Published online Jun 21, 2007. doi: 10.3748/wjg.v13.i23.3153
Revised: March 10, 2007
Accepted: March 12, 2007
Published online: June 21, 2007
Imaging of both benign and malignant anorectal diseases has traditionally posed a challenge to clinicians, and as a result history and physical exam have been relied on heavily. CT scanning and endorectal ultrasound have become popular in assessment of anatomy and staging of tumors, but have limitations. Magnetic resonance imaging (MRI) has the capability to fill in the gaps left open by more conventional imaging modalities and continues to be promising as the definitive imaging technique in the pelvis, especially with advancement of emerging technologies in this field. A comprehensive review of this topic has been undertaken. Anorectal disease is divided into three broad categories: cancer, fistula/abscess, and pelvic floor disorders. A review of the literature is performed to evaluate the use of MRI and other imaging modalities in these three areas. Preoperative imaging is useful in the evaluation of all three areas of anorectal disease. MRI is an effective tool in delineating anatomy and, when correlating with the specific clinical scenario, is an effective adjunct in clinical decision-making in order to optimize outcome. MRI continues to be a promising and novel approach to imaging various afflictions of the anorectum and the pelvic floor. Its role is more well-established in some areas than in others, and there are still significant limitations. As technology advances, MRI will shed more light on a complex anatomical area.
- Citation: Berman L, Israel GM, McCarthy SM, Weinreb JC, Longo WE. Utility of magnetic resonance imaging in anorectal disease. World J Gastroenterol 2007; 13(23): 3153-3158
- URL: https://www.wjgnet.com/1007-9327/full/v13/i23/3153.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i23.3153
Imaging of anorectal disease has always posed a challenge to clinicians. This is an area of the body with a complex array of muscle groups and tissue planes which can easily mask extension of a tumor, conceal the path of a complex fistula, or hide the subtle anatomic defect responsible for disordered defecation. Because of these difficulties, clinicians rely heavily on history and physical examination to guide diagnosis and management of anorectal disorders. There are certain areas where imaging can provide crucial information, and these include malignancy, fistula, and pelvic floor disorders. Traditionally, the modalities of choice for imaging anorectal disease have been ultrasound and CT scan. Each of these has unique limitations, but they provide helpful information, especially when used in combination. With the advent of recent technology including endoanal coils and phased array imaging, magnetic resonance imaging (MRI) has begun to play a more prominent role in imaging anorectal disease. In many cases it surpasses the ability of ultrasound and CT scan, and often accomplishes in one test what would have previously only been possible with multiple, if any. This paper will review the imaging modalities that are used in anorectal cancer, fistula disease and pelvic floor disorders, with a focus on MRI.
The management of rectal cancer has evolved over the years, with preoperative imaging playing an increasingly prominent role. Formerly, patients with rectal cancer would often be diagnosed clinically and proceed to the operating room without any further preoperative workup. Surgeons had limited knowledge of tumor characteristics and the presence of metastatic disease, which led to a high rate of incomplete disease resection.
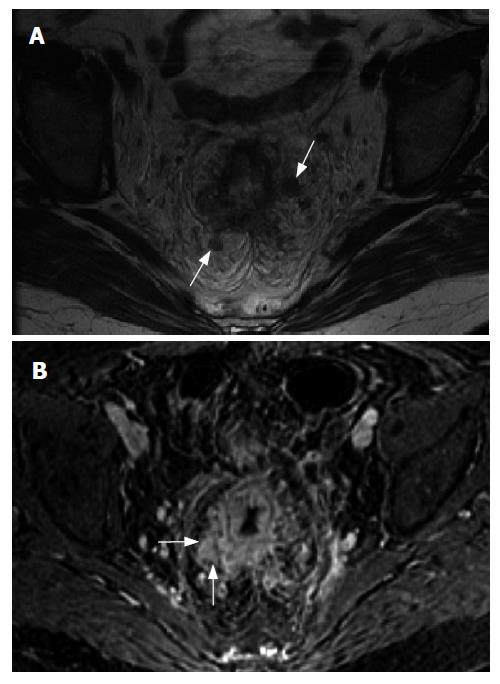
With the advent of neo-adjuvant chemotherapy, pre-operative imaging has become even more essential. If patients are imaged pre-operatively and the extent of their disease determined in this manner, they can be reliably placed into one of several treatment categories. If the tumor is truly superficial (T1 or less) and there is no locally advanced or nodal disease, the patient may be a candidate for transanal endoscopic excision of the tumor[1]. For a deeper tumor which is still confined to the rectum (T2 or T3) and with no nodal disease, total mesorectal excision and postoperative chemoradiation is likely the best choice. For locally advanced tumors which may involve the mesorectal fascia (Figure 1) or very distal tumors, neo-adjuvant therapy is indicated to increase chances of resectability or allow for a sphincter-preserving operation and avoid a permanent colostomy[2].
Preoperative imaging is key in determining the degree of local invasion of a tumor, the presence of nodal metastases, whether there is invasion of the meso-rectal fascia, and the presence of a circumferential resection margin. There are several imaging modalities which have been utilized for this purpose, and there is currently no consensus as to which ones identify preoperative stage most reliably.
Endorectal ultrasound may be the most reliable method for evaluating the degree of tumor invasion into the rectal wall (accuracy 69%-97%)[3]. It is more accurate for superficial tumors than more advanced rectal cancer, since there is a sudden drop off in tissue resolution a certain distance away from the probe. Overstaging can be a problem, as scarring and hematomas from previous biopsy sites can be mistaken for tumor. Also, ultrasound provides limited evaluation of lymph node involvement and the mesorectal excision plane. Lastly, it is highly operator-dependent which can lead to inconsistencies.
Traditionally, CT scan has been used to assess the anatomy of the entire pelvis as well as identify distant metastases. CT has been shown to have high accuracy for locally advanced tumors, but its limited contrast resolution does not allow for detailed evaluation of the rectal wall. It is therefore suboptimal for staging of more superficial tumors. CT is relatively low-cost and more quickly and easily available than MRI, and can survey the whole body in seconds for transmural spread, involvement of surrounding structures, and the presence of distant metastases[4].
Initially, MRI was comparable to CT in the limited degree of resolution of the layers of the rectal wall it provided. With the advent of new MRI technology such as endoluminal and phased array coils, this method has been gradually replacing CT in many institutions for the assessment of local disease. It is superior in identifying layers of the rectal wall and provides a more accurate tumor stage (71%-91%)[5]. Endoluminal coil MRI is not widely available. It also shares some of the limitations of endorectal ultrasound with a limited field of view which does not include mesorectal fascia and surrounding pelvic structures. Also, the positioning of the coil can be a problem in patients with high tumors or tumors which significantly narrow the rectal lumen.
Phased array coil MRI is a surface coil system with four or more coils in the anterior and posterior positions. Each coil produces separate images which are combined to provide high-resolution images. It, therefore, achieves more detailed tissue resolution over a larger field of view. Its accuracy for tumor staging has been reported to be comparable to that of endorectal ultrasound, providing optimal visualization of the anal sphincter complex as well as the anal canal including the levator ani muscle and puborectalis[6]. With the use of external coils, MRI has an accuracy of 65%-86% for tumor staging of rectal cancer[7,8]. It also provides good visualization of the mesorectal fascia, which allows for prediction of a circumferential resection margin (CRM). In one study of 76 patients, accurate prediction of the CRM was close to 100%[9]. It is well established that tumoral invasion of the CRM leads to a high rate of recurrence[10]. The subset of patients with T3 tumors who have CRM involvement benefit from neoadjuvant treatment, and surface coil MRI has been consistently shown to accurately identify CRM involvement. This modality may, therefore, have the most potential in terms of its ability to reliably determine whether immediate resection or neoadjuvant therapy is indicated in patients with borderline tumor stage.
Identifying nodal disease is problematic in any of the previously discussed imaging modalities, since micrometastases are often found in normal sized lymph nodes. Radiologic criteria for abnormal nodes rely on the size and shape of the nodes, and alterations in these characteristics are often not present in rectal cancer[11]. Alternative methods for identifying nodal involvement include positron emission tomography (PET) scanning, which has proven more useful in identifying distant metastases or recurrent rectal cancer than the primary. MR imaging with ultrasmall superparamagnetic iron oxide (USPIO) contrast agents is a new method for the evaluation of nodal metastasis. This is an agent that is taken up by the reticular endothelial system (RES) in normal lymph nodes and decreases T2 signal intensity. In pathologic nodes where the RES is replaced by tumor deposits there are deficits in USPIO uptake and, therefore, increased signal. This technique has been validated in urologic tumors, but its role in rectal cancer is not yet defined[12].
Once patients have been radiographically evaluated and it is determined that neoadjuvant therapy is indicated, the next challenge lies in tumor restaging. This is a significant problem, since radiation causes scarring and fibrosis which can easily be mistaken for tumor and lead to overestimation of the tumor stage. Alternatively, there may be residual cancer beneath normal mural structure which can be missed on MRI and lead to understaging. Chen et al[13] evaluated 50 patients with rectal cancer who had undergone preoperative chemoradiation and found that MRI had a 52% accuracy rate in tumor stage and 68% in node stage. Most of the inaccuracy was caused by overstaging. Kuo et al[14] in a similar series of 36 patients, found accuracy rates of 74% for tumor stage and 64% for node stage, with a similar bias towards overstaging. The authors of both studies concluded that although MRI is valuable in the initial staging of rectal cancer, its accuracy is greatly decreased after chemoradiation.
There are few alternatives to traditional imaging for restaging in patients who have undergone neoadjuvant therapy. Diffusion-weighted magnetic resonance imaging (DWI) creates images with signal intensity that is sensitized to the random motion of free water molecules[15]. Tumor water mobility is altered by chemoradiation; DWI can thereby differentiate radiation-induced fibrosis from residual tumor. Functional imaging modalities such as PET-CT are also useful for restaging, taking advantage of the functional aspects of tumor biology and interpreting this in the context of the radiographic appearance.
Even with preoperative imaging and neoadjuvant chemoradiation, local recurrence rates have still been quoted to be as high as 32%[16]. Considering the high rate of recurrence after curative resection, continued surveillance is crucial. This can be achieved with a variety of biochemical markers and imaging modalities. Local recurrence is a significant problem after resection of rectal cancer, and more consistent preoperative radiologic evaluation is likely to lead to improved rates of complete resection and decreased local recurrence. The role for MRI in elucidating this disease process continues to evolve. As MRI technology advances, it will increase the accuracy of patient stratification into appropriate treatment groups with or without neoadjuvant therapy, thereby improving the efficacy of operative intervention.
Peri-anal abscess and fistula disease are relatively common conditions which can be challenging to manage surgically because of their high recurrence rate after operative therapy. Traditionally, these patients are diagnosed clinically, and examination under anesthesia is the primary method of defining the extent of the disease process. This method often leads to misinterpretation of fistula anatomy and failure to detect complex fistulas, especially in patients with inflammatory bowel disease or recurrent fistula disease. It has been shown that previous fistula surgery, complexity of fistula anatomy, failure to identify the internal opening, wrongly diagnosed primary tracks, and missing secondary tracks are all independent risk factors associated with poor outcome after surgery[17]. It has more recently become the standard of care to obtain preoperative imaging in order to better define fistula anatomy, design a more effective operation, and avoid recurrent disease. Anorectal MRI, CT scan, endoanal ultrasound, and anal fistulography are routinely used in the pre-operative evaluation of these patients.
Anal fistulography and CT scan are suboptimal imaging modalities for this purpose. Fistulography is limited in several ways[18]. Firstly, subtle extensions from the primary tract may not fill with contrast if they are plugged with debris, or if they are simply too remote. Also, there is no visualization of the anal sphincter complex or levator plate. Thus, the relationship of the fistula to the anal sphincters, or a supra- or infra-levator location, cannot be identified[19]. CT attenuation of the anal sphincter and pelvic floor is similar to that of the fistula itself, therefore, it is impossible to see unless it is filled with air or contrast material. CT is only useful in diagnosing fistula-associated abcesses[19].
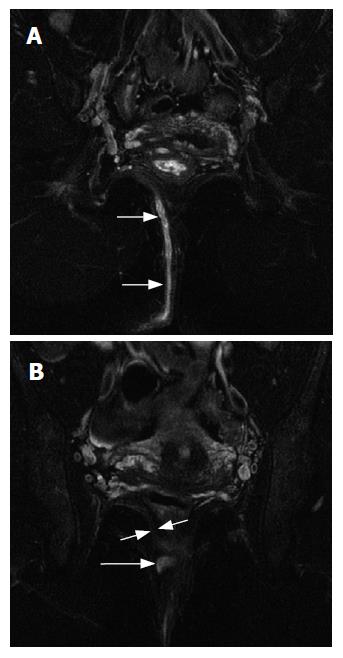
MRI and anal endosonography may be the only viable options for preoperative imaging of anal fistulas (Figure 2). Endosonography is a quick exam which is usually well-tolerated by patients. It is very accurate in identifying the location of the internal opening of the fistula, since this is usually at the tip of the ultrasound probe. Intersphincteric fistulas are very well visualized, and trans-sphincteric fistulas are seen as tracts that cross the external sphincter to reach the ischioanal fossa. Fistula extensions are seen as hypoechoic fluid collections[19].
MRI can be performed with surface coils or endoluminal coils, or a combination of both. The best spatial resolution is achieved by using a dedicated endoanal coil, and this can be combined with a surface coil to increase the field of view. Endoluminal coils are especially useful in evaluating patients with recurrent fistulas or Crohn’s disease. The precise location and size of the internal opening can be clearly described, and ano- or recto-vaginal fistulas can also be visualized. Information about sphincter integrity can also be obtained, which is useful in patients who have had previous fistula surgery and may not have an intact anal sphincter complex. These coils can at times be difficult to place because of anal stenosis or anal pain in this patient population, and in these cases the surface coil alone can provide adequate information[19].
Buchanan et al[20] performed a prospective trial involving 104 patients with suspected fistula in ano who underwent digital examination, anal endosonography, and body-coil MR imaging. Each modality was used independently to classify fistula disease, and compared with an outcome-derived reference standard, which was determined based on MR findings, surgical findings, and outcome after surgery. The proportion of correctly classified fistula anatomy was lowest by clinical examination (61%), better by ultrasound (81%), and best by MRI (97%). Ultrasound showed good resolution of fistulas and their relation to the internal and external anal sphincter muscles, but it did have a limited field of view. The authors concluded that MRI is the most accurate way to define fistula anatomy, but it is also expensive and requires expert interpretation which may not be available in all centers. Anal endosonography is more readily accessible and interpretable and provides a reasonable alternative in the absence of MRI. Discrepancies in endosonography are probably related to operator expertise, as ultrasound is highly operator dependent.
Phased array (PA) MR imaging can provide even more detailed and accurate information when compared with body coil MR. Beets-Tan et al[21] performed high-resolution PA coil MR imaging in 56 patients prior to fistula surgery. Patients were analyzed in groups according to whether they had a primary fistula in the absence of Crohn’s disease (simple fistula), a recurrent fistula or primary fistula with Crohn’s disease (complex fistula). Surgeons started the operation without knowledge of the MRI findings, but findings were revealed to them intra-operatively and then they proceeded with further exploration when necessary. MRI imaging provided important information in 12 of the 56 patients, with the highest benefit being in the groups with Crohn’s and recurrent fistula disease (40% and 24%, respectively) and less benefit in the group with primary fistulas (8%). The authors concluded that in patients with complex or recurrent disease, MRI has a more marked benefit over ultrasound or clinical examination alone, since it has increased sensitivity for detecting abscess and horseshoe extensions.
High-resolution MR fistulography uses image subtraction in a protocol containing a contrast-enhanced, three-dimensional fast low-angle shot (FLASH) sequence. Images are obtained before and after intravenous injection of gadolinium helate contrast agents, and then the images are subtracted which show only enhancing tissues, i.e. the wall of the fistula. Schaefer et al[22] studied 36 patients with clinically diagnosed fistula disease and performed subtraction MR-fistulography preoperatively. MR results were withheld from the surgeons at the time of operation and operative findings were compared with MR findings. Overall there was an 89% complete agreement. Lack of agreement occurred in four patients, all of whom had multiple fistulas and abscesses in the setting of Crohn's disease. The authors concluded that this relatively new technique may be especially useful in evaluating complex anal fistulas in patients with inflammatory bowel disease.
Overall, it seems that MR imaging, with body coil, phased array coils, or subtraction fistulography, has an unequivocal benefit in the preoperative evaluation of patients with complex fistula disease. MRI has the ability to accurately classify disease preoperatively, and alert the surgeon to disease that might have otherwise been missed in patients with recurrent fistulas or Crohn’s disease. For simple primary fistulas, examination under anesthesia alone can be just as effective.
Disorders of the posterior pelvic floor may present with obstructed defecation or fecal incontinence. The pathophysiology of these disorders can involve impaired coordination of skeletal and autonomic muscle activity, or simply muscle weakness and atrophy which can be secondary to obstetric injury or neuropathy. Treatment is dependent on accurate diagnosis of the problem, and imaging is essential in this aspect. Given that this disease process is dynamic in nature, it is necessary to obtain dynamic and anatomic imaging. Traditional methods include endoanal ultrasound and evacuation proctography. Endosonography depicts anal sphincter anatomy and defecography visualizes dynamic pelvic floor motion during simulated defecation.
There are significant limitations in both of these modalities, however. Ultrasound, with its limited field of view, is unable to identify external anal sphincter (EAS) defects and differentiate these from normal anatomic variants. This can be a problem specifically with anterior defects that can occur after obstetric trauma[23]. Its weakness in identifying EAS atrophy is related to an inability to distinguish between similarly echogenic muscle and surrounding peri-rectal fat. Defecography has been criticized for lack of inter-observer reproducibility and the poor relationship of defecographic abnormalities to symptoms[24]. It provides no information about anatomy of pelvic floor musculature or other surrounding organs, and for younger patients, the degree of radiation is a significant limitation[25].
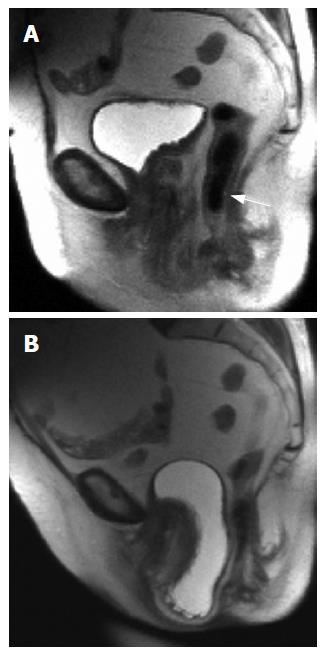
MR imaging has been examined as an alternative to these limited modalities, because of the intrinsic soft tissue contrast that is available with MRI. MRI with an endoanal coil and MRI fluoroscopy has been studied in the evaluation of anatomic and functional deficits. The value of dynamic MRI have been enhanced over the past few years with the advent of surface coils and fast T2-weighted imaging techniques. These advances allow for rapid imaging of the entire pelvic floor at rest and during straining to localize defects (Figure 3)[26].
Fletcher et al[27] studied six patients with fecal incontinence and seven with obstructed defecation. These patients were evaluated with a combination of endoanal coil MRI, endoanal ultrasound, MR fluoroscopy, and scintigraphic defecography. Endoanal MRI was performed using a disposable endorectal colon coil to obtain images in the axial, coronal and sagittal plane. The coil was then removed and 120 mL of ultrasound gel instilled into the rectum. A four-element, phased array coil system was used to obtain dynamic MR proctography during rest, squeeze, and defecation. Once the gel had been fully expressed, further images were taken at rest and during Valsalva to assess for levator hernias and extracolonic abnormalities. In this group of patients, endoanal ultrasound and MRI were comparable in imaging anal sphincter defects, but only MRI revealed atrophy of the external anal sphincter and puborectalis. MR fluoroscopy was found to be superior to scintigraphy in identifying excessive perineal descent. The authors concluded that pelvic MRI is the single modality which can accurately assess anatomical and functional pelvic floor disorders.
MR defecography was also examined by Rentsch et al[1], who studied 20 patients with varied pelvic floor disorders including both fecal incontinence and obstructed defecation. Dynamic images were obtained at a frequency of one image per second during evacuation of a contrast-enhanced gel from the rectum. This technique resulted in the diagnosis of descending perineum, rectocele, cystocele, enterocele, intussusception, and puborectalis dyskinesia. Diagnoses were consistent with clinical results in 77% and additional diagnoses were revealed in 34%. The authors concluded that dynamic MR imaging had a significant impact on the diagnosis and treatment of these disorders.
MR imaging is advantageous in assessing obstructed defecation and fecal incontinence. It provides better details of structural anatomy with good soft tissue contrast to define anatomical planes, and also has superior temporal resolution for the examination of functional abnormalities. Its role may be most essential in patients with complex multi-compartment disorders, as it provides an accurate and detailed representation of the whole pelvis, and can guide interdisciplinary treatment. Although MRI has certain advantages, endoanal ultrasound and evacuation defecography are more widely available and have had a significant impact on the diagnostic approach to pelvic floor disorders.
Preoperative imaging is essential to effective diagnosis and treatment of anorectal cancer, complex fistulas, and pelvic floor disorders. The extent of a tumor can be fully described, and patients appropriately referred for neoadjuvant treatment when indicated. Complex fistulas can be more readily identified and fully explored in the operating room with knowledge gained by preoperative imaging. Pelvic floor disorders are more effectively diagnosed and managed when functional and anatomic studies are performed. The role of imaging has been well established as a valuable tool to be used in conjunction with a thorough history and physical examination in these patients. Advances in imaging technology, largely MRI, contribute to the improvements in outcome that have been seen. There are, however, still significant limitations, such as tumor restaging in patients who have undergone neoadjuvant therapy. Perhaps further advances in technology will enable us to distinguish scar tissue from neoplastic growth, or identify residual tumor lying beneath normal mural structures. Despite the progress that has been made in elucidating anatomical and functional defects in a complex system, there is still significant need for more research.
S- Editor Zhu LH L- Editor Zhu LH E- Editor Lu W
| 1. | Akasu T, Kondo H, Moriya Y, Sugihara K, Gotoda T, Fujita S, Muto T, Kakizoe T. Endorectal ultrasonography and treatment of early stage rectal cancer. World J Surg. 2000;24:1061-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 109] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Chen ET, Mohiuddin M, Brodovsky H, Fishbein G, Marks G. Downstaging of advanced rectal cancer following combined preoperative chemotherapy and high dose radiation. Int J Radiat Oncol Biol Phys. 1994;30:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 131] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Beets-Tan RG, Beets GL. Rectal cancer: review with emphasis on MR imaging. Radiology. 2004;232:335-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 240] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 4. | Matsuoka H, Nakamura A, Masaki T, Sugiyama M, Takahara T, Hachiya J, Atomi Y. Preoperative staging by multidetector-row computed tomography in patients with rectal carcinoma. Am J Surg. 2002;184:131-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Kinkel K, Lu Y, Both M, Warren RS, Thoeni RF. Detection of hepatic metastases from cancers of the gastrointestinal tract by using noninvasive imaging methods (US, CT, MR imaging, PET): a meta-analysis. Radiology. 2002;224:748-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 363] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 6. | Bianchi PP, Ceriani C, Rottoli M, Torzilli G, Pompili G, Malesci A, Ferraroni M, Montorsi M. Endoscopic ultrasonography and magnetic resonance in preoperative staging of rectal cancer: comparison with histologic findings. J Gastrointest Surg. 2005;9:1222-1227; discussion 1227-1228;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Gagliardi G, Bayar S, Smith R, Salem RR. Preoperative staging of rectal cancer using magnetic resonance imaging with external phase-arrayed coils. Arch Surg. 2002;137:447-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Iafrate F, Laghi A, Paolantonio P, Rengo M, Mercantini P, Ferri M, Ziparo V, Passariello R. Preoperative staging of rectal cancer with MR Imaging: correlation with surgical and histopathologic findings. Radiographics. 2006;26:701-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Beets-Tan RG, Beets GL, Vliegen RF, Kessels AG, Van Boven H, De Bruine A, von Meyenfeldt MF, Baeten CG, van Engelshoven JM. Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet. 2001;357:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 549] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 10. | Brown G, Davies S, Williams GT, Bourne MW, Newcombe RG, Radcliffe AG, Blethyn J, Dallimore NS, Rees BI, Phillips CJ. Effectiveness of preoperative staging in rectal cancer: digital rectal examination, endoluminal ultrasound or magnetic resonance imaging? Br J Cancer. 2004;91:23-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Mönig SP, Baldus SE, Zirbes TK, Schröder W, Lindemann DG, Dienes HP, Hölscher AH. Lymph node size and metastatic infiltration in colon cancer. Ann Surg Oncol. 1999;6:579-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Bellin MF, Roy C, Kinkel K, Thoumas D, Zaim S, Vanel D, Tuchmann C, Richard F, Jacqmin D, Delcourt A. Lymph node metastases: safety and effectiveness of MR imaging with ultrasmall superparamagnetic iron oxide particles--initial clinical experience. Radiology. 1998;207:799-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 170] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Chen CC, Lee RC, Lin JK, Wang LW, Yang SH. How accurate is magnetic resonance imaging in restaging rectal cancer in patients receiving preoperative combined chemoradiotherapy? Dis Colon Rectum. 2005;48:722-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 173] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 14. | Kuo LJ, Chern MC, Tsou MH, Liu MC, Jian JJ, Chen CM, Chung YL, Fang WT. Interpretation of magnetic resonance imaging for locally advanced rectal carcinoma after preoperative chemoradiation therapy. Dis Colon Rectum. 2005;48:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Kremser C, Judmaier W, Hein P, Griebel J, Lukas P, de Vries A. Preliminary results on the influence of chemoradiation on apparent diffusion coefficients of primary rectal carcinoma measured by magnetic resonance imaging. Strahlenther Onkol. 2003;179:641-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Sagar PM, Pemberton JH. Surgical management of locally recurrent rectal cancer. Br J Surg. 1996;83:293-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 167] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Garcia-Aguilar J, Belmonte C, Wong WD, Goldberg SM, Madoff RD. Anal fistula surgery. Factors associated with recurrence and incontinence. Dis Colon Rectum. 1996;39:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 320] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 18. | Kuijpers HC, Schulpen T. Fistulography for fistula-in-ano. Is it useful? Dis Colon Rectum. 1985;28:103-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 126] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Halligan S, Stoker J. Imaging of fistula in ano. Radiology. 2006;239:18-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 20. | Buchanan GN, Halligan S, Bartram CI, Williams AB, Tarroni D, Cohen CR. Clinical examination, endosonography, and MR imaging in preoperative assessment of fistula in ano: comparison with outcome-based reference standard. Radiology. 2004;233:674-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 199] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 21. | Beets-Tan RG, Beets GL, van der Hoop AG, Kessels AG, Vliegen RF, Baeten CG, van Engelshoven JM. Preoperative MR imaging of anal fistulas: Does it really help the surgeon? Radiology. 2001;218:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 197] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 22. | Schaefer O, Lohrmann C, Langer M. Assessment of anal fistulas with high-resolution subtraction MR-fistulography: comparison with surgical findings. J Magn Reson Imaging. 2004;19:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Hussain SM, Stoker J, Laméris JS. Anal sphincter complex: endoanal MR imaging of normal anatomy. Radiology. 1995;197:671-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 79] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Wald A, Jafri F, Rehder J, Holeva K. Scintigraphic studies of rectal emptying in patients with constipation and defecatory difficulty. Dig Dis Sci. 1993;38:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Stoker J, Halligan S, Bartram CI. Pelvic floor imaging. Radiology. 2001;218:621-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 120] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Fielding JR. MR imaging of pelvic floor relaxation. Radiol Clin North Am. 2003;41:747-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Fletcher JG, Busse RF, Riederer SJ, Hough D, Gluecker T, Harper CM, Bharucha AE. Magnetic resonance imaging of anatomic and dynamic defects of the pelvic floor in defecatory disorders. Am J Gastroenterol. 2003;98:399-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |