naturforsch C. 2000;55:278-281. [PubMed] |
| 3. | Gong LS, Zhang YD, Liu S. Target distribution of magnetic albumin nanoparticles containing adriamycin in transplanted rat liver cancer model. Hepatobiliary Pancreat Dis Int. 2004;3:365-368. [PubMed] |
| 4. | Gupta PK, Hung CT. Magnetically controlled targeted chemotherapy. In: N Willmott and J Daly, eds. Microspheres and Regional Cancer Therapy. CRC Press 1993; 71–116. |
| 5. | Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer. 1999;80:827-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 6. | Tang ZY, Sun FX, Tian J, Ye SL, Liu YK, Liu KD, Xue Q, Chen J, Xia JL, Qin LX. Metastatic human hepatocellular carcinoma models in nude mice and cell line with metastatic potential. World J Gastroenterol. 2001;7:597-601. [PubMed] |
| 7. | Shuto T, Kinoshita H, Hirohashi K, Kubo S, Tanaka H, Tsukamoto T, Okuda T. Indications for, and effectiveness of, a second hepatic resection for recurrent hepatocellular carcinoma. Hepatogastroenterology. 1996;43:932-937. [PubMed] |
| 8. | Blum HE. Molecular targets for prevention of hepatocellular carcinoma. Dig Dis. 2002;20:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Kogure T, Ueno Y, Iwasaki T, Shimosegawa T. The efficacy of the combination therapy of 5-fluorouracil, cisplatin and leucovorin for hepatocellular carcinoma and its predictable factors. Cancer Chemother Pharmacol. 2004;53:296-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Tanioka H, Tsuji A, Morita S, Horimi T, Takamatsu M, Shirasaka T, Mizushima T, Ochi K, Kiura K, Tanimoto M. Combination chemotherapy with continuous 5-fluorouracil and low-dose cisplatin infusion for advanced hepatocellular carcinoma. Anticancer Res. 2003;23:1891-1897. [PubMed] |
| 11. | Pohl J, Zuna I, Stremmel W, Rudi J. Systemic chemotherapy with epirubicin for treatment of advanced or multifocal hepatocellular carcinoma. Chemotherapy. 2001;47:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Murata K, Shiraki K, Kawakita T, Yamamoto N, Okano H, Nakamura M, Sakai T, Deguchi M, Ohmori S, Nakano T. Low-dose chemotherapy of cisplatin and 5-fluorouracil or doxorubicin via implanted fusion port for unresectable hepatocellular carcinoma. Anticancer Res. 2003;23:1719-1722. [PubMed] |
| 13. | Dizon DS, Kemeny NE. Intrahepatic arterial infusion of chemotherapy: clinical results. Semin Oncol. 2002;29:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Xu H, Song T, Bao XQ. Site-directed research of magnetic nanoparticles in magnetic drug targeting. Journal of Magnetism and Magnetic Materials. 2005;293:514-519. [DOI] [Full Text] |
| 15. | Lübbe AS, Bergemann C, Huhnt W, Fricke T, Riess H, Brock JW, Huhn D. Preclinical experiences with magnetic drug targeting: tolerance and efficacy. Cancer Res. 1996;56:4694-4701. [PubMed] |
| 16. | Andrew DG, Giles R. Mathematical modeling of magnetically targeted drug delivery. Magnetism and Magnetic Materials. 2005;293:455-463. [DOI] [Full Text] |
| 17. | Lübbe AS, Alexiou C, Bergemann C. Clinical applications of magnetic drug targeting. J Surg Res. 2001;95:200-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 650] [Cited by in RCA: 389] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 18. | Zhang YD, Gong LS, Pan YF. Target distribution of magnetic nanoparticle containing drug in vivo and its therapeutical efficiency for liver cancer. Zhongguo Yixue Gongcheng Zazhi. 2003;11:18-21. |
| 19. | Clark JW, Glicksman AS, Wanebo HJ. Systemic and adjuvant therapy for patients with pancreatic carcinoma. Cancer. 1996;78:688-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013-2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 21. | Keane MM, Ettenberg SA, Nau MM, Russell EK, Lipkowitz S. Chemotherapy augments TRAIL-induced apoptosis in breast cell lines. Cancer Res. 1999;59:734-741. [PubMed] |
| 22. | Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3914] [Cited by in RCA: 3937] [Article Influence: 145.8] [Reference Citation Analysis (0)] |
| 23. | Sartorius UA, Krammer PH. Upregulation of Bcl-2 is involved in the mediation of chemotherapy resistance in human small cell lung cancer cell lines. Int J Cancer. 2002;97:584-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 166] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Cohen-Saidon C, Razin E. The involvement of Bcl-2 in mast cell apoptosis. Novartis Found Symp. 2005;271:191-195; discussion 195-199;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Duan XX, Ou JS, Li Y, Su JJ, Ou C, Yang C, Yue HF, Ban KC. Dynamic expression of apoptosis-related genes during development of laboratory hepatocellular carcinoma and its relation to apoptosis. World J Gastroenterol. 2005;11:4740-4744. [PubMed] |
| 26. | Boyce M, Degterev A, Yuan J. Caspases: an ancient cellular sword of Damocles. Cell Death Differ. 2004;11:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4724] [Cited by in RCA: 4686] [Article Influence: 156.2] [Reference Citation Analysis (0)] |