Published online Jun 14, 2007. doi: 10.3748/wjg.v13.i22.3133
Revised: February 22, 2007
Accepted: March 8, 2007
Published online: June 14, 2007
AIM: To develop a practical method for isolation, purification and culture of hepatic Kupffer cells (KCs) and to observe their suppressive effects on the proliferation of alloreactive T cells.
METHODS: Perfusion in situ in vivo combined with density gradient centrifugation was applied in isolation, purification and culture of hepatic KC. The suppression by KCs on the T cell proliferation in mixed lymphocyte reaction (MLR) was observed.
RESULTS: This method resulted in a satisfactorily high yield of (1.1 ± 0.2) × 107 KCs per liver, (93.5% ± 1.8%) viable cells, over 90% purity and positive for ED-2. After the first 24 h in culture, a great number of KCs which exhibited typical characteristics were observed. Using 3H-TdR incorporation assay, non-irradiated KCs significantly suppressed allo-MLR. The KCs recovered from accepted liver allografts in groups D and E were more effective in suppressing allo-MLR.
CONCLUSION: A standardized procedure for isolation of highly purified rat KCs is proposed and KCs have suppressive effects on the proliferation of alloreactive T cells, especially those derived from accepted liver allografts.
- Citation: Liu H, Cao H, Wu ZY. Isolation of Kupffer cells and their suppressive effects on T lymphocyte growth in rat orthotopic liver transplantation. World J Gastroenterol 2007; 13(22): 3133-3136
- URL: https://www.wjgnet.com/1007-9327/full/v13/i22/3133.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i22.3133
Kupffer cells (KCs), the resident macrophage population in the liver, which comprise one of the major population (20%) of the hepatic nonparenchymal cell fraction, may play an important role in immunomodulation and the induction of tolerance after liver transplantation. The inherent tolerogenicity of the liver poses important questions about how immune reactivity in the liver is regulated. The interaction between KC and lymphocytes in the liver sinusoids was described first by Gassel[1], who showed that KCs within the hepatic sinusoidal space are in physical contact with two lymphocytes at a time. Therefore, increasing attention has focused on the key role of hepatic allograft-derived KCs in regulating immune responses and facilitating tolerance induction. The primary aim of this study was to develop a practical method for isolation, purification and culture of hepatic KCs and to observe their suppressive effects on the proliferation of alloreactive T cells.
Inbred male Sprague-Dawley rats weighing 230-250 g and Wistar rats weighing 250-270 g served as donors and recipients, respectively. All animals were purchased from the Animal Resource Center of Science Academy of China in Shanghai. Animals were cared for under a protocol approved by the Jiao Tong University Animal Ethics Committee.
Mouse anti-rat ED2 antibody was purchased from Serotec Inc. (UK) and goat anti-mouse ED2 antibody was provided by Jing Mei Biological Inc. (Beijing, China). The other reagents included RPMI-1640 (Gibco Inc., USA), IV-type collagenase (Sigma Inc., USA), Percoll’s solution (Pharmacia Inc., Switzerland), etc.
Orthotopic rat liver transplantation was performed by the cuff technique without hepatic artery reconstruction as described by Kamada[2]. Recipients were divided into five groups: group A (control group, SD→SD); group B (SD→Wistar without any immunosuppression); group C (SD→Wistar, CsA from d 1 to d 5); group D (SD→Wistar, CsA from d 1 to d 5 and anti-CD40L mAb on d 0 and d 2); and group E (SD→Wistar, the same as group D in combination with donor specific blood transfusion). CsA was diluted in normal saline to 1 g/L and injected into the recipients subcutaneously once a day (1.5 mg/kg per day). Anti-CD40L mAb was administered intraperitoneally once a day (1 mg/kg per day).
Non-parenchymal cell (NPC) suspensions were acquired by collagenase in situ collagenase perfusion of the liver and then KCs were isolated by sedimentation in a two-step Percoll gradient with selective adherence of cells to plastic flasks. The morphologic features of KCs were observed under light microscopy and the viability was determined by trypan blue exclusion. In addition, purity of the KC fraction was determined by ED-2 staining.
We prepared suspensions of spleen cells by squeezing mechanically dissociated spleen through a 50-μm stainless steel screen followed by erythrocyte lysis with Tris-ammonium chloride and washings in PPMI 1640. T cells were purified further by passage over nylon wool columns.
Mixed lymphocyte reaction (MLR) was performed: 2.5 × 105 SD T cells and 2.5 × 105 Wistar T cells from normal rats were cocultured in 96-well plate in the presence of 3 × 104 KCs or γ-ray irradiated KCs as control. All cultures were incubated for 6 d at 37°C in a 5% CO2-humidified air atmosphere and 18 h before harvesting, One μCi of 3H-thymidine was added into each well. Incorporation of 3H-TdR into DNA was assayed by liquid scintillation counting system. Based on the above experiment, 3 × 104 KCs derived from the different groups on the 6th day after operation were added into the above-mentioned MLR system and their suppressive effects on T cell proliferation in vitro were observed.
The data of liquid scintillation counting were expressed in mean ± SD and ANOVA was applied for comparison in different groups. A P value less than 0.05 was considered significant.
Viability and purity: The technique of cell isolation in this study yielded about (1.1 ± 0.2) × 107 KCs per liver with (93.5 ± 1.8)% viability determined by trypan blue exclusion. The purity of KC fraction was consistently ≥ 90% determined by morphology in combination with ED2 staining.
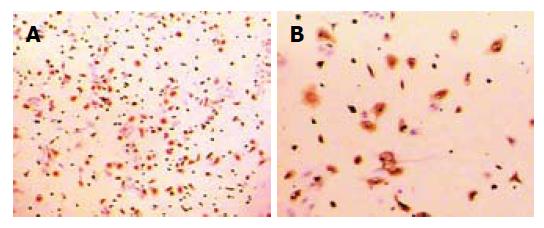
Morphology and immunohistobiochemical staining: The freshly isolated cells had a ball-like shape, 20 μm in diameter when viewed under light microscopy. About 3-4 h later, most of them adhered to the wall of plastic flasks and 48 h later they became larger, prominent and showed typical macrophage morphologic features with irregular shape, transparent cytoplasm and kidney-like nucleus (Figure 1A and B). By immunohistobiochemical technique, the cells were stained positively for ED2 (Figure 2A and B).
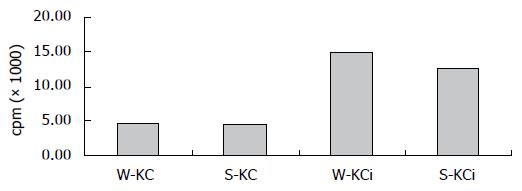
Effects of irradiated and non-irradiated KCs on T lymphocyte proliferation: To observe the effect of KCs on T cell response, we examined T cell proliferation in two-way MLR. By 3H-TdR incorporation assay, non-irradiated KCs showed significantly suppressive effects on T cell proliferation in MLR. However, when irradiated KCs were used, there was a marked decrease in the inhibitory effect upon the MLR (Figure 3).
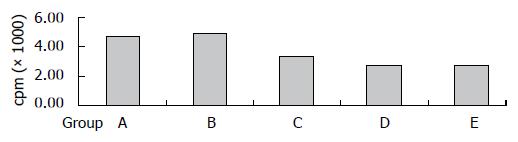
Effects of KCs derived from liver allograft on T lymphocyte proliferation: The KCs derived from liver allograft of different groups were added into the MLR system in order to determine the effects of those cells. The results showed that there was a marked decrease of cpm value in MLR when KCs from groups D and E were present, i.e., KCs derived from those two groups had more significantly suppressive effects on T cell proliferation. The cpm of different groups was 4810 ± 132, 5036 ± 315, 3480 ± 201, 2825 ± 118 and 2786 ± 122, respectively (Figure 4).
Kupffer cells (KCs), the most important resident macrophages of the liver, comprise one of the major populations (about 20%) of the hepatic nonparenchymal cell fraction, and the tolerogenic properties of the liver are generally accepted[3]. Therefore increased attention has focused on the possible role and mechanism of KCs from liver allograft in tolerance induction[4,5]. In recent years, some authors have put forward a novel KC-dependent immunomodulatory mechanism which may be strengthened markedly after liver transplantation, especially those donor-derived KCs expressing Fas ligand which exhibit immune privilege in vivo[6]. But precise mechanisms accounting for this phenomenon have not been illustrated.It is of great importance to establish a kind of stable, economical and reliable technique of KC isolation and culture for the study of tolerance induction and immune regulation. Various methods have been applied and basic steps include collagenase in situ perfusion of the liver, sedimentation in two-step Percoll gradient and selective adherence. The isolated KCs should be kept with biological and immunological characteristics in vivo such as phagocytosis, lysosomal enzymes and expression of specific antigens[7-9].
By collagenase in situ perfusion in combination with gradient centrifugation, we successfully established a method of isolating, purifying and culturing KCs. And the technique in this study yielded about (1.1 ± 0.2) × 107 KCs per liver with a 93.5% ± 1.8% viability and a consistently ≥ 90% purity. Twenty-four hours later, single layer of KCs formed with an irregular shape and kidney-like nucleus. Forty-eight h later KCs increased and became larger, more prominent and showed typical macrophage morphologic features with irregular shape, transparent cytoplasm and kidney-like nucleus. With immunohistobiochemical techniques, the cells were stained positively for ED2. The isolated KCs could survive as long as about 4 weeks while keeping biological and immunological characteristics in vitro.
In comparison with the literature, we made some modification in isolation and culture of KCs. Firstly, we blocked the suprahepative inferior vena cava (IVC) while perfusing collagenase in situ. Secondly, we increased perfusion velocity in order to wash off thoroughly erythrocytes in sinusoid. And thirdly, digestion of liver cells in vitro was applied. Collagenase and Pronase E were used at the same time when isolating KCs both at home and abroad[10-12]. Pronase E will destroy CD14 molecules on the surface of KCs which are independent in the activation of macrophages by LPS so that the isolated KCs may not keep physiological functions[13,14]. Therefore, only collagenase was used in perfusion of liver. The results showed that the technique in our study was able to yield sufficient number of KCs with high viability and purity.
On the basis of the successful isolation and culture of KCs, we observed suppressive effects of KCs on T lymphocyte proliferation in vitro. Non-irradiated KCs showed significant suppressive effects on T cell proliferation in MLR while there was a marked decrease in the inhibitory effect upon the MLR when irradiated KCs pretreated by 100Gy γ-ray were used. When KCs derived from liver allograft of groups D and E were added into the MLR system, there was a marked decrease of cpm value, i.e., KCs derived from those two groups had more significantly suppressive effect on T cell proliferation. Administration of KCs derived from chronically accepted liver allografts might be able to significantly prolong the survival of hepatic allografts in an acute rejection model in an alloantigen-specific manner[15-17]. There are different explanations for this phenomenon. FasL expression of KCs is increased after liver transplantation which is associated with T lymphocyte apoptosis through Fas-FasL pathway[18]. At the same time, KCs can increase the secretion of IL-10 and TGF-β by up-regulating the expression of Th2/Th3 cytokines mRNA so that KCs may regulate the differentiation of Th2/Th3 cells[19,20]. And KC itself secretes an array of cytokines such as IL-4, IL-10, IL-12 and TGF-β which may drive the differentiation of T cell subsets towards Th2/Th3[21]. Because Th2 cells are not sensitive to FasL-induced apoptosis, selective Th2 survival coupled with rapid death of Th1 cells may be a mechanism for differential regulation of the two T cell subsets by KCs[22,23]. Due to the restriction of instruments in our lab, mechanisms proposed have not yet been validated convincingly.
In conclusion, KCs had significantly suppressive effects on allo-reactive T lymphocyte proliferation, especially those recovered from chronically accepted liver allografts. The hepatic professional and non-professional APC including KCs, liver sinusoidal endothelial cells(LSEC), DC, even hepatocytes may play key roles in regulating immune responses and facilitating tolerance induction after liver transplantation[24]. The precise mechanism needs to be further investigated in the future.
S- Editor Liu Y L- Editor Ma JY E- Editor Liu Y
| 1. | Gassel HJ, Engemann R, Thiede A, Hamelmann H. Replacement of donor Kupffer cells by recipient cells after orthotopic rat liver transplantation. Transplant Proc. 1987;19:351-353. [PubMed] |
| 2. | Kamada N, Calne RY. A surgical experience with five hundred thirty liver transplants in the rat. Surgery. 1983;93:64-69. [PubMed] |
| 3. | Thomson AW, O'Connell PJ, Steptoe RJ, Lu L. Immunobiology of liver dendritic cells. Immunol Cell Biol. 2002;80:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Everett ML, Collins BH, Parker W. Kupffer cells: another player in liver tolerance induction. Liver Transpl. 2003;9:498-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Meyer D, Löffeler S, Otto C, Czub S, Gassel HJ, Timmermann W, Thiede A, Ulrichs K. Donor-derived alloantigen-presenting cells persist in the liver allograft during tolerance induction. Transpl Int. 2000;13:12-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Sun Z, Wada T, Maemura K, Uchikura K, Hoshino S, Diehl AM, Klein AS. Hepatic allograft-derived Kupffer cells regulate T cell response in rats. Liver Transpl. 2003;9:489-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Olynyk JK, Clarke SL. Isolation and primary culture of rat Kupffer cells. J Gastroenterol Hepatol. 1998;13:842-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Smedsrød B, Pertoft H, Eggertsen G, Sundström C. Functional and morphological characterization of cultures of Kupffer cells and liver endothelial cells prepared by means of density separation in Percoll, and selective substrate adherence. Cell Tissue Res. 1985;241:639-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Valatas V, Xidakis C, Roumpaki H, Kolios G, Kouroumalis EA. Isolation of rat Kupffer cells: a combined methodology for highly purified primary cultures. Cell Biol Int. 2003;27:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Gong JP, Wu CX, Liu CA, Li SW, Shi YJ, Yang K, Li Y, Li XH. Intestinal damage mediated by Kupffer cells in rats with endotoxemia. World J Gastroenterol. 2002;8:923-927. [PubMed] |
| 11. | Peng Y, Gong JP, Liu CA, Li XH, Gan L, Li SB. Expression of toll-like receptor 4 and MD-2 gene and protein in Kupffer cells after ischemia-reperfusion in rat liver graft. World J Gastroenterol. 2004;10:2890-2893. [PubMed] |
| 12. | Ikejima K, Enomoto N, Seabra V, Ikejima A, Brenner DA, Thurman RG. Pronase destroys the lipopolysaccharide receptor CD14 on Kupffer cells. Am J Physiol. 1999;276:G591-G598. [PubMed] |
| 13. | Cavalieri B, Perrelli MG, Aragno M, Ramadori P, Poli G, Cutrìn JC. Ischaemic preconditioning modulates the activity of Kupffer cells during in vivo reperfusion injury of rat liver. Cell Biochem Funct. 2003;21:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Enomoto K, Nishikawa Y, Omori Y, Tokairin T, Yoshida M, Ohi N, Nishimura T, Yamamoto Y, Li Q. Cell biology and pathology of liver sinusoidal endothelial cells. Med Electron Microsc. 2004;37:208-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Callery MP, Kamei T, Flye MW. Kupffer cell blockade inhibits induction of tolerance by the portal venous route. Transplantation. 1989;47:1092-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Bittmann I, Bottino A, Baretton GB, Gerbes AL, Zachoval R, Rau HG, Löhrs U. The role of graft-resident Kupffer cells and lymphocytes of donor type during the time course after liver transplantation--a clinico-pathological study. Virchows Arch. 2003;443:541-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Akamatsu Y, Ohkohchi N, Doi H, Satomi S. Effect of elimination of donor Kupffer cells and/or recipient macrophages on acute rejection in liver transplantation. Hepatogastroenterology. 2003;50:1105-1110. [PubMed] |
| 18. | Kwekkeboom J, Kuijpers MA, Bruyneel B, Mancham S, De Baar-Heesakkers E, Ijzermans JN, Bouma GJ, Zondervan PE, Tilanus HW, Metselaar HJ. Expression of CD80 on Kupffer cells is enhanced in cadaveric liver transplants. Clin Exp Immunol. 2003;132:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Ohkohchi N. Suppression of Kupffer cell function is a key for liver transplantation from the non-heart-beating donor. Transplant Proc. 2001;33:3728-3731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Kupiec-Weglinski JW, Busuttil RW. Ischemia and reperfusion injury in liver transplantation. Transplant Proc. 2005;37:1653-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 185] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 21. | Rentsch M, Puellmann K, Sirek S, Iesalnieks I, Kienle K, Mueller T, Bolder U, Geissler E, Jauch KW, Beham A. Benefit of Kupffer cell modulation with glycine versus Kupffer cell depletion after liver transplantation in the rat: effects on postischemic reperfusion injury, apoptotic cell death graft regeneration and survival. Transpl Int. 2005;18:1079-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Martinez OM, Rosen HR. Basic concepts in transplant immunology. Liver Transpl. 2005;11:370-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Zhu XH, Qiu YD, Shen H, Shi MK, Ding YT. Effect of matrine on Kupffer cell activation in cold ischemia reperfusion injury of rat liver. World J Gastroenterol. 2002;8:1112-1116. [PubMed] |
| 24. | Nakamitsu A, Hiyama E, Imamura Y, Matsuura Y, Yokoyama T. Kupffer cell function in ischemic and nonischemic livers after hepatic partial ischemia/reperfusion. Surg Today. 2001;31:140-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |