Published online Jun 14, 2007. doi: 10.3748/wjg.v13.i22.3137
Revised: February 1, 2007
Accepted: February 8, 2007
Published online: June 14, 2007
We report a panel of severe inflammatory and vascular intraocular disorders occurring during interferon-alpha (IFN-α) treatment in eight hepatitis C virus (HCV)-infected patients. These events include three cases of Vogt-Koyanagi-Harada like (VKH) disease (an association of panuveitis, retinal detachment, ear and meningeal detachment and skin and hair changes), two cases of central retinal vein occlusion, one case of central retinal artery occlusion, one case of severe hypertensive retinopathy and one case of bilateral ischemic optic neuropathy with severe visual impairment. Rare as they are, such severe ophthalmological complications require a close follow-up of HCV-infected patients under IFN-α treatment with ophthalmological monitoring if any ocular manifestation occurs.
- Citation: Sène D, Touitou V, Bodaghi B, Saadoun D, Perlemuter G, Cassoux N, Piette JC, Hoang PL, Cacoub P. Intraocular complications of IFN-α and ribavirin therapy in patients with chronic viral hepatitis C. World J Gastroenterol 2007; 13(22): 3137-3140
- URL: https://www.wjgnet.com/1007-9327/full/v13/i22/3137.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i22.3137
Treatment of chronic hepatitis C virus (HCV) infection included initially standard interferon-α (IFN-α) given three times a week. Since 2000, it has been proposed to administer pegylated IFN-a once a week[1]. Most frequent side effects include flu-like syndrome, asthenia, and weight loss. Ophthalmological complications are rare. The most typical ocular adverse effect is the IFN-α related retinopathy, which is characterized by cotton wool spots and retinal haemorrhages especially around the optic nerve. Visual loss is usually absent or limited and reversible after interruption of the therapy[2,3]. Involvement of the posterior segment of the eye is rare but may lead to permanent visual loss in the absence of appropriate therapy[4]. In the present report, we prospectively recorded and analyzed eight patients who presented with severe ophthalmological complications during the treatment with IFN-α and ribavirin for chronic hepatitis C. Clinical and angiographic findings were monitored. IFN-α was discontinued in all cases.
All patients were chronically HCV-infected (HCV RNA positive). Epidemiological, clinical and biological features were prospectively recorded. Patients were referred to the ophthalmologist only in case of ocular symptoms. They were managed and followed in a single ophthalmological department. Ophthalmological examination included visual acuity, slit lamp examination, fundoscopy, and fluorescein angiography if necessary. Final diagnosis and therapeutic management were collegially assumed. Severe ophthalmological complications during IFN-α therapy included inflammatory ocular diseases [3 cases of Vogt-Koyanagi-Harada (VKH) like disease] and vascular disorders (5 cases), including central retinal vein occlusion (CRVO) in 2, central retinal artery occlusion (CRAO) in 2, severe hypertensive retinopathy in 1 and ischemic optical neuritis in 1. The three cases of VKH-like disease have been already reported elsewhere[5].
The intraocular inflammatory disorders reported herein are three cases of VKH disease. The VKH disease is a rare autoimmune disorder with an ocular involvement, mainly granulomatous panuveitis associated with exudative retinal detachments, with skin and hair (vitiligo, poliosis and alopecia) changes and ear and meningeal involvement (meningitis, cranial nerve palsy, focal signs, dysacusis, hearing loss). The diagnosis is confirmed by the retinal fluorescein angiography that shows typical pin-points and bilateral serous retinal and pigmented epithelial detachments.
VKH-like disease after 4 mo of pegylated (PEG)-IFNα-2b therapy: A 43-year-old woman, HCV-infected (genotype 3) since 1982 (after blood transfusion), had a non-symptomatic mixed cryoglobulinemia and significant liver histological damages at liver biopsy (Metavir A1F2), which required pegylated IFN-α (PEG-IFN-α) (1.5 μg/kg per week) and ribavirin (10.5 mg/kg per day). Four months later, she was admitted for decreased vision of the left eye. Visual acuity was 20/20 OD and 20/200 OS. Fundus examination disclosed a left macular edema and bilateral serous retinal detachment. Fluorescein angiography revealed several pin-points, bilateral serous retinal and pigmented epithelial detachments. VKH-like disease was diagnosed. PEG-IFN and ribavirin were both discontinued. Intravenous pulses of methylprednisolone (1 g/d, 3 d) were performed, followed by oral prednisone (1 mg/kg per day). One month later, visual acuity remained 20/20 P2 for the right eye and improved to 20/40 P2 for the left eye. Retinal detachments disappeared. Seven months later, visual acuity was 20/20 P2 OD, 20/25 P2 OS and prednisone was slowly tapered. After one year of treatment, steroids were stopped without any ocular relapse.
Clinical features of the patients with VKH disease occurring during IFN therapy are reported in Table 1.
| Case 1 | Case 2 | Case 3 | |
| Age (yr) | 43 | 51 | 42 |
| Sex | F | F | M |
| HCV genotype liver biopsy (Metavir)1 | 3 | 1 | 1 |
| A1F2 | A1F2 | A3F1 | |
| Anti-HCV therapy | PEG-IFN α-2b + Ribavirin | PEG-IFN α-2b + Ribavirin | PEG-IFN α-2b + Ribavirin |
| Interval before first ocular manifestations2 | 4 mo | 3 mo | 4 mo |
| Ocular manifestations | -Visual acuity 20/200 OS | -Bilateral vision loss | -Bilateral vision loss |
| -Macular edema and a bilateral serous retinal detachment. | -Bilateral uveitis, major papillar and retinal edema | -Episcleritis and bilateral uveitis | |
| Retinal fluorescein angiography | Pin-points and bilateral serous retinal and pigmented epithelium detachments, suggestive of a Vogt-Koyanagi-Harada like [VKH] disease | ||
| Therapeutic management | -PEG-IFN and ribavirin disruption | -PEG-IFN and ribavirin disruption | -PEG-IFN and ribavirin disruption |
| -Methylprednisolone IV and per os | - Methylprednisolone IV and per os | - Methylprednisolone IV and per os | |
| Course | -Complete recovery under low dose steroids (< 10 mg/d) | -Low improvement of ocular lesions | -Partial improvement of ocular lesions |
| -Steroids were stopped after one year of treatment without ocular relapse. | -Cortico-dependency > 25 mg/d | -Cortico-dependency > 25 mg/d | |
| -Failure of cyclosporine course | -Re-introduction of PEG-IFN and ribavirin 5 mo later3 | ||
| -Introduction of azathioprine | -Full recovery of ocular manifestations 10 mo after IFN was reintroduced | ||
Five patients presented with severe intraocular compli-cations under PEG-IFN + Ribavirin treatment, including 2 cases of central retinal vein occlusion, one case of central retinal artery occlusion, one case of acute anterior ischemic optic neuritis and one case of severe exudative hypertensive retinopathy. The common hallmark of all these cases is the complete or severe and definitive vision loss despite the treatment withdrawal and an adequate therapeutic management. This reflects the severity of such complications. Clinical features of the patients with intraocular vascular side effects during IFN therapy are reported in Table 2.
| Main features | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 |
| Age (yr) | 51 | 70 | 55 | 40 | 40 |
| Sex | M | M | F | M | M |
| HCV genotype | 1 | 2 | |||
| Liver biopsy (Metavir) | ND | F4 | A2F1 | A2F2 | F4 (clinical cirrhosis) |
| Antecedents | Sarcoidosis | Arterial hypertension | - | Splenic lymphoma with villous lymphocytes | Hypertension with past hypertensive retinopathy |
| Smoking | Mixed cryoglobulin- associated glomerulonephritis | ||||
| Severe arterial hypertension | |||||
| Dyslipidemia, smoking | |||||
| Anti-HCV therapy | PEG-IFN α-2b + Ribavirin | PEG-IFN α-2b + Ribavirin | PEG-IFN α-2b + Ribavirin | Standard IFN α-2b + ribavirin | Standard IFN α-2b + Ribavirin |
| Interval before first ocular manifestations | 7 mo | 5 mo | 6 mo | 18 mo | 6 mo |
| Ocular manifestations | -Initial visual acuity: OD (< 20/200), OS (20/20) | -OD vision loss (20/200 OD; 20/20 OS) | -Bilateral vision loss (20/400 OD, 20/80 OS) | -Visual acuity OS: 10/10; OD: < 20/200 | -Bilateral vision loss (20/64 P2 OD, 20/200 OS) |
| -Papillar edema, macular edema and retinal hemorrhages | -Bilateral macular edema and retinal hemorrhages | ||||
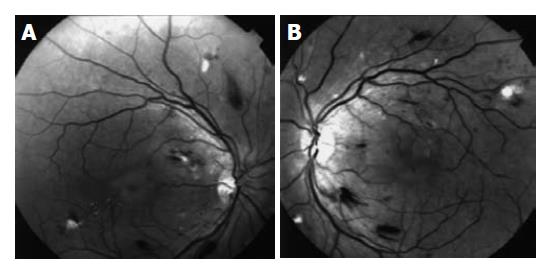
| -Cotton wool spots | -Cotton-wool spots (IFN-α-induced retinopathy) (Figure 1A and B) | ||||
| Diagnosis | Central retinal vein occlusion OD | Central retinal vein occlusion OD | Acute anterior ischemic optic neuritis | Central retinal artery occlusion OD | Exsudative hypertensive and IFN -induced retinopathy |
| Treatment | -Withdrawal of PEG-IFN and ribavirin | -Withdrawal of PEG-IFN and ribavirin | -Withdrawal of PEG-IFN and ribavirin | -Withdrawal of standard IFN and ribavirin | -Withdrawal of standard IFN and ribavirin |
| -Steroids and IV heparin | -Steroids, IV heparin and aspirin | -Steroids | -Steroids and IV heparin | -Better control of hypertension (nadolol, benazepril) | |
| Course | -6 mo later, radiary neurotomy | -2 mo later, radiary neurotomy | -At the end of follow-up, severe visual impairment (< 20/400 OD; 20/80 OS) | -4 mo later, slow improvement (20/64 OD) | -2 mo later, significant improvement of the visual acuity (20/40 OD; 20/40 OS) |
| -At the end of follow-up, definitive loss of vision OD (< 20/200) | -At the end of follow-up, definitive vision loss OD | -Died of severe sepsis 5 mo later | -Introduction of PEG- IFN α-2b and Ribavirin without recurrence after more than 1 yr follow-up |
Most of ophthalmological side effects occurring during IFN-α treatment are benign, transient, and are mainly represented by the classical IFN-related retinopathy. In the present work, we describe two mechanisms of severe and sight-threatening ocular complications during IFN therapy: the first group of complications includes inflammatory disorders and the second group vascular intraocular diseases. Concerning the cases of inflammatory intraocular disorders, we report three cases of VKH-like disease-defined by the association of a panuveitis with exudative retinal detachment, skin and hair changes and ear and meningeal involvement- occurring during IFN-α treatment for HCV infection. In all cases, high doses of steroids were required and sometimes associated with an immunosuppressive drug (azathioprine, cyclophosphamide, and cyclosporine). These complications mostly resulted in a definitive and severe visual impairment.
VKH-like disease during interferon therapy is rare, and to our knowledge, apart from our three patients[5], only three cases of VKH under an IFN-α course have been reported[6,7]. This evidence and the third patient of our report who benefited from a second course of PEG-IFN for both HCV-infection and VKH disease, show the complex relationship between IFN-α course and VKH disease. However, considering the severity of the visual impairment which can be induced by such a syndrome, ophthalmological examination should be systematically proposed during IFN treatment. In case of intraocular inflammation, the diagnosis of VKH-like disease must be considered and interferon therapy can be disrupted.
Retinal vascular disorders associated with IFN treatment include central retinal venous and central retinal arterial occlusion, and severe hypertensive retinopathy. Only few cases have been reported[8,9]. Arterial and venous occlusions were associated with a severe visual defect that did not improve despite the combination of heparin, steroids and the withdrawal of the IFN therapy. Most of arterial occlusive events occurred in presence of ill-controlled vascular risk factors, such as hypertension, dyslipidemia and smoking.
Finally, cases of ischemic optic neuritis have been reported in HCV-infected patients under IFN-α[10] and may impair dramatically visual functions. Predisposing factors are not clearly identified, except for classical vascular risk factors. These data point out the necessary control of known vascular risk factors before and during IFN-α treatment.
Other ophthalmological complications of IFN-α therapy include transient blurred vision, increased intraocular pression, neovascular glaucoma, and “specific IFN-α”-related retinopathy characterized by cotton wool spots, retinal hemorrhages, and microaneurysms[11-13]. Functional abnormalities seemed also to be frequent under IFN-α but without clinical expression[12].
Besides previous case reports of ocular side effects of IFN-α therapy, our study raises the possibility of severe ophthalmological manifestations (occlusion of retinal, choroidal or optic nerve vessels, or VKH-like diseases). These results confirm the potential severity of intraocular complications during IFN-α therapy in HCV-infected patients. A close ophthalmological monitoring and efficient control of systemic and ocular vascular risk factors (hypertension, diabetes, and dyslipidemia) seem mandatory before further IFN reintroduction.
S- Editor Wang J L- Editor Ma JY E- Editor Lu W
| 1. | Zeuzem S, Feinman SV, Rasenack J, Heathcote EJ, Lai MY, Gane E, O'Grady J, Reichen J, Diago M, Lin A. Peginterferon alfa-2a in patients with chronic hepatitis C. N Engl J Med. 2000;343:1666-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 883] [Cited by in RCA: 851] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 2. | Zegans ME, Anninger W, Chapman C, Gordon SR. Ocular manifestations of hepatitis C virus infection. Curr Opin Ophthalmol. 2002;13:423-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Abe T, Nakajima A, Satoh N, Koizumi T, Sakuragi S, Ono T, Komatsu M, Masamune O. Clinical characteristics of hepatitis C virus-associated retinopathy. Jpn J Ophthalmol. 1995;39:411-419. [PubMed] |
| 4. | Perlemuter G, Cacoub P, Sbaï A, Hausfater P, Thibault V, Le TH, Wechsler B, Buffet C, Piette JC. Hepatitis C virus infection in systemic lupus erythematosus: a case-control study. J Rheumatol. 2003;30:1473-1478. [PubMed] |
| 5. | Touitou V, Bodaghi B, Cassoux N, Tran TH, Rao NA, Cacoub P, LeHoang P. Vogt-Koyanagi-Harada disease in patients with chronic hepatitis C. Am J Ophthalmol. 2005;140:949-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Papastathopoulos K, Bouzas E, Naoum G, Vergados I, Tsiodras S. Vogt-Koyanagi-Harada disease associated with interferon-A and ribavirin therapy for chronic hepatitis C infection. J Infect. 2006;52:e59-e61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Sylvestre DL, Disston AR, Bui DP. Vogt-Koyanagi-Harada disease associated with interferon alpha-2b/ribavirin combination therapy. J Viral Hepat. 2003;10:467-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Ríos-Rull P, Rubio M, Ojeda E, Hernández Navarro F. [Hepatitis-C-positive mixed essential cryoglobulinemia, autoimmune hemolytic anemia, and immune thrombocytopenic purpura]. Sangre (Barc). 1994;39:225. [PubMed] |
| 9. | Nadir A, Amin A, Chalisa N, van Thiel DH. Retinal vein thrombosis associated with chronic hepatitis C: a case series and review of the literature. J Viral Hepat. 2000;7:466-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Purvin VA. Anterior ischemic optic neuropathy secondary to interferon alfa. Arch Ophthalmol. 1995;113:1041-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 67] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Guyer DR, Tiedeman J, Yannuzzi LA, Slakter JS, Parke D, Kelley J, Tang RA, Marmor M, Abrams G, Miller JW. Interferon-associated retinopathy. Arch Ophthalmol. 1993;111:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 172] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Manesis EK, Moschos M, Brouzas D, Kotsiras J, Petrou C, Theodosiadis G, Hadziyannis S. Neurovisual impairment: a frequent complication of alpha-interferon treatment in chronic viral hepatitis. Hepatology. 1998;27:1421-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Farel C, Suzman DL, McLaughlin M, Campbell C, Koratich C, Masur H, Metcalf JA, Robinson MR, Polis MA, Kottilil S. Serious ophthalmic pathology compromising vision in HCV/HIV co-infected patients treated with peginterferon alpha-2b and ribavirin. AIDS. 2004;18:1805-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Touitou V, Escande C, Bodaghi B, Cassoux N, Wechsler B, Lemaitre C, Tran TH, Fardeau C, Piette JC, LeHoang P. [Diagnostic and therapeutic management of Vogt-Koyanagi-Harada syndrome]. J Fr Ophtalmol. 2005;28:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |