Published online Jun 14, 2007. doi: 10.3748/wjg.v13.i22.3095
Revised: April 8, 2007
Accepted: April 16, 2007
Published online: June 14, 2007
AIM: To evaluate the results of the treatment of simple liver cysts (solitary and multiple) and polycystic liver disease (PLD) using percutaneous sclerotherapy and/or surgical procedures in a single tertiary referral centre.
METHODS: Retrospective analysis of 54 patients referred for evaluation and possible treatment of simple liver cysts (solitary and multiple) and PLD, from January 1997 to July 2006.
RESULTS: Simple liver cysts were treated in 41 pts (76%) with a mean size of 12.6 cm. The most common reason for referral was abdominal pain or discomfort (85%). Percutaneous sclerotherapy was performed as initial treatment in 30 pts, showing cyst recurrence in 6 pts (20%). Surgical treatment was initially performed in 11 pts with cyst recurrence in 3 pts (27%). PLD was treated in 13 pts (24%) with a mean size of the dominant cyst of 13 cm. Percutaneous sclerotherapy for PLD was performed in 9 pts with recurrence in 7 pts (77.8%). Surgical treatment for PLD was undertaken in 4 pts (30.8%) with recurrence in all. Eventually, 2 pts with PLD in the presence of polycystic kidney disease underwent liver- and kidney transplantation because of deterioration of liver and kidney function.
CONCLUSION: The majority of patients with simple liver cysts and PLD are referred for progressive abdominal pain. As initial treatment, percutaneous sclerotherapy is appropriate. Surgical deroofing is indicated in case of cyst recurrence after percutaneous sclerotherapy. However, the results of percutaneous sclerotherapy and surgical treatment for PLD are disappointing. Partial liver resection is indicated when there is suspicion of a pre-malignant lesion.
- Citation: Erdogan D, van Delden OM, Rauws EA, Busch OR, Lameris JS, Gouma DJ, van Gulik TM. Results of percutaneous sclerotherapy and surgical treatment in patients with symptomatic simple liver cysts and polycystic liver disease. World J Gastroenterol 2007; 13(22): 3095-3100
- URL: https://www.wjgnet.com/1007-9327/full/v13/i22/3095.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i22.3095
Liver cysts comprise a heterogeneous group of lesions classified as congenital or acquired[1]. Nowadays, most cysts are detected as an incidental finding when imaging of the liver is performed for abdominal complaints[2]. In most cases, liver cysts are of the simple type and are asymptomatic without clinical significance. Nevertheless, a minority attains large size and may cause progressive abdominal pain and/or discomfort. Although the diagnosis is readily made by radiological imaging, the mode of treatment is controversial. Nowadays, treatment usually consists of percutaneous aspiration of the cyst followed by instillation of a sclerosant (sclerotherapy)[3], or surgical treatment, either laparoscopically[4] or during laparotomy, in case of recurrence after radiological intervention. Only few published studies have focused on the outcome of treatment of simple liver cysts and PLD (including adult polycystic liver disease and adult polycystic kidney disease) after percutaneous or surgical interventions.
The aim of this study was to assess the outcome of patients with simple liver cysts and polycystic liver disease treated with percutaneous sclerotherapy or by surgical intervention, and to propose a treatment algorithm for these lesions.
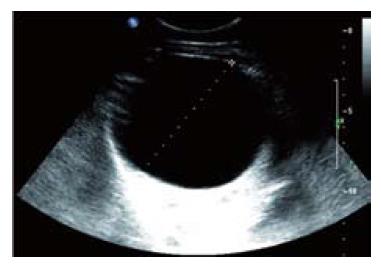
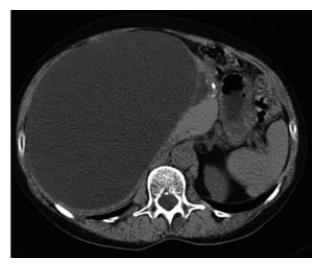
Between January 1997 and July 2006, a total of 54 conse-cutive patients were referred for evaluation and treatment of simple liver cysts (including solitary and multiple cysts) or polycystic liver disease, to the Department of Radiology, Gastroenterology and Hepatology and/or Surgery, Academic Medical Center, Amsterdam, The Netherlands. The study group consisted of 49 women (90.7%) and 5 males (9.3%) with a mean age of 56.3 ± 13.6 years (range 21-81). Medical records were reviewed for demographic features, presenting symptoms or indications for further analysis, in addition to size and location of treated cysts. The liver cysts were classified into two groups: simple (solitary or multiple) liver cysts or polycystic liver disease (PLD). The latter included PLD in the presence of polycystic kidney disease and autosomal dominant PLD). The following items were recorded: mode of treatment consisting of ultrasound-guided percutaneous sclerotherapy or surgical treatment, complications, duration of hospital stay and clinical outcome. Simple liver cysts typically presented on ultrasound (US) as anechoic, unilocular, sharply demarcated lesions with imperceptible walls showing posterior acoustic enhancement[5]. On CT, a simple liver cyst appeared as a well-demarcated lesion with fluid density without enhance-ment after contrast administration. Polycystic liver disease typically appears as multiple homogeneous lesions with fluid density without wall or content enhancement after contrast administration[6].
Percutaneous sclerotherapy was performed under local anesthesia. The cyst was aspirated by needle puncture under US guidance and examined for bile content to exclude communication with the biliary system. A pigtail catheter was then inserted for complete drainage aspiration and 95% ethanol or tetracycline was injected to destruct the epithelial lining of the cyst wall. The volume of the aspirated cyst fluid was recorded and only partially replaced by ethanol or tetracycline. The injected solution was left inside the cyst for approximately 2 h before drainage. The patients were observed for 24 h after percutaneous sclerotherapy.
Laparoscopic deroofing was performed under general anesthesia with the patient in a supine position. After insertion of the Veress needle just below the umbilicus, a CO2 pneumoperitoneum was installed and three trocars were inserted. The cyst was aspirated and deroofed with excision of the walls near the liver tissue by an electro-surgical hook knife[7].
Statistical analysis was performed using SPSS® (SPSS 12.0.1, Chicago, Illinois, USA). Continuous variables were expressed as mean ± SD and differences were analysed using the Mann-Whitney U test. Categorical variables were analysed using Pearson’s χ2 test or Fisher exact test (when a table had a cell with an expected frequency of less than 5). P < 0.05 was considered significant.
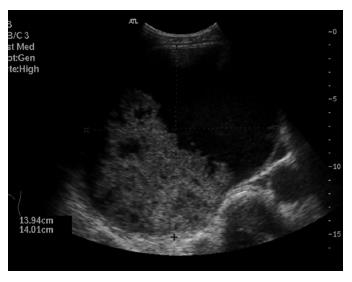
Simple liver cysts were diagnosed in 76% (41/54) of patients. The mean age at initial diagnosis was 57.5 yrs. The most common reason for referral was abdominal complaints in 35 patients (85%), consisting of pain in the right upper quadrant or epigastrium, discomfort or feeling of abdominal distension. Three of these patients presented with an acute onset of abdominal pain which could be attributed to intracystic bleeding as the appearance on US or CT was suggestive of a bleed within the cyst. Two patients were analysed because of jaundice and cholestasis, respectively, with abdominal imaging studies showing internal septations and a nonhomogeneous appearance. In 2 of the 41 patients (5%), suspicion of a malignant lesion (because of prior surgery for colorectal cancer) was the indication for further analysis. One patient presented with fever due to infection of the cyst. One patient with a prior medical history of a simple liver cyst on imaging was referred for analysis on the suspicion of intracystic bleeding in the absence of abdominal complaints.
Abdominal US was performed in all patients, additional contrast enhanced CT in 23 (56%) and MRI in two patients (Table 1). In case of an uncomplicated, simple liver cyst, abdominal imaging showed the characteristic features as described above (methods section) (Figures 1 and 2). Patients with intracystic bleeding as complication mainly showed a nonhomogeneous appearance of the lesion or internal echogenic material, with septations within the cyst (Figure 3). In most of these patients, the diagnosis of a hepatobiliary cystadenoma was considered but rejected because they all were known with simple liver cysts on previous imaging studies. In two patients, endoscopic retrograde cholangiography (ERC) was used to assess possible communication with the intrahepatic biliary system. The mean size of the cysts was 12.6 cm. The cysts requiring treatment were located centrally in 18 patients (44%) with extension into the left and right liver lobes. In 14 patients (34%), the cyst was located in the right liver lobe. The mean follow-up time, from initial presentation elsewhere or in our center to initial treatment, was 31.5 mo (range 1-156 mo).
| Simple liver cyst | PLD | Total | |
| n (male:female) | 41 (4:37) | 13 (1:12) | 54 (5:49) |
| Age (yr) | 57.5 ± 14.9 | 52.8 ± 7.8 | 56.3 ± 13.6 |
| (range) | (21-81) | (40-67) | (21-81) |
| Mean lesion size (cm) | 12.6 ± 6.3 | 13 ± 4.8 | 12.7 ± 5.6 |
| (range) | (3.5-25) | (8-21) | (3.5-25) |
| Diagnostic imaging | |||
| (US:CT:ERCP:MRI) | 41:23:2:2 | 13:11:1:0 | 54:34:3:2 |
| Location of treated cyst | |||
| (right:left:bilateral) | 14:09:18 | 07:02:04 | 21:11:22 |
Percutaneous sclerotherapy was applied in 30 patients (73%). Of these, 2 patients had undergone percutaneous aspirations elsewhere to determine whether abdominal symptoms could be attributed to the cyst. Although the complaints had completely resolved after the intervention, recurrence after approximately 6 weeks with concomitant recurrence of abdominal pain occurred.
Four patients had unsuccessful, previous percutaneous sclerotherapy elsewhere with the larger cysts showing recurrence within 6 to 9 mo along with abdominal complaints. In the present series, the mean aspirated volume of the cyst was 2223 ± 1772 mL (range 50-5000). The mean volume of the sclerosant used for cyst ablation was 179 ± 95.8 mL (range 25-350 mL). In all these patients, an immediate decrease in size was demonstrated after percutaneous sclerotherapy on ultrasound examination. Complications were encountered in 2 patients (6.7%), including an intracystic bleed which did not require further treatment and one infection of the treated cyst which required re-admittance for intravenous antibiotic treatment. During follow-up, cyst recurrence was seen on US in 6 of the 30 patients (20%) within 4 mo. Of these, only 1 patient (1/30; 3.3%) showed concomitant recurrence of symptoms and in this patient, percutaneous sclerotherapy was eventually repeated twice. In the remaining 5 patients, relief of abdominal symptoms was attained despite small cyst recurrence, be it that the recurrent cysts were all significantly smaller than before treatment. These patients were discharged from further follow-up when no further increase in size of the recurrent cyst was detected. The mean hospital stay in patients after percutaneous sclerotherapy for a simple liver cyst was 3.4 d (median 2; range 2-23). The mean time of follow-up after treatment was 15 mo (2-35 mo).
Of all 41 patients, surgical treatment was performed in 11 patients (27%) with a mean duration of operation of 132.5 min (range 70-340). Of these patients, 4 patients had had previous treatment for relief of abdominal complaints, including prior percutaneous sclerotherapy in a hospital elsewhere in 3 patients and laparoscopic deroofing in 1 patient. Cyst recurrence in these patients was seen within 5 mo after the initial procedure. Surgical procedures included cyst wall deroofing and omentoplasty in 8 patients, of which 5 had laparoscopic procedures and 3 had an open approach. Two patients underwent laparotomy and enucleation of the cyst and 1 patient underwent local excision of a cyst near the common hepatic duct. Reasons for an open procedure were a superior or posterior location of the cyst in the liver or the fact that a malignancy could not be ruled out. A postoperative complication occurred in 1 patient (1/11; 9%) consisting of bile leakage requiring percutaneous drainage of a bile collection.
Cyst recurrence after surgical treatment was seen in three patients (3/11; 27%). Additional percutaneous sclerotherapy was carried out in 2 of these patients because of concomitant progressive abdominal complaints (2/11; 18.2%). Eventually, complete regression of these cysts was achieved (Table 2). The mean hospital stay in patients after surgical treatment for a simple liver cyst was 13.9 d (median 8; range 4-35).
| Simple liver cyst | PLD | Total | ||||
| (n = 41) | (n = 13) | (n = 54) | ||||
| Surgical | Percutaneous sclerotherapy | Surgical | Percutaneous sclerotherapy | Surgical | Percutaneous sclerotherapy | |
| Treatment1 | 11 (27) | 30 (73) | 4 (31) | 9 (69) | 15 (28) | 39 (72) |
| Cyst recurrence | 3 (27) | 6 (20) | 4 (100) | 7 (78) | 7 (47) | 13 (33) |
| Additonal treatment after recurrence | 2 (18) | 1 (3) | 3 (75) | 4 (44) | 5 (33) | 5 (13) |
In patients with simple liver cysts (including solitary and multiple cysts), no significant differences in recurrence rate were observed after surgical treatment compared to recurrence after percutaneous sclerotherapy [27.3% (3/11) vs 20% (6/30), respectively; P = 0.680]. However, additional treatment was required in 18.2% (2/11) of patients with simple liver cysts after surgical treatment compared to 3.3% (1/30) of simple liver cysts after percutaneous sclerotherapy (P = 0.170).
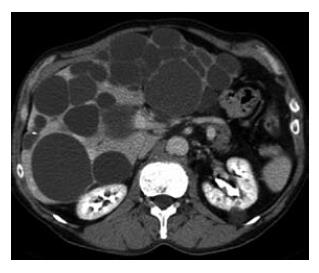
Thirteen patients (13/54; 24%) were diagnosed with PLD and all experienced progressive abdominal complaints. Abdominal US was performed in all patients. Eleven patients underwent abdominal CT (11/13; 85%) showing multiple unilocular cysts throughout the liver (Figure 4). In two patients, the imaging findings were suggestive of an intracystic bleeding and the diagnosis was confirmed by percutaneous drainage of dark brown, haemorrhagic fluid. The mean size of the largest dominant cyst was 13 cm and these larger cysts were located in the right liver lobe in 7 patients (54%) (Table 1).
In PLD, percutaneous sclerotherapy was carried out in 9 patients (69%). Only 3 patients had previous percutaneous drainage of a dominant cyst. Nevertheless, these cysts recurred within 4 mo. The maximum volume of intracystic fluid drained in this series was 5200 ml. No complications were seen after percutaneous sclerotherapy. Cyst recurrence was seen in 7 patients (77.8%). Of these, 4 patients required repeat percutaneous sclerotherapy because of progressive abdominal pain.
Surgical treatment was undertaken in 4 patients (30.8%) with PLD, and the mean duration of operation was 110 ± 47 min (60-170 min). All these patients had previous treatment in their medical history; three patients had undergone several attempts at percutaneous sclerotherapy and laparoscopic deroofing, and 1 patient had undergone percutaneous aspiration only.
In these patients, cyst recurrence occurred within one year after the various procedures. The surgical procedures included laparoscopic deroofing in 2 patients and laparotomy with deroofing in another 2 patients. Postoperative complications were seen in 2 patients, consisting of intracystic bleeding in one patient and bile leakage from a peripheral bile duct requiring biliary stenting in the other patient. Cyst recurrence after surgical treatment was seen in all patients after surgical treatment for PLD (Table 2). Eventually, liver transplantation was required in 2 patients because of deterioration of liver and kidney function in one patient, and failed percutaneous sclerotherapy in the other patient.
The series described in this study, represents a selected group of patients who underwent treatment because of symptomatic large liver cysts. These patients mainly presented with abdominal symptoms (88%) and showed a mean cyst size of 13 cm. The large size of the cysts in this population is due to referral bias, because patients with abdominal complaints and large liver cysts are more likely to be referred for further diagnosis and/or treatment. Most of our patients (76%) were diagnosed with simple liver cysts. The precise incidence is difficult to determine, but is reported to be around 2% to 5%, according to ultrasound studies[1,8]. A predominance in women is found with a mean age between 50 and 60 years, which is in accordance with our results[9].
Abdominal US or CT are the first choice of imaging for abdominal pain and are highly accurate for simple liver cysts[10]. Complications such as intracystic bleeding, rupture to the peritoneal cavity or intracystic infection may give rise to diagnostic problems because of their unusual appearance. Intracystic bleeding was responsible for abdominal complaints in five (15%) patients with liver cysts in our series. In one patient with a preoperative suspicion of a hepatobiliary cystadenoma, intraoperative frozen section of the cyst wall showed features of a simple cyst without ovarian stroma, and deroofing revealed hemorrhagic intracystic fluid. In this particular case, a simple cyst was misdiagnosed as cystadenoma[11]. The precise etiology of bleeding in a simple cyst remains unclear, but rupture of blood vessels inside the cyst wall due to rapid enlargement is thought to be a likely mechanism[12]. In such cases, abdominal multiphase contrast enhanced computed tomography (CT) may be required to further characterize the nature of the lesion. CT or MRI imaging is also useful for exact localization and establishing the relationship of the cyst with surrounding vascular structures, when surgery is considered.
PLD is a rare clinical entity characterized by multiple simple cysts throughout the liver with variable size. Approximately 80% of PLD occurs in patients older than 60 years[2,13], as was also found in our patients. This entity may be associated with polycystic kidney disease or autosomal dominant PLD. Therefore, the kidneys and the pancreas should be assessed during imaging studies to determine whether these organs are affected too. Patients with PLD usually lack symptoms and are diagnosed during physical examination or incidentally, when imaging studies are performed for other reasons. Routine liver function tests usually are normal but may show elevated cholestatid parameters due to external compression of the bile ducts[14].
Although most patients with liver cysts are asympto-matic, a minority develops symptoms due to enlargement. As mentioned above, treatment should be considered only for progressive abdominal pain, or when complications have occurred. Also in case of suspicion on a hepatobiliary cystadenoma, surgical resection should be performed. Complete evaluation including upper GI endoscopy must be undertaken to exclude other causes of abdominal symptoms before symptoms may be attributed to the cyst. When the diagnosis has been established, several options ranging from no intervention to surgical treatment can be considered. Percutaneous sclerotherapy is first choice treatment because of its minimally invasive character and safety of the technique.
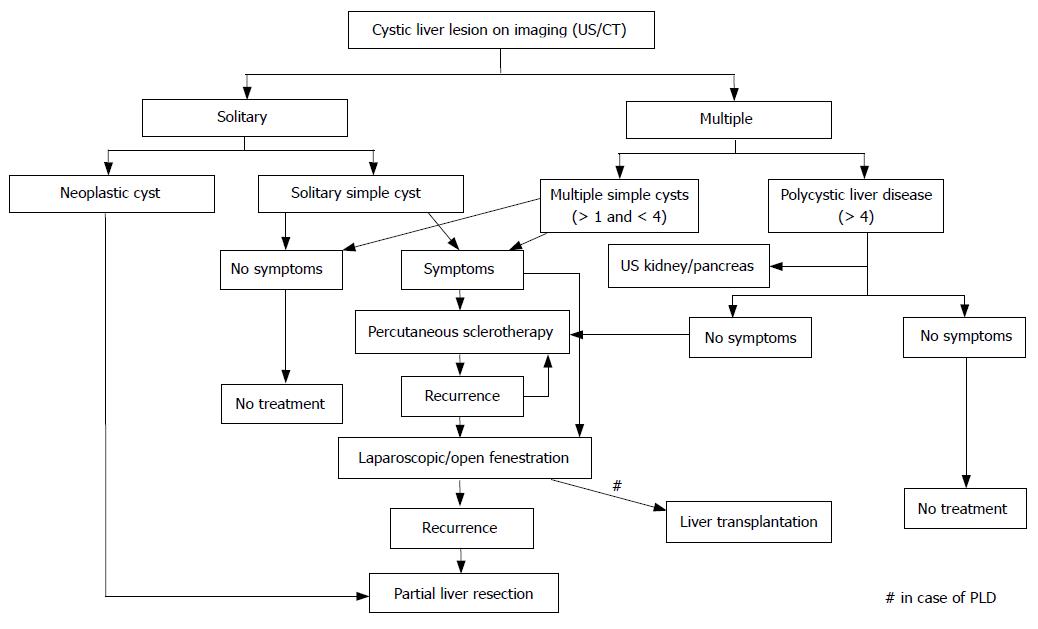
Percutaneous drainage of the cyst only results in temporary relief of abdominal pain and may be used as a trial treatment, to determine whether abdominal symptoms are attributable to the cyst[15]. For definitive treatment, concomitant instillation of tetracycline or ethanol in the cyst is applied to ablate the epithelial lining of the cyst which reduces recurrence remarkably[3,16]. Nevertheless, in our series, recurrence of the cyst after single percutaneous sclerotherapy of simple liver cysts (including solitary and multiple cysts) was seen in 20% of patients, as is consistent with other reports[3]. This failure rate may be explained by instilling an insufficient amount of sclerosant or by insufficient exposure of the cyst lining to the sclerosant, especially in large cysts. Of note is that percutaneous sclerotherapy for PLD is unsuccessful in the short-term, as was reflected by a recurrence of 77.8% within a few months in this series. Percutaneous sclerotherapy in PLD is indicated to assess whether symptoms are attributable to a dominant cyst or to bridge the patient to liver transplantation (Figure 5).
The second choice of treatment after percutaneous sclerotherapy has failed, comprises cyst wall deroofing[17]. This technique is indicated when a cyst recurs after percutaneous sclerotherapy with concomitant increase of abdominal complaints. The technique is based on removal of part of the cyst wall, usually the part lying external to the liver surface, allowing free drainage of intracystic fluid into the peritoneal cavity. Alternatively, enucleation of the cyst or a formal partial liver resection may be an option. Obviously, enucleation is the more parenchyma sparing method while a relatively avascular dissection plane exisits between the cyst wall and the surrounding liver parenchyma.
In previous reports, laparoscopic approach of cysts located in segments VI, VII and VIII were considered a contraindication for this non-invasive procedure[15,18]. However, according to a recent study, location of the cyst should not be a contraindication anymore for a laparoscopic procedure[4]. During the surgical procedure, the cyst content should be aspirated to determine whether communication with the intrahepatic biliary system is present. This may prevent bile leakage from cut bile ducts running from the parenchyma into the cyst wall. As mentioned above, complicated liver cysts after intracystic bleeding may show internal septations and/or wall nodules on imaging studies, and therefore, resection is advised if a neoplastic cyst cannot be ruled out.
In conclusion, the majority of patients with simple liver cysts or PLD are referred for progressive abdominal pain. As initial treatment, percutaneous sclerotherapy is appropriate and may determine whether the symptoms are attributable to the cyst. Surgical deroofing of the cyst wall, either laparoscopically or during laparotomy, is indicated when percutaneous treatment has failed. However, the results of percutaneous sclerotherapy and surgical treatment for PLD are disappointing. In case of suspicion of a complicated cyst or malignant lesion, complete excision of the cyst or partial liver resection is indicated.
S- Editor Liu Y L- Editor Alpini GD E- Editor Liu Y
| 1. | Caremani M, Vincenti A, Benci A, Sassoli S, Tacconi D. Ecographic epidemiology of non-parasitic hepatic cysts. J Clin Ultrasound. 1993;21:115-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 82] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Gaines PA, Sampson MA. The prevalence and characterization of simple hepatic cysts by ultrasound examination. Br J Radiol. 1989;62:335-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 117] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Simonetti G, Profili S, Sergiacomi GL, Meloni GB, Orlacchio A. Percutaneous treatment of hepatic cysts by aspiration and sclerotherapy. Cardiovasc Intervent Radiol. 1993;16:81-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Fabiani P, Iannelli A, Chevallier P, Benchimol D, Bourgeon A, Gugenheim J. Long-term outcome after laparoscopic fenestration of symptomatic simple cysts of the liver. Br J Surg. 2005;92:596-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Spiegel RM, King DL, Green WM. Ultrasonography of primary cysts of the liver. AJR Am J Roentgenol. 1978;131:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 41] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Mortelé KJ, Ros PR. Cystic focal liver lesions in the adult: differential CT and MR imaging features. Radiographics. 2001;21:895-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 255] [Article Influence: 10.6] [Reference Citation Analysis (74)] |
| 7. | Hsu KL, Chou FF, Ko SF, Huang CC. Laparoscopic fenestration of symptomatic liver cysts. Surg Laparosc Endosc Percutan Tech. 2005;15:66-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Mathieu D, Vilgrain V, Mahfouz AE, Anglade MC, Vullierme MP, Denys A. Benign liver tumors. Magn Reson Imaging Clin N Am. 1997;5:255-288. [PubMed] |
| 9. | Sanchez H, Gagner M, Rossi RL, Jenkins RL, Lewis WD, Munson JL, Braasch JW. Surgical management of nonparasitic cystic liver disease. Am J Surg. 1991;161:113-118; discussion 113-118;. [PubMed] |
| 10. | Liang P, Cao B, Wang Y, Yu X, Yu D, Dong B. Differential diagnosis of hepatic cystic lesions with gray-scale and color Doppler sonography. J Clin Ultrasound. 2005;33:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Erdogan D, Lamers WH, Offerhaus GJ, Busch OR, Gouma DJ, van Gulik TM. Cystadenomas with ovarian stroma in liver and pancreas: an evolving concept. Dig Surg. 2006;23:186-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Yamaguchi M, Kuzume M, Matsumoto T, Matsumiya A, Nakano H, Kumada K. Spontaneous rupture of a nonparasitic liver cyst complicated by intracystic hemorrhage. J Gastroenterol. 1999;34:645-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Carrim ZI, Murchison JT. The prevalence of simple renal and hepatic cysts detected by spiral computed tomography. Clin Radiol. 2003;58:626-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 206] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 14. | Schwed DA, Edoga JK, Stein LB. Biliary obstruction due to spontaneous hemorrhage into benign hepatic cyst. J Clin Gastroenterol. 1993;16:84-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Morino M, De Giuli M, Festa V, Garrone C. Laparoscopic management of symptomatic nonparasitic cysts of the liver. Indications and results. Ann Surg. 1994;219:157-164. [PubMed] |
| 16. | Kairaluoma MI, Leinonen A, Ståhlberg M, Päivänsalo M, Kiviniemi H, Siniluoto T. Percutaneous aspiration and alcohol sclerotherapy for symptomatic hepatic cysts. An alternative to surgical intervention. Ann Surg. 1989;210:208-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 76] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Jones WL, Mountain JC, Warren KW. Symptomatic non-parasitic cysts of the liver. Br J Surg. 1974;61:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Katkhouda N, Hurwitz M, Gugenheim J, Mavor E, Mason RJ, Waldrep DJ, Rivera RT, Chandra M, Campos GM, Offerman S. Laparoscopic management of benign solid and cystic lesions of the liver. Ann Surg. 1999;229:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 127] [Article Influence: 4.9] [Reference Citation Analysis (0)] |