Published online Jun 14, 2007. doi: 10.3748/wjg.v13.i22.3122
Revised: April 1, 2007
Accepted: April 9, 2007
Published online: June 14, 2007
AIM: To evaluate the efficacy of telomerase activity assay and peritoneal lavage cytology (PLC) examination in peritoneal lavage fluid for the prediction of peritoneal metastasis in gastric cancer patients, and to explore the relationship between telomerase activity and proliferating cell nuclear antigen expression.
METHODS: Telomeric repeated amplification protocol (TRAP)-enzyme-linked immunosorbent assay (ELISA) was performed to measure the telomerase activity in 60 patients with gastric cancer and 50 with peptic ulcer. PLC analysis of the 60 patients with gastric cancer was used for comparison. The proliferating cell nuclear antigen (PCNA) in gastric carcinoma was immunohistochemically examined.
RESULTS: The telomerase activity and PLC positive rate in peritoneal lavage fluid from patients with gastric cancer was 41.7% (25/60), and 25.0% (15/60), respectively. The positive rate of telomerase activity was significantly higher than that of PLC in the group of pT4 (15/16 vs 9/16, P < 0.05), P1-3 (13/13 vs 9/13, P < 0.05) and diffuse type (22/42 vs 13/42, P < 0.05). The patients with positive telomerase activity, peritoneal metastasis, and serosal invasion had significantly higher levels of average PCNA proliferation index (PI), (55.00 ± 6.59 vs 27.43 ± 7.72, 57.26 ± 10.18 vs 29.15 ± 8.31, and 49.82 ± 6.74 vs 24.65 ± 7.33, respectively, P < 0.05).
CONCLUSION: The TRAP assay for telomerase activity is a useful adjunct for cytologic method in the diagnosis of peritoneal micrometastasis and well related to higher proliferating activity of gastric cancer. The results of this study also suggest a promising future therapeutic strategy for treating peritoneal dissemination based on telomerase inhibition.
- Citation: Da MX, Wu XT, Guo TK, Zhao ZG, Luo T, Qian K, Zhang MM, Wang J. Clinical significance of telomerase activity in peritoneal lavage fluid from patients with gastric cancer and its relationship with cellular proliferation. World J Gastroenterol 2007; 13(22): 3122-3127
- URL: https://www.wjgnet.com/1007-9327/full/v13/i22/3122.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i22.3122
In spite of improvement in postoperative survival with advances in surgical techniques and multimodal adjuvant therapy, gastric cancer nevertheless, remains a top killer among cancers of the gastrointestinal tract[1]. The postoperative survival rate of patients with advanced gastric cancer is very low[2-4]. Peritoneal dissemination is the most frequent pattern of recurrence in patients with gastric carcinoma[5]. About 50%-60% of gastric cancer patients with serosal invasion after curative resection eventually developed peritoneal metastases[6,7]. The incidence of peritoneal recurrence after curative resection was 13.46% and the survival after peritoneal recurrence was 4.9 mo in average[8]. The 5-year survival rate of patients with positive peritoneal lavage cytology was only 2%[9]. However, the mechanism of peritoneal dissemination has not yet been clearly defined so far. Free cancer cells exfoliated from cancer lesions have been postulated as the main cause of metastasis[5,8-10].
Recent advances on the molecular mechanisms of peritoneal dissemination have elucidated some of the target molecules and the development of new multimodal therapies in an attempt to eliminate peritoneal exfoliated free cancer cells has also improved survival[11]. Of course, it is very important to develop reliable methods for the accurate selection of patients for whom adjuvant intraperitoneal therapy will be required. PLC examination has been generally considered as the gold standard for predicting peritoneal recurrences and is seen as one of the most accurate prognostic tools[10]. In the absence of macroscopic peritoneal metastases, positive cytological results have been shown to correlate with the survival of patients with advanced gastric cancer[12]. However, the sensitivity of this assay is relatively low[13-15], ranging from 21%-35%[9]. Some patients with negative cytological findings have been diagnosed later with peritoneal metastasis[15]. Hence, further studies are required to eliminate the above limitations to improve the accuracy of this technique.
Telomerase is a ribonucleoprotein polymerase that adds TTAGGG repeats to telomeric ends[16]. Telomerase activity is closely linked to attainment of cellular immortality, while lack of such activity contributes to cellular senescence. Telomerase is inactive in most normal somatic tissues except for germ and some stem cells, but has been found to be reactivated in approximately 85% of human malignancies[17,18]. Telomerase activity can be detected in trace amount of tissues, such as that collected in fine-needle aspiration cytology or even in the body fluid of cancer patients[19]. Recently, telomerase expression also was found in peritoneal fluid of patients with gastric cancer[20-22]. The presence of telomerase was associated with advanced disease or peritoneal dissemination.
In the present study, we reported the telomerase activity of peritoneal lavage fluid and expression of PCNA in gastric tissues. We aimed to compare the efficacy of telomerase activity assay and conventional cytological examination of peritoneal lavage fluid for prediction of peritoneal metastasis in gastric cancer, and to explore the relationship between telomerase activity and proliferating cell nuclear antigen expression.
Between January 2004 and January 2005, we observed 60 patients with gastric cancer who underwent surgical resection at West China Hospital, including 40 men and 20 women, aged from 30-78 years (mean of 58 years). Another 50 patients with peptic ulcer who underwent surgical resection at same hospital were also studied as control, including 27 men and 23 women, ranged in age from 26 to 60 years (mean of 43 years). No patient had received preoperative radiation therapy or chemotherapy. Whole body bone scan and sonography were performed for all of the patients to rule out distant metastases. Histological type was established on haematoxylin/eosin stained sections according to Lauren’s classification. Well and moderately differentiated tubular adenocarcinomas were referred to as the intestinal type, while poorly differentiated, signet ring cells and mucinous adenocarcinomas were referred to as the diffuse type. The histological type included diffuse type in 42 and intestinal type in 18. According to the criteria of the International Union Against Cancer (UICC) TNM Classification 5th edition, the depth of invasion was classified as follows: T1 in 3, T2 in 17, T3 in 24, and T4 in 16. Macroscopically evident peritoneal metastasis (P) and microscopically evident serosal invasion were also confirmed by histopathology.
Specimens of lesion tissue and peritoneal lavage fluid were collected at the time of operation from patients undergoing surgical resection. Immediately after the laparotomy, before the manipulation of the process, 50 mL of isotonic sodium chloride was instilled into the left subphrenic or Douglas cavities with 16F catheter and recovered after being gently stirred. If ascites was identified within the peritoneum, the fluids were sucked directly. The lavage fluids were collected in heparinized tubes and centrifuged at 3000 r/min for 10 min at 4°C (centrifugate radius: 90 mm). The supernatant was discarded. The superficial “white” layer of the sediment was picked up with a Pasteur pipette and was smeared on three glass slides and fixed for 1 min in Delaunay’s solution (1 L Delaunay’s contains 500 mL of absolute alcohol, 500 mL of pure acetone, and 1 mL of 3 mol/L trichloracetic acid) for Papanicolaou staining. At the end of this examination, the rest of the material was suspended in phosphate buffered saline (PBS) and the centrifugation step was repeated. To avoid contamination with red blood cells that could potentially interfere with polymerase chain reaction (PCR), cells were washed 2-4 times with ice-cold hypotonic solution (50 mmol/L KCl) and then kept frozen at -80°C until telomerase assay. Tissue specimens were obtained from the mucosal surface of the resected lesions. Lesion material (10-20 g) was dissected from cancers without interfering with resection margins or the macroscopic appearance of the lesion. Necrotic areas from the centre of the lesions were avoided. After surgical removal, all the samples were snap frozen immediately and stored at -80°C until further use. Telomerase assay and cytological examination were performed independently in a blinded manner.
The streptavidin peroxidase method was used to quantify PCNA protein expression. All reagents were purchased from Beijing Zhongshan Biotechnology Co., Ltd. Resected gastric cancer samples were fixed in 10% buffered formalin and embedded in paraffin, and 4-μm sections were cut, dewaxed and rehydrated by sequential immersion in xylene, graded ethanol and water. Mouse anti-PCNA monoclonal antibody (Santa Cruz, USA) and biotinylated goat anti-mouse IgG antibody (Santa Cruz, USA) were used as the first and second antibodies, respectively. Endogenous peroxidase activity was blocked by 3% hydrogen peroxide for 15 min in methanol. Dewaxed tissue sections were heated in a steamer for 2 min to retrieve the antigens and cooled to room temperature. Tissue sections after the enzyme digestion were then treated with the primary antibodies against PCNA. After washing in PBS and exposure to 10% normal goat serum for 10 min to reduce the nonspecific antibody binding, the slides were incubated at 37°C with mouse monoclonal antibody against PCNA (Santa Cruz, USA) at 1:100 dilution, in humid chambers for 1 h. After further PBS washing, slides were incubated at 37°C with biotinylated goat anti-mouse IgG antibody in humid chambers for 20 min. Primary antibodies were visualized with an Envision System (DAKO, Denmark). After further PBS washing, slides were incubated with substrate diaminobenzidine and hydrogen peroxide for 10 min. Finally, sections were counterstained with hematoxylin. Negative control experiments were carried out as above by normal rabbit serum for the primary antibodies. Sections from previously studied cases of gastric cancer known to express PCNA were used as positive controls.
The assessment of all the samples was conducted blindly by calculating the average ratio of positive cells in 10 vision fields (the plasma staining brown yellow) under a × 400 microscope. The sections were initially scanned at low power to determine the areas that were evenly labeled. The cases were evaluated independently by two pathologists, and discrepancies in estimation were reconciled by concurrent review using a multiheaded microscope. At least 10 high-power fields were chosen randomly, and 2000 cells were counted. Tumor sections were considered negative if staining was absent or present in 10% of the tumor cells. In each specimen, PI was expressed as a percentage of positive nuclei per total nuclei counted in the section.
Telomerase activity was measured using a telomerase polymerase chain reaction (PCR) ELISA kit (Roche Diagnostics GmbH, Germany) according to the manufacturer’s protocol. The frozen samples were suspended in 200 mL lysis reagent, pre-cooled on ice by retropipetting at least 3 times and incubated on ice for 30 min. The lysate was centrifuged at 16 000 ×g for 20 min at 4°C. The supernatant was removed carefully and transferred to a fresh tube for the TRAP assay. For each sample to be tested and the controls, 25 μL reaction mixture was transferred into a tube suitable for PCR amplification. The extended products were amplified by PCR using Taq polymerase, the P1-TS, P2 primers and nucleotides. After a 30 min incubation at 25°C to allow the telomerase mediated extension of the TS primer and followed by 94°C for 10 min to inactivate the telomerase, the reaction mixture was subjected to 30 PCR cycles at 94°C for 30 s, 50°C for 30 s, and 72°C for 90 s on a DNA thermocycler (GeneAmp PCR System 9700, ABI, USA). Using the ELISA method, the amplified products were immobilized onto streptavidin-coated microtiter plates via biotin-streptavidin interaction, and then detected by anti-digoxigenin antibody conjugated to peroxidase. After the addition of the peroxidase substrate (3, 3’, 5, 5’-tetramethyl benzidine), the amount of TRAP products were determined by measurement of their absorbance at 450 nm (with a reference wave length of 690 nm). The maximum value of absorbance for the negative control should be 0.25 A450 nm-A690 nm units. The absorbance readings obtained with the positive control (supplied with the kit) should be higher than 1.5 A450 nm-A690 nm units after 20 min substrate reaction. Samples are regarded as telomerase-positive if the difference in absorbance (ΔA) is higher than 0.2 A450 nm-A690 nm units.
Data were analyzed by t test and χ2 test. P < 0.05 was considered statistically significant. The SPSS software version 12.0 was used for statistical analysis.
In the tissue specimens, telomerase activity was positive in 31 (51.7%) of 60 patients with gastric cancer, and 2 (4%) of 50 patients with peptic ulcer (P < 0.005). In the peritoneal lavage fluid, telomerase activity was found positive in 25 (41.7%) of 60 patients with gastric cancer, and none in peptic ulcer subjects (P < 0.005). Although the telomerase activity positive rate in tissues tended to be higher than that in the peritoneal lavage fluid, there was no significant difference statistically (P > 0.05). The sensitivity, specificity, positive predictive value, and negative predictive value of telomerase activity in peritoneal lavage fluid from patients with gastric cancer were 84%, 91%, 91%, and 83%, respectively.
Conventional cytological examinations were performed routinely for gastric cancer samples tested by the telomerase activity assay. All 50 control specimens were cytologically negative. The positive rate of telomerase activity in peritoneal lavage fluid collected from patients with gastric cancer was 41.7% (25/60), which was well related to serosal invasion, Lauren’s classification, depth of infiltration and peritoneal metastasis of cancer, and rose with the increased depth of infiltration and serosa-involved areas (P < 0.05). The positive rate of PLC was 25.0% (15/60), which was obviously high in the group with macroscopic peritoneal metastasis (the group of P1-3), and also increased with the increased depth of infiltration and serosa-involved areas (P < 0.05). All PLC positive cases were telomerase test positive in their peritoneal lavage fluids. Of the 45 PLC negative cases, 4 cases with macroscopic peritoneal metastasis (P1-3) and 6 cases with serosal invasion were telomerase test positive. Although the positive rate of telomerase activity in peritoneal lavage fluids from patients with gastric cancer was not significantly higher than that of PLC in general, it was significantly higher than that of PLC in the group of pT4, P1-3 and diffuse type (Table 1).
| Clinicopathological | Telomerase activity | PLC | |||
| features | n | Positive cases n (%) | P | Positive cases n (%) | P |
| Lauren’s classification | 0.01 | 0.104 | |||
| Intestinal | 18 | 3 (16.7) | 2 (11.1) | ||
| Diffuse | 42 | 22 (52.4)e | 13 (31.0) | ||
| Invasion depth | 0 | 0.001 | |||
| PT1-2 | 20 | 0 (0) | 0 (0) | ||
| PT3 | 24 | 10 (41.7) | 6 (25.0) | ||
| PT4 | 16 | 15 (93.8)ae | 9 (56.3)a | ||
| Peritoneal metastasis | 0.035 | 0 | |||
| P0 | 47 | 12 (25.5) | 6 (12.8) | ||
| P1-3 | 13 | 13 (100.0)e | 9 (69.2) | ||
| Serosal invasion | 0.014 | 0.092 | |||
| Absent | 23 | 5 (21.7) | 3 (13.0) | ||
| Present | 37 | 20 (54.1) | 12 (32.4) | ||
| Serosa-involved | 0 | 0 | |||
| areas (cm2) | |||||
| 0 | 23 | 1 (4.3) | 0 (0) | ||
| < 10 | 7 | 1 (14.3) | 0 (0) | ||
| 10-20 | 17 | 12 (70.6) | 7 (41.2) | ||
| > 20 | 13 | 11 (84.6)c | 8 (61.5)c | ||
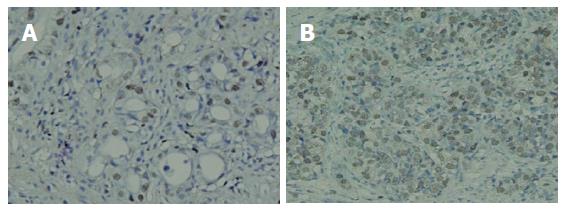
As shown in Figure 1, the PCNA protein was expressed intensely, mainly in the nucleus of gastric cancer cells. PCNA expression was positive in 51 (85%) of 60 patients with gastric carcinoma. In 25 patients with positive telomerase activity, the PI was significantly higher than those with negative telomerase activity (55.00 ± 6.59 vs 27.43 ± 7.72, P < 0.05). The 13 patients with macroscopic peritoneal metastasis had significantly higher levels of PI than negative ones (57.26 ± 10.18 vs 29.15 ± 8.31, P < 0.05). Furthermore, the PI in 37 patients with serosal invasion was significantly higher than in those without serosal invasion (49.82 ± 6.74 vs 24.65 ± 7.33, P < 0.05).
To date, there has been no effective therapy for peritoneal carcinomatosis. Therefore, attention has been paid to detecting peritoneal free cancer cells in patients with advanced gastric carcinoma without overt peritoneal metastasis to prevent peritoneal metastasis[23]. Several studies have shown that cytological examination of peritoneal lavage fluid is still the gold standard for assessing the presence of free cancer cells in the peritoneal cavity[10,12]. However, cytological examination lacks sensitivity for detecting free cancer cells[9,15]. Telomerase activity in body fluids, as measured by the TRAP assay, is a sensitive potential tumor marker that might help increase the cancer detection rate and the cancer treatment success rate when combined with conventional cytopathological methods[24,25].
High expression of telomerase was demonstrated in gastric cancer. Hiyama et al[26] first reported telomerase activity in advanced gastric cancer tissue and its metastases. The survival time of patients with detectable telomerase activity in their tumors was significantly shorter than those without telomerase activity. In another study by Tahara et al[27], reactivation of telomerase was found to be involved at the early stage of gastric carcinogenesis and its expression correlated well with malignant progression of the cancer. In the present study, telomerase activity was detected in 51.7% (31/60) of gastric cancer tissue specimens and 41.7% (25/60) of these cases in the peritoneal lavage fluid. These results were comparable to the findings of other studies on gastric cancer[20,21].
This study demonstrated significant correlations between positive rate of telomerase activity in peritoneal lavage fluid from patients with gastric cancer and invasion depth of cancer, serosal invasion, histological types, serosa-involved areas, presence and extent of peritoneal metastasis, which are important biological features of gastric cancer and reflect the invasiveness of tumor cells. In particular, our data demonstrated that 100% (13/13) of gastric cancers with peritoneal metastasis had detectable telomerase activity in peritoneal lavage fluid, although the sample size was relatively small. Thus, telomerase activity expression in peritoneal lavage fluid can be used as an indication of the invasive potential and poor prognosis of the gastric cancer.
With regard to clinically evident peritoneal metastasis, 100% (13/13) of these cases showed detectable telomerase activity, while only 69.2% (9/13) of these cases produced positive cytological results, the positive rate of telomerase activity was also significantly higher than that of PLC in the group of pT4 and diffuse type, thus confirming that telomerase assay is more sensitive than PLC methods. The positive rate of tolemerase activity of the 37 specimens with serosal invasion was 54.1% (20/37), while PLC positive rate of those was 32.4% (12/37). As we know, serosal invasion appears to be mainly responsible for the exfoliation of free cancer cells into the peritoneal cavity[11]. It has been reported that approximately half of patients with serosal invasion develop peritoneal recurrence even if curative resection is performed[6,7,28]. In general, although using the presence of telomerase activity did not show results that surpass PLC methods, the test might offer the possibility of detecting cancer cells in cytology-negative specimens. Our data suggest that the telomerase test in peritoneal fluids can be used as an adjuvant to PLC test in the diagnosis of peritoneal micrometastasis, particularly in cases of negative cytology. In these cases, a review of peritoneal histocytology is advised. Statistically, the sensitivity and specificity of telomerase activity in peritoneal lavage fluid for gastric cancer was 84% and 91%, respectively. Our study also indicates that telomerase activity is a candidate molecular marker for the presence of free cancer cells in peritoneal cavity.
Telomerase activity was not detected in peritoneal lavage fluids in some cases of gastric cancer, which might be attributable to several reasons. One possible explanation may be that the concentration of free cancer cells in peritoneal lavage fluids was insufficient. We can not completely rule out the possibility of technical error or that perhaps some peritoneal lavage fluids did not contain cancer cells. However, the peritoneal lavage fluid can also be contaminated with lymphocytes or other blood cells in the presence of inflammation or ulceration[29]. The presence of these cells may give rise to false-positive results in TRAP assay[30]. Further studies are required to eliminate the above limitations to improve the accuracy of this technique.
The rate of PCNA expression in 60 gastric carcinoma specimens was 85% (51/60).The patients with positive telomerase activity, peritoneal metastasis, and serosal invasion showed significantly higher levels of PI. It was suggested that telomerase activity was well related to higher proliferating activity of gastric cancer, which was the very important reason of peritoneal carcinomatosis and serosal invasion.
In conclusion, to detection of telomerase activity in peritoneal lavage fluid and tumor cells by cytology could be useful to predict subclinical metastasis to the peritoneum in patients with gastric cancer. Telomerase activity assay could be a useful adjunct for cytologic method in the diagnosis of peritoneal micrometastasis and well related to higher proliferating activity of gastric cancer. Our results also suggest a promising future therapeutic strategy for peritoneal dissemination of gastric cancer based on telomerase inhibition.
Peritoneal dissemination is the most common pattern of metastasis in advanced gastric carcinoma. There has been no standard treatment for peritoneal carcinomatosis and the results are poor. The mechanism of peritoneal dissemination has not yet been clearly defined so far. Free cancer cells exfoliated from cancer lesions have been postulated as the main cause of metastasis.
A close association has been demonstrated between positive results for peritoneal free cancer cells and low survival rate. Cytological examination has been the gold standard for detecting free cancer cells in the peritoneal lavage fluid. However, the sensitivity is relatively low, and the reliability of morphologic diagnosis is limited. Telomerase activity in body fluids is a sensitive potential tumor marker that might help increase the cancer detection rate. A PCR-based assay called telomeric repeat amplification protocol (TRAP), is quite sensitive and can detect as few as 10 telomerase positive cells.
The TRAP assay for telomerase activity in the peritoneal lavage fluid could be a useful potential marker in the diagnosis of peritoneal micrometastasis and well related to higher cellular proliferative activity of gastric cancer. It suggested a promising future therapeutic strategy for treating peritoneal dissemination based on telomerase inhibition.
Measurement of telomerase activity could be a useful adjunct of cytologic method for detecting free cancer cells in the peritoneal lavage fluid during operation.
Telomerase is a critical enzyme responsible for cellular immortality. Telomerase activity is closely linked to attainment of cellular immortality, while lack of such activity contributes to cellular senescence. With the exception of germ and some stem cells, normal somatic cells do not have telomerase activity. Conversely, telomerase activity has been observed in 80%-90% of malignancies.
The aim of this study was to evaluate the efficacy of telomerase activity assay and conventional cytological examination in peritoneal lavage fluid for the prediction of peritoneal metastasis in gastric cancer. The authors compared the positive rate of telomerase activity with that of cytology and found that telomerase activity has stronger correlations with clinicopathological factors than cytology. The authors concluded that telomerase activity could be a useful adjunct for cytologic method in the diagnosis of peritoneal micrometastasis and malignant progression.
S- Editor Liu Y L- Editor Ma JY E- Editor Liu Y
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13286] [Cited by in RCA: 13555] [Article Influence: 677.8] [Reference Citation Analysis (1)] |
| 2. | Chen Z, Zheng T, Chen J. [Evaluation of ten-year results of cancer prevention and treatment in Changle City with high incidence of gastric cancer]. ZhonghuaLiu ZaZhi. 2000;22:311-313. [PubMed] |
| 3. | Roder DM. The epidemiology of gastric cancer. Gastric Cancer. 2002;5 Suppl 1:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 246] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 4. | Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer. 1999;83:18-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 5. | Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 551] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 6. | Broll R, Weschta M, Windhoevel U, Berndt S, Schwandner O, Roblick U, Schiedeck TH, Schimmelpenning H, Bruch HP, Duchrow M. Prognostic significance of free gastrointestinal tumor cells in peritoneal lavage detected by immunocytochemistry and polymerase chain reaction. Langenbecks Arch Surg. 2001;386:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Nakanishi H, Kodera Y, Yamamura Y, Kuzuya K, Nakanishi T, Ezaki T, Tatematsu M. Molecular diagnostic detection of free cancer cells in the peritoneal cavity of patients with gastrointestinal and gynecologic malignancies. Cancer Chemother Pharmacol. 1999;43 Suppl:S32-S36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Lee CC, Lo SS, Wu CW, Shen KH, Li AF, Hsieh MC, Lui WY. Peritoneal recurrence of gastric adenocarcinoma after curative resection. Hepatogastroenterology. 2003;50:1720-1722. [PubMed] |
| 9. | Bando E, Yonemura Y, Takeshita Y, Taniguchi K, Yasui T, Yoshimitsu Y, Fushida S, Fujimura T, Nishimura G, Miwa K. Intraoperative lavage for cytological examination in 1,297 patients with gastric carcinoma. Am J Surg. 1999;178:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 240] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | Suzuki T, Ochiai T, Hayashi H, Nakajima K, Yasumoto A, Hishikawa E, Shimada H, Horiuchi F, Ohki S, Isono K. Importance of positive peritoneal lavage cytology findings in the stage grouping of gastric cancer. Surg Today. 1999;29:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Yonemura Y, Endo Y, Obata T, Sasaki T. Recent advances in the treatment of peritoneal dissemination of gastrointestinal cancers by nucleoside antimetabolites. Cancer Sci. 2007;98:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Suzuki T, Ochiai T, Hayashi H, Hori S, Shimada H, Isono K. Peritoneal lavage cytology findings as prognostic factor for gastric cancer. Semin Surg Oncol. 1999;17:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Vogel I, Kalthoff H. Disseminated tumour cells. Their detection and significance for prognosis of gastrointestinal and pancreatic carcinomas. Virchows Arch. 2001;439:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Hermanek P. pTNM and residual tumor classifications: problems of assessment and prognostic significance. World J Surg. 1995;19:184-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 108] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Abe S, Yoshimura H, Tabara H, Tachibana M, Monden N, Nakamura T, Nagaoka S. Curative resection of gastric cancer: limitation of peritoneal lavage cytology in predicting the outcome. J Surg Oncol. 1995;59:226-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Moyzis RK, Buckingham JM, Cram LS, Dani M, Deaven LL, Jones MD, Meyne J, Ratliff RL, Wu JR. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci USA. 1988;85:6622-6626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1496] [Cited by in RCA: 1602] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 17. | Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1993] [Cited by in RCA: 2000] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 18. | Hiyama E, Kodama T, Shinbara K, Iwao T, Itoh M, Hiyama K, Shay JW, Matsuura Y, Yokoyama T. Telomerase activity is detected in pancreatic cancer but not in benign tumors. Cancer Res. 1997;57:326-331. [PubMed] |
| 19. | Hiyama E, Hiyama K. Clinical utility of telomerase in cancer. Oncogene. 2002;21:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 140] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Abstracts of the 41st Annual Meeting of the American Society of Clinical Oncology (ASCO). May 13-17, 2005. Orlando, Florida, USA. J Clin Oncol. 2005;23:1s-1087s. [PubMed] |
| 21. | Lee HJ, Myung SJ, Park YH, Cho YK, Jung HY, Lee GH, Hong WS, Yang SK, Kim JH, Min YI. [Measurement of telomerase activity and telomerase reverse transcriptase expression in gastric fluid and tissue for early diagnosis of stomach cancer]. Korean J Gastroenterol. 2003;42:183-189. [PubMed] |
| 22. | Mori N, Oka M, Hazama S, Iizuka N, Yamamoto K, Yoshino S, Tangoku A, Noma T, Hirose K. Detection of telomerase activity in peritoneal lavage fluid from patients with gastric cancer using immunomagnetic beads. Br J Cancer. 2000;83:1026-1032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Nakanishi H, Kodera Y, Torii A, Hirai T, Yamamura Y, Kato T, Kito T, Tatematsu M. Detection of carcinoembryonic antigen-expressing free tumor cells in peritoneal washes from patients with gastric carcinoma by polymerase chain reaction. Jpn J Cancer Res. 1997;88:687-692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Tseng CJ, Jain S, Hou HC, Liu W, Pao CC, Lin CT, Horng SG, Soong YK, Hsueh S. Applications of the telomerase assay in peritoneal washing fluids. Gynecol Oncol. 2001;81:420-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Hiyama E, Yokoyama T, Tatsumoto N, Hiyama K, Imamura Y, Murakami Y, Kodama T, Piatyszek MA, Shay JW, Matsuura Y. Telomerase activity in gastric cancer. Cancer Res. 1995;55:3258-3262. [PubMed] |
| 27. | Tahara H, Kuniyasu H, Yokozaki H, Yasui W, Shay JW, Ide T, Tahara E. Telomerase activity in preneoplastic and neoplastic gastric and colorectal lesions. Clin Cancer Res. 1995;1:1245-1251. [PubMed] |
| 28. | Bonenkamp JJ, Songun I, Hermans J, van de Velde CJ. Prognostic value of positive cytology findings from abdominal washings in patients with gastric cancer. Br J Surg. 1996;83:672-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 168] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Lee WY. Limitations of detection of malignancy in pleural effusions using ELISA-based TRAP assay: comparison with cytological examination. Cytopathology. 2005;16:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Zendehrokh N, Dejmek A. Telomere repeat amplification protocol (TRAP) in situ reveals telomerase activity in three cell types in effusions: malignant cells, proliferative mesothelial cells, and lymphocytes. Mod Pathol. 2005;18:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |