Published online Jun 14, 2007. doi: 10.3748/wjg.v13.i22.3071
Revised: March 20, 2007
Accepted: March 28, 2007
Published online: June 14, 2007
AIM: To examine the in vivo phenotype associated with hepatic metastatic lymph node 64 (MLN64) over-expression.
METHODS: Recombinant-adenovirus-mediated MLN64 gene transfer was used to overexpress MLN64 in the livers of C57BL/6 mice. We measured the effects of MLN64 overexpression on hepatic cholesterol content, bile flow, biliary lipid secretion and apoptosis markers. For in vitro studies cultured CHO cells with transient MLN64 overexpression were utilized and apoptosis by TUNEL assay was measured.
RESULTS: Livers from Ad.MLN64-infected mice exhibited early onset of liver damage and apoptosis. This response correlated with increases in liver cholesterol content and biliary bile acid concentration, and impaired bile flow. We investigated whether liver MLN64 expression could be modulated in a murine model of hepatic injury. We found increased hepatic MLN64 mRNA and protein levels in mice with chenodeoxycholic acid-induced liver damage. In addition, cultured CHO cells with transient MLN64 overexpression showed increased apoptosis.
CONCLUSION: In summary, hepatic MLN64 over-expression induced damage and apoptosis in murine livers and altered cholesterol metabolism. Further studies are required to elucidate the relevance of these findings under physiologic and disease conditions.
- Citation: Tichauer JE, Morales MG, Amigo L, Galdames L, Klein A, Quiñones V, Ferrada C, R AA, Rio MC, Miquel JF, Rigotti A, Zanlungo S. Overexpression of the cholesterol-binding protein MLN64 induces liver damage in the mouse. World J Gastroenterol 2007; 13(22): 3071-3079
- URL: https://www.wjgnet.com/1007-9327/full/v13/i22/3071.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i22.3071
MLN64 (metastatic lymph node 64) cDNA was originally discovered as a highly expressed and amplified gene in certain breast, gastric, and esophageal cancers[1-3]. Although MLN64 could play a causative role in tumorigenesis, its amplification probably reflects the close genomic proximity (within 36 kb) to the oncogene c-erb-B2 (her-2/neu), which is invariantly coamplified[3,4]. The N-terminal of MLN64, the so-called MENTAL domain, includes four transmembrane helices, whereas the C-terminal domain contains the StAR-related lipid transfer domain (START)[5,6]. The latter domain is present in proteins involved in diverse cell functions[6,7] and exhibits 37% identity with StAR[5,6]. There is a family of proteins with homology to StAR, each containing the 200-210-aa START domain, in which MLN64 is known as StarD3[5,6].
Like StAR, the isolated MLN64 START domain binds[5] and transfers[8] cholesterol in vitro to the mitochondria and stimulates steroidogenesis when cotransfected with the cytochrome P450scc[8,9]. However, full-length MLN64 is less active in steroidogenic assays since its transmembrane domain localizes it to late endosomes with the START domain facing the cytosol[8,10]. For this reason, the role of MLN64 in steroidogenesis is still unclear, but it has been suggested that proteolysis could release the START domain to allow delivery of cholesterol to mitochondria[8]. This putative role for MLN64 in steroidogenesis has led to speculation that high levels of MLN64 observed in some breast carcinomas could contribute to the progression of these tumors through increased intratumoral steroidogenesis[4].
The localization and topology of MLN64 in late endosomes suggest that this protein participates in the efflux of cholesterol from late endosomes and lysosomes, possibly together with other proteins such as Niemann-Pick type C (NPC)1 and NPC2[10,11]. NPC disease is a lysosomal cholesterol-storage neurodegenerative disorder that is caused by deficiency of either NPC1 or NPC2[12,13]. Interestingly, expression of a truncated MLN64 protein lacking the START domain caused accumulation of free cholesterol in lysosomes of COS-1 and CHO cells, causing an NPC-like cellular phenotype[8]. These results suggest that MLN64 plays a role in the maintenance of endosomal cholesterol flow and intracellular cholesterol homeostasis. However, the overexpression of full-length MLN64 also induced an increase in sterol accumulation in COS cells, arguing against a role for MLN64 in cholesterol efflux from this compartment[14]. Moreover, mice with targeted mutation of the MLN64 START domain were neurologically intact and fertile, and exhibited only modest alterations in cellular sterol metabolism[15], leaving unanswered the question of MLN64 function in vivo.
MLN64 expression is detected in all tissues[16]. In the liver, MLN64 could mobilize cholesterol into mitochondria where it should be oxidized in the first step of the acidic pathway for bile acid synthesis[17]. Indeed, Pandak et al[18] and Ren et al[19,20] demonstrated that overexpression of the closest homologue of MLN64 in liver cells, StAR, leads to an important increase in bile acid synthesis both in vitro and in vivo. However, MLN64 overexpression in primary rat hepatocytes produced only a 20% increase in the rate of bile acid synthesis, suggesting that the hepatic expression of this protein is not a major determinant of the transport of cholesterol to the mitochondria during bile acid synthesis[19].
Even though MLN64 has been suggested to parti-cipate in intracellular cholesterol mobilization and steroidogenesis, its physiological function remains unclear. Interestingly, MLN64 cDNA has also been associated with other cellular processes such as the actin-mediated dynamics of late endocytic organelles[14] and apoptosis, since a differential-display analysis identified the gene as being downregulated in retinoic acid-induced apoptosis in T-cell lymphoma[21]. In the present study, we studied the in vivo phenotype associated with MLN64 overexpression in liver using an adenovirus-mediated overexpression strategy. We found that mice with hepatic MLN64 overexpression exhibited significant liver damage and apoptosis, and some minor alterations to cholesterol metabolism.
C57BL/6J mice originally purchased from Jackson Laboratory (Bar Harbor, ME) were bred to generate our own colony. All mice had free access to water and a chow diet (< 0.02% cholesterol; Prolab RMH 3000, PMI Feeds, St. Louis, MO). In some experiments, 2-mo-old C57BL/6J mice were fed chow supplemented with 2% chenodeoxycholic acid (CDCA) for 1 or 2 d.
Protocols were performed according to accepted criteria for the humane care of experimental animals, and were approved by the review board for animal studies of our institution.
The recombinant adenovirus encoding the full-length murine MLN64 cDNA (Ad.MLN64), under control of the cytomegalovirus (CMV) promoter, was generated by homologous recombination in bacterial cells using the AdEAsy system (generously provided by Dr. Bert Vogelstein, The Johns Hopkins University, Baltimore, MD)[22]. The control adenovirus Ad.E1Δ, containing no transgene, was kindly donated by Dr. Karen Kozarsky (SmithKline Beecham Pharmaceutics, King of Prussia, PA). Large-scale production of recombinant adenoviruses was performed from infected HEK 293 cells as described previously[23].
For viral administration, mice of 2 mo-old were anesthetized by ether inhalation, the femoral vein was exposed, and 1x10e11 viral particles (in 0.1 mL of isotonic saline buffer) of control or recombinant adenoviruses were injected intravenously. An additional control group received 0.1 mL of saline buffer only. Animals were studied 12-24 h after adenoviral infection.
Five micrograms of total liver RNA from individual animals was reverse transcribed using random hexamer primers. Semiquantitative RT-PCR was performed in the presence of [α-32P]dCTP for bax and mdm2 genes using primers based on mouse and rat cDNA sequences available in GeneBank databases (Table 1). After an initial 5 min 94°C denaturation step, thermal cycling involved 28 cycles at 94°C for 30 s, 52°C for 30 s, and 72°C for 30 s for bax mRNA analysis and 29 cycles at 94°C for 1 min, 52°C for 1 min, and 72°C for 1 min for mdm2 mRNA analysis.
| Gene | Primers | Fragment size |
| bax | TATTGGTGAGTCGGATTGC (S) | 230 bp |
| TGGACGGTCAGTGTCTGG (AS) | ||
| mdm2 | CCAACATGTCTGTGTCTACCG (S) | 216 bp |
| ACAATGTGCTGCTGCTTCTC (AS) | ||
| mln64 | TCGACATCTTTGTTCTGGCT (S) | 148 bp |
| GAGCAACTCAGAAAGGATGAC (AS) | ||
| 18 S | GTAACCCGTTGAACCCCATT (S) | 151 bp |
| CCATCCAATCGGTAGTAGCG (AS) |
Gene expression levels were compared to coampli-fication of 18S internal standards (Ambion, Austin, TX). PCR products were resolved by 2% agarose gel electrophoresis and transferred to a nylon filter. Radiolabeled bands were quantified using an imaging system (Molecular Imager GS-525, BIO-RAD, Hercules, CA), with the results normalized to the signal generated by radiolabeled 18S PCR products.
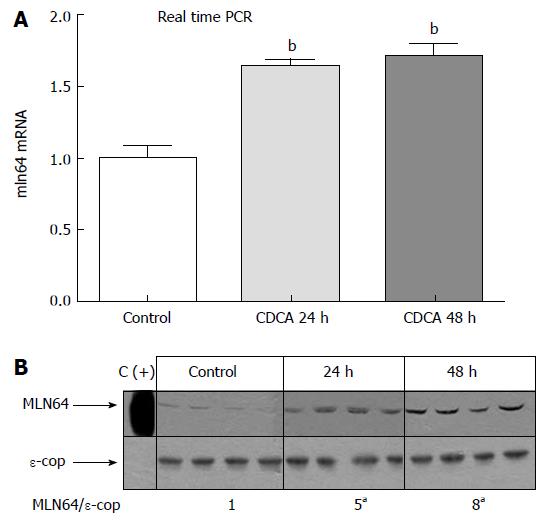
Two micrograms of total RNA from each liver sample were pretreated with DNAse I (Invitrogen, Carlsbad, CA) to remove any contaminating genomic DNA, and then reverse-transcribed to cDNA with random hexamers (Invitrogen, Carlsbad, CA). Real-time PCR was performed with a Stratagene Mx3000P Real-Time PCR System and Brilliant SYBR Green QPCR Mater Mix (Stratagene, La Jolla, CA) using 50 ng cDNA per 20 μL reaction volume, a SYBR green master mix and mln64-specific primers (Table 1). Changes in gene expression for mln64 were determined and normalized to 18S rRNA expression levels.
For MLN64, total membrane extracts and 10 000 ×g mitochondria/lysosome enriched-pellets from murine liver were prepared[24], and proteins were fractionated by 10% SDS-PAGE. After transfer to nitrocellulose, proteins were immunoblotted for MLN64 using anti-MLN64 antiserum[10] and a commercial antibody ab3478 (Abcam, Cambridge, UK). An anti-ε-COP (Coatomer Protein Complex Subunit Epsilon) antibody (obtained from Dr. Monty Krieger, Massachusetts Institute of Technology, Cambridge, MA) was used as a control for the membrane protein loading.
Liver homogenates were prepared for analyzing p53, p21, and Bax protein expressions[25]. For caspase-3 and-12 immunoblotting, liver homogenates were fractionated into total membranes and cytosol as described previously[23]. Proteins (50 μg/sample) were separated by 12% SDS-PAGE and immunoblotted using anti-caspase-12 (BD Biosciences Pharmingen, San Diego, CA), anti-caspase-3, anti-p53, anti-p21 and anti-Bax (Santa Cruz Biotechnology, Santa Cruz, CA). An anti-albumin antibody was used for protein normalization.
Antibody binding to protein samples was visualized using enhanced chemiluminiscence and measured using an imaging system (Molecular Imager GS-525, BIO-RAD).
Mice were anesthetized by intraperitoneal injection of sodium pentobarbital. After laparotomy, the cyst duct was ligated and a fistula was made in the common bile duct using a polyethylene catheter. Hepatic bile specimens were collected for 30 min, then plasma and liver samples were obtained as described previously[26,27].
Serum alkaline phosphatase (AP) and alanine aminotrans-ferase (ALT) were measured by standard methods. Hepatic and biliary cholesterol as well as biliary phospholipids and bile acids were determined by standard protocols[28,29].
Fresh-frozen liver tissues cryosectioned at 4-5 μm were fixed in acetone, rinsed three times in phosphate-buffered saline (PBS), permeabilized with 0.1% Triton X-100 for 15 min, blocked overnight in 10% goat serum in PBS, and incubated for 2 h at 37°C with the polyclonal antibody against MLN64 (1:500 dilution). The secondary antibody was fluorescein-isothiocyanate-conjugated goat antirabbit IgG (1:150 dilution). After washing in PBS, samples were mounted on coverslips using Fluoromount-G (EMS, Fort Washington, MD). Stained sections were examined by immunofluorescence microscopy.
Liver tissue was fixed in 4% paraformaldehyde for 48 h and then embedded in paraffin, sectioned, and placed on glass slides. Hematoxylin and eosin staining was then performed according to standard procedures.
TUNEL (TdT-mediated dUTP nick end labeling) staining was performed on sections of liver samples using a commercially available kit (TUNEL Apoptosis Detection Kit, Upstate, Charlottesville, VA) according to the manufacturer’s recommendations with minor modifications. Briefly, cryosections of fresh-frozen tissues were fixed in 4% paraformaldehyde in PBS for 30 min at room temperature. The tissue was permeabilized with 0.1% Triton X-100 and 0.1 mol/L sodium acetate for 2 min at 4°C, and then washed twice with PBS. Tissues were subjected to proteinase K treatment for 15 min. The DNA of apoptotic cells was enzymatically labeled with the terminal deoxynucleotidyl transferase for 1 h at 37°C and visualized by staining with fluorescein.
Liver tissue samples were lysed with hypotonic lysis buffer (50 mmol/L EDTA, 1% SDS, 50 mg/L proteinase K in 50 mmol/L Tris-HCl, pH 8) for 15 min on ice, and then incubated for 4 h at 37°C. DNA was extracted with an equal volume of phenol/chloroform followed by chloroform isoamylalcohol (24:1) extraction, the aqueous phase was collected, and DNA was precipitated by adding 150 mmol/L sodium acetate and two volumes of ethanol at -20°C for 1 h. The precipitate was pelleted by centrifugation at 10 000 ×g, and then washed in cold 70% ethanol. After centrifugation, each pellet was dissolved in autoclaved distilled water and electrophoresed on a 1.5% agarose gel containing 2 μg/mL ethidium bromide. DNA fragments were visualized in an ultraviolet transilluminator.
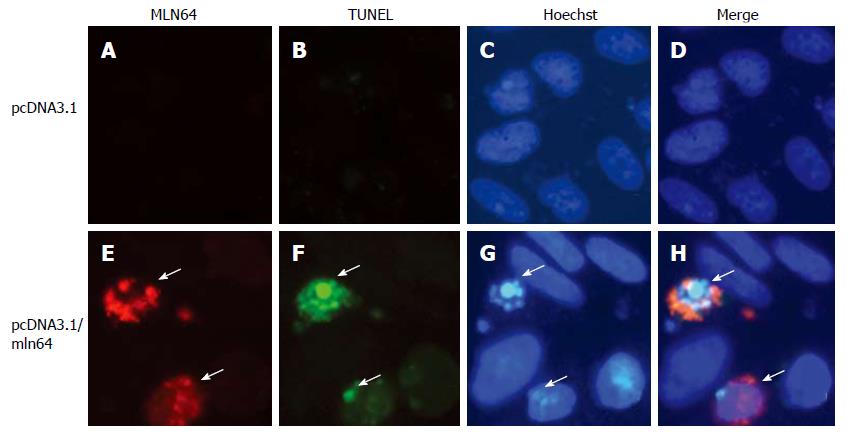
Chinese hamster ovary (CHO)-K1 cells were grown in Ham F-12 media supplemented with 10% fetal bovine serum (FBS), 100 μg/mL streptomycin, and 100 U/mL penicillin and maintained at 37°C, in an atmosphere of 5% CO2, and saturated humidity. CHO cells were transfected using a lipofectAMINE 2000 reagent (Invitrogen, Carlsbad, CA) with a pcDNA3.1 empty plasmid or a pcDNA3.1/MLN64 vector that was constructed by ligation of a cDNA HindIII fragment encoding mouse MLN64 in pcDNA3.1 (Invitrogen, Carlsbad, CA). After 24 h cells were subjected to immunofluorescence, TUNEL analysis, and Hoechst staining.
For immunofluorescence, CHO cells were fixed in 4% paraformaldehyde in PBS for 1 h, permeabilized with 0.1% Triton X-100/0.1% sodium citrate in PBS for 2 min, blocked 20 min in 0.02% gelatin in PBS and incubated 2 h with anti-MLN64 ab3478 antibodies (Abcam, Cambridge, UK) at 1:1000 dilution, followed by incubation with a Alexa-594-conjugated goat anti-rabbit IgG (Molecular Probes, Eugene, OR, dilution 1:1000). TUNEL analysis was performed using a commercial kit following manufacturer’s recommendations (Roche, Mannheim, Germany). For Hoechst 33258 staining, cells were incubated with 1:50 000 dilution of Hoechst 33258 (Polysciences, Warrington, Pennsylvania) for 15 min at 37°C. Fluorescence was analyzed and photographed under an appropriate microscope (Olympus BX51 TF, Tokyo, Japan).
Results are expressed as mean ± standard error values. The statistical significance of differences between the means of the experimental groups was evaluated using Student’s t-test for unpaired data and with one-way analysis of variance (ANOVA) with a post-hoc Tukey multiple-comparison test (Prism 3.0, GraphPad). A difference was considered statistically significant when the probability value was P < 0.05.
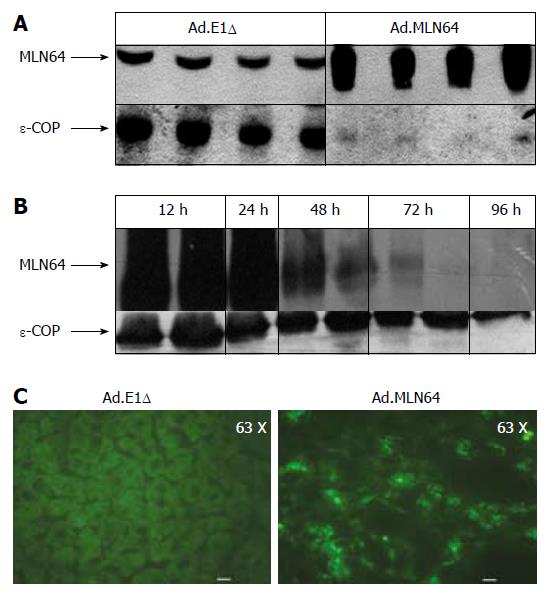
To determine the effects of hepatic MLN64 over-expression, Ad.MLN64 and the control Ad.E1Δ were infected into C57BL/6 mice. Western blot analysis (Figure 1A) of mitochondria/lysosome enriched preparations demonstrated that MLN64 protein was increased several times in the livers of Ad.MLN64-treated C57BL/6 mice at 24 h after infection. Overexpressed MLN64 was detected as a predominant 50-kDa protein[16]. The increase in hepatic MLN64 protein levels induced by the infusion of Ad.MLN64 was time-dependent (Figure 1B), reaching its maximal effect after 24 h of infection, and decreasing to undetectable levels after 96 h. Thus, all the subsequent experiments were performed at 24 h after Ad.MLN64 infection.
The liver MLN64 staining was essentially intracellular in Ad.MLN64-infected mice, exhibiting an abundant punctate pattern (Figure 1C) consistent with MLN64 protein localization in vesicular compartments as reported previously in other cell types[8,10].
As described above, hepatic MLN64 expression was highly increased at 12-24 h after infection with Ad.MLN64, but it dramatically decreased 96 h thereafter (Figure 1). This short half-life for transgene expression is an unusual finding for adenovirus-mediated gene expression in the liver, given that both previous studies[30,31] and our own investigations have found substantial levels of protein expression for other genes using this type of adenovirus still at 2 wk after infection.
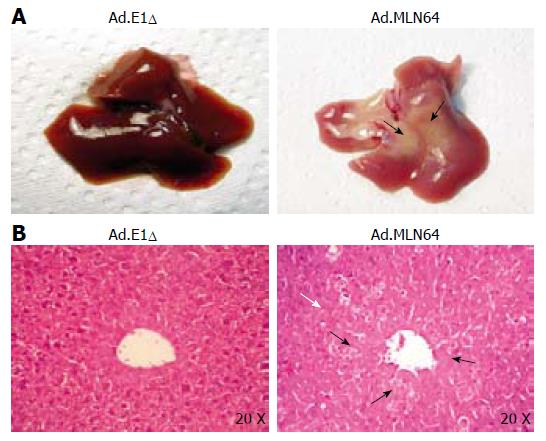
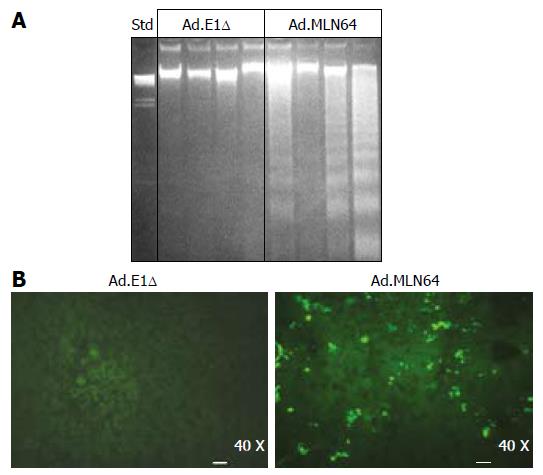
To explore the potential mechanisms underlying the rapid decrease in MLN64 overexpression, we evaluated liver damage and apoptosis in MLN64-infected mice. The first evidence of hepatic damage in these animals was in the gross morphology of the liver, with the formation of yellowish-white zones suggesting the presence of hepatic necrosis at 24 h after infection (Figure 2A). Furthermore, serum ALT and AP were significantly elevated in mice infected with virus-encoding CMV-MLN64 compared with both noninfected mice and those infected with the control Ad.E1Δ virus (Table 2). Interestingly, we found a dose-effect response in serum ALT and AP levels utilizing lower doses of viral particles (results not shown). Histological examination of liver sections demonstrated the presence of multiple necrotic zones as well as numerous condensed and fragmented nuclei, which are characteristic of apoptosis (Figure 2B). DNA fragmentation analysis and TUNEL assays revealed the presence of a DNA “ladder” pattern and TUNEL-positive cells in livers of MLN64-overexpressing mice (Figure 3), confirming that the apoptosis was induced by MLN64 overexpression.
The acute hepatocyte apoptosis/necrosis induced by MLN64 overexpression was fully reversible, with livers appeared healthy and normal at 1 wk after Ad.MLN64 infection, when no MLN64 expression was detected (data not shown). Remarkably, mortality was not increased in Ad.MLN-64-infected mice compared to controls.
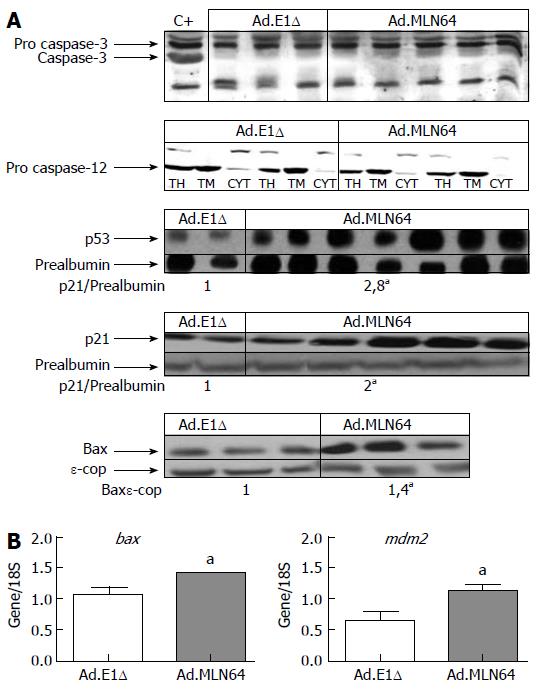
To further clarify the mechanism of MLN64-induced apoptosis, we evaluated the hepatic expressions of various apoptosis-related genes (Figure 4). Western blot analysis in MLN64-overexpressing mice revealed a significant increase in the expressions of proapoptotic proteins p53, p21, and Bax, whereas the activated forms of caspase-3 and caspase-12 were not detected (Figure 4A). Semiquanti-tative PCR analysis revealed that bax and mdm2 mRNA levels were increased in MLN64-overexpressing mice (Figure 4B).
To determine if overexpression of MLN64 in cultured cells, by a system that does not use an adenovirus to overexpress the protein, directly results in increased apoptosis, we transiently transfected CHO-K1 cells and analyzed apoptosis by TUNEL and Hoechst staining. We observed a clear increase in TUNEL positive cells in MLN64-overexpressing cells (Figure 5). Furthermore, nuclear morphology assessed by Hoechst staining of these cells confirmed an ongoing apoptotic process with brightly stained nucleus and chromatin condensation (Figure 5). The percentage of MLN64 transfected CHO cells undergoing apoptosis was 32%, whereas no positive TUNEL cells were detected in control CHO transfected cells with pcDNA3.1. Similar results were obtained in HEK 293 cells with MLN64 transient overexpression (results not shown). These results support a potential role for MLN64 in determining cell death.
Since excess cellular free cholesterol is a potent inducer of cell apoptosis[32,33], and MLN64 overexpression in COS cells causes cholesterol accumulation[14], we determined the hepatic total and unesterified cholesterol levels in mice with adenovirus-mediated hepatic MLN64 overexpression. As indicated in Table 3, MLN64 overexpression increased the hepatic free-cholesterol content by 21% relative to control animals.
| Hepatic cholesterol | Biliary lipid secretion | ||||||
| Unesterified | Ester | Bile flow | Cholesterol | Bile salts | Phospholipids | ||
| Group | Liver weight | (mg/g liver weight) | (mg/g liver weight) | (μL/min/100 g body weight) | (nmol/min/100 g body weight) | (nmol/min/100 g body weight) | (nmol/min/100 g body weight) |
| Ad.E1Δ | 1.32 ± 0.04 (n = 11) | 1.97 ± 0.08 | 0.20 ± 0.08 | 9.43 ± 0.76 (n = 8) | 2.75 ± 0.33 | 254.1 ± 34.2 | 84.25 ± 5.5 |
| Ad.MLN64 | 1.14 ± 0.04 (n = 16)a | 2.38 ± 0.08a | 0.21 ± 0.07 | 4.14 ± 0.61a | 1.38 ± 0.19a | 308.5 ± 45.4 | 54.64 ± 13.5 |
To begin to explore the pathophysiological relevance of our findings, we analyzed MLN64 liver expression in cholestatic hepatocyte injury induced by a diet high in CDCA, which is a well-characterized model of apoptosis-related liver damage induced by bile acids[34,35]. Figure 6 shows that MLN64 mRNA and protein levels increased significantly compared to control levels, demonstrating that MLN64 expression is indeed modulated in a liver-damage model involving apoptosis.
Since the phenotype of MLN64-infected mice-including liver damage, apoptosis, and the increase in plasma AP levels-suggested that animals were in a cholestatic condition, we studied the effects of hepatic MLN64 overexpression on bile flow and biliary lipid secretion (Table 3). The total bile flow decreased by 56% in Ad.MLN64-infected mice. The biliary output of cholesterol decreased by approximately 46% and biliary phospholipid secretion showed a trend to decrease. Biliary bile acid secretion remained within normal range, with a trend to increase. Consistent with the decrease in bile flow without changes in biliary bile acid output, biliary bile acid concentrations were increased twofold in Ad.MLN64-infected mice (71.07 ± 7.2 mmol/L in Ad.MLN64 mice vs 35.33 ± 7.4 mmol/L in Ad.E1Δ mice). The bile acid pool size and composition remained unchanged in Ad.MLN64-infected mice (data not shown).
This study demonstrates that adenovirus-mediated MLN64 overexpression in the mouse liver induces liver damage, most likely due to activation of the apoptosis cascade. Consistent with acute and reversible hepatocyte apoptosis induced by MLN64 overexpression, mortality was not increased and livers appeared healthy 7 d after Ad.MLN64 infection when no MLN64 overexpression was detected. In addition, MLN64 hepatic overexpression was associated with changes in hepatic cholesterol content, bile flow, and biliary bile acid concentration.
The precise function of MLN64 is unknown. The MENTAL region of MLN64, which contains four transmembrane domains, associates this protein with the membrane of endosomal compartments[10], and its C-terminal START domain makes it capable of mobilizing cholesterol[8,10]. Removal of the MLN64 N-terminal region increased steroidogenesis in COS-1 transfected cells[16] and enhanced the generation of steroid hormone by placental mitochondria in in vitro assays[8]. However, mice with targeted mutation of the MLN64 START domain did not exhibit a sterol-metabolism-related phenotype[15], questioning the physiological importance of the MLN64 START domain in cholesterol trafficking and metabolism.
The most significant finding of our study was the dramatic induction of liver damage in MLN64-infected mice, mainly through apoptosis as demonstrated by both TUNEL and DNA fragmentation analyses. We also detected an increase in ALT and AP plasma levels at 24 h after Ad.MLN64 infection, which is consistent with the current understanding of liver cell apoptosis; it is not a silent process and may also trigger liver inflammation and necrosis[36]. Adenovirus-mediated MLN64 overexpression was concentrated in some zones of the liver (results not shown). This was correlated with the visualization of hepatic damage in specific zones of the liver and suggests that acute and complete MLN64 down-regulation occurs mainly by apoptosis only in cells overexpressing MLN64. This response should induce liver regeneration in the injured zones and could explain that livers appear healthy 7 d after Ad.MLN64-infection.
We detected an increase in the hepatic levels of p53, p21, Bax, and Mdm2, suggesting that the apoptosis affects various signaling pathways. Surprisingly, active caspase-3 levels were not increased in MLN64-overexpressing livers. Caspase-3 is one of the central effector caspases when apoptosis is initiated by a variety of stimuli[37], and caspase-3 activation in liver has been described in several models of damage, such as iron deposition, steatohepatitis, and treatment with hepatocarcinogens[38,39]. The failure to detect caspase-3 activation in the present study could be due to a very low number of cells undergoing apoptosis or a low sensitivity of the caspase-3 antibody, or both. Alternatively, MLN64 overexpression may have induced apoptosis through non-caspase-3-mediated pathways.
The actual mechanism by which MLN64 mediates apoptosis is not clear and requires further study. Exami-nation of the activation of other caspases, such as caspase 6, 7, 8, 9 and 10 should help elucidate is the observed apoptosis is caused by the intrinsic pathway or extrinsic pathway[37]. One attractive hypothesis for MLN64-induced apoptosis is that this protein stimulates the delivery of free cholesterol into the endoplasmic reticulum (ER) membrane, triggering protein unfolding[32]. Consistent with this idea, liver unesterified cholesterol levels were increased and hepatic LDL receptor expression, which is normally controlled by the cholesterol levels in the ER, was downregulated in MLN64-overexpressing mice (results not shown). However, caspase-12, a specific effector that is activated during the ER stress response[37], was not activated in our MLN64-overexpressing mice. Still, we can not totally exclude a role for the ER stress response in MLN64 overexpression-induced apoptosis given that is has been shown that caspase-12 and caspase 4 are not always required for caspase-dependent ER stress induced-apoptosis[40]. Another possibility is that the lysosomal pathway of apoptosis[41] was activated in livers of MLN64-overexpressing mice. In this pathway, which can be activated by death receptors, lipid mediators, and photodamage, lysosomal proteases such as Cathepsin b (Ctsb) can be released by the lysosomes into the cytosol, where they contribute to the apoptotic cascade upstream of mitochondria[41]. MLN64 overexpression in the endolysosomal compartment may alter membrane structure and composition so as to favor the lysosomal release of proteases, which leads to apoptosis by activation of mitochondria-dependent apoptotic pathways without caspase-3 involvement. Indeed, it has been shown that MLN64 overexpression induces the formation of enlarged endosomes, an effect probably mediated by its MENTAL domain[42]. Interestingly, in vitro studies involving TNF-treated murine fibrosarcoma cells have shown that Ctsb is responsible for apoptosis-associated changes in the absence of caspase activity[43]. Further studies using Ctsb-/- mice and Ctsb inhibitors will help to ascertain the importance of lysosomal Ctsb release to this response and whether Ctsb inactivation attenuates the liver damage and apoptosis induced by MLN64 overexpression. Also, additional work is required to determine whether the increase in free-cholesterol levels and the MLN64 subcellular localization are important in the apoptotic response induced by MLN64 overexpression.
Our results using CHO cells with transient MLN64 overexpression supports a role for MLN64 in apoptosis. This cell culture system, which does not use the adenovirus to overexpress itself, is extremely useful in addressing concerns about the amount of adenovirus or adenoviral contaminants as the cause for the hepatotoxic effects. In addition, our results show that MLN64 effect on apoptosis is present in other cell types from diverse origin, such as CHO cells, which are derived from hamster ovary and not only in mouse hepatic-derived cells. We found that 30% of MLN64 overexpressing CHO were undergoing an apoptotic process. This results show that although there is a significant percentage of the population undergoing apoptosis, much higher that in cells that overexpress any protein, not all cells are suffering cell death. This may explain why previous overexpression studies of MLN64 in CHO, COS and Hela cells and primary rat hepatocytes have not seen an increase in cell death[8,14,19].
The (patho) physiological relevance of our findings is further supported by the results obtained in mice with CDCA-induced liver injury[34,35]. In fact, we found a significant increase in hepatic MLN64 protein and mRNA levels in the bile acid-induced liver injury model. This correlative evidence in vivo suggested a potential direct role on MLN64 expression in hepatocyte death due to CDCA administration.
MLN64 overexpression also increased the hepatic free-cholesterol content. This finding is consistent with a role for MLN64, proposed by Ren et al[19], in delivering cholesterol from lysosomes to the plasma membrane, where most of the cellular free cholesterol resides[44]. Another possibility is that cholesterol that accumulates in late endosomes primarily drives MLN64 protein to this subcellular compartment[14]. Further analyses are required to determine whether increased hepatic free cholesterol in MLN64-infected mice is localized in the plasma membrane or in intracellular compartments, and how this phenomenon is related to the apoptotic process.
In conclusion, we have shown that MLN64 over-expression induces apoptosis in the murine liver and alters cholesterol-metabolism-related parameters. Further studies are required to elucidate the physiological relevance of these findings.
The authors thank Dr. David Cohen and Dr. Flavio Nervi for technical support and helpful discussions.
S- Editor Liu Y L- Editor Robert SE E- Editor Ma WH
| 1. | Tomasetto C, Régnier C, Moog-Lutz C, Mattei MG, Chenard MP, Lidereau R, Basset P, Rio MC. Identification of four novel human genes amplified and overexpressed in breast carcinoma and localized to the q11-q21.3 region of chromosome 17. Genomics. 1995;28:367-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 198] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | Akiyama N, Sasaki H, Ishizuka T, Kishi T, Sakamoto H, Onda M, Hirai H, Yazaki Y, Sugimura T, Terada M. Isolation of a candidate gene, CAB1, for cholesterol transport to mitochondria from the c-ERBB-2 amplicon by a modified cDNA selection method. Cancer Res. 1997;57:3548-3553. [PubMed] |
| 3. | Moog-Lutz C, Tomasetto C, Régnier CH, Wendling C, Lutz Y, Muller D, Chenard MP, Basset P, Rio MC. MLN64 exhibits homology with the steroidogenic acute regulatory protein (STAR) and is over-expressed in human breast carcinomas. Int J Cancer. 1997;71:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Alpy F, Boulay A, Moog-Lutz C, Andarawewa KL, Degot S, Stoll I, Rio MC, Tomasetto C. Metastatic lymph node 64 (MLN64), a gene overexpressed in breast cancers, is regulated by Sp/KLF transcription factors. Oncogene. 2003;22:3770-3780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Tsujishita Y, Hurley JH. Structure and lipid transport mechanism of a StAR-related domain. Nat Struct Biol. 2000;7:408-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 405] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 6. | Alpy F, Tomasetto C. Give lipids a START: the StAR-related lipid transfer (START) domain in mammals. J Cell Sci. 2005;118:2791-2801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 300] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 7. | Soccio RE, Breslow JL. StAR-related lipid transfer (START) proteins: mediators of intracellular lipid metabolism. J Biol Chem. 2003;278:22183-22186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 234] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 8. | Zhang M, Liu P, Dwyer NK, Christenson LK, Fujimoto T, Martinez F, Comly M, Hanover JA, Blanchette-Mackie EJ, Strauss JF. MLN64 mediates mobilization of lysosomal cholesterol to steroidogenic mitochondria. J Biol Chem. 2002;277:33300-33310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 118] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Bose HS, Whittal RM, Huang MC, Baldwin MA, Miller WL. N-218 MLN64, a protein with StAR-like steroidogenic activity, is folded and cleaved similarly to StAR. Biochemistry. 2000;39:11722-11731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Alpy F, Stoeckel ME, Dierich A, Escola JM, Wendling C, Chenard MP, Vanier MT, Gruenberg J, Tomasetto C, Rio MC. The steroidogenic acute regulatory protein homolog MLN64, a late endosomal cholesterol-binding protein. J Biol Chem. 2001;276:4261-4269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 130] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Strauss JF, Liu P, Christenson LK, Watari H. Sterols and intracellular vesicular trafficking: lessons from the study of NPC1. Steroids. 2002;67:947-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277:228-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1130] [Cited by in RCA: 1132] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 13. | Naureckiene S, Sleat DE, Lackland H, Fensom A, Vanier MT, Wattiaux R, Jadot M, Lobel P. Identification of HE1 as the second gene of Niemann-Pick C disease. Science. 2000;290:2298-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 649] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 14. | Hölttä-Vuori M, Alpy F, Tanhuanpää K, Jokitalo E, Mutka AL, Ikonen E. MLN64 is involved in actin-mediated dynamics of late endocytic organelles. Mol Biol Cell. 2005;16:3873-3886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Kishida T, Kostetskii I, Zhang Z, Martinez F, Liu P, Walkley SU, Dwyer NK, Blanchette-Mackie EJ, Radice GL, Strauss JF. Targeted mutation of the MLN64 START domain causes only modest alterations in cellular sterol metabolism. J Biol Chem. 2004;279:19276-19285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Watari H, Arakane F, Moog-Lutz C, Kallen CB, Tomasetto C, Gerton GL, Rio MC, Baker ME, Strauss JF. MLN64 contains a domain with homology to the steroidogenic acute regulatory protein (StAR) that stimulates steroidogenesis. Proc Natl Acad Sci USA. 1997;94:8462-8467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 177] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1372] [Cited by in RCA: 1490] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 18. | Pandak WM, Ren S, Marques D, Hall E, Redford K, Mallonee D, Bohdan P, Heuman D, Gil G, Hylemon P. Transport of cholesterol into mitochondria is rate-limiting for bile acid synthesis via the alternative pathway in primary rat hepatocytes. J Biol Chem. 2002;277:48158-48164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Ren S, Hylemon P, Marques D, Hall E, Redford K, Gil G, Pandak WM. Effect of increasing the expression of cholesterol transporters (StAR, MLN64, and SCP-2) on bile acid synthesis. J Lipid Res. 2004;45:2123-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Ren S, Hylemon PB, Marques D, Gurley E, Bodhan P, Hall E, Redford K, Gil G, Pandak WM. Overexpression of cholesterol transporter StAR increases in vivo rates of bile acid synthesis in the rat and mouse. Hepatology. 2004;40:910-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Wang KC, Cheng AL, Chuang SE, Hsu HC, Su IJ. Retinoic acid-induced apoptotic pathway in T-cell lymphoma: Identification of four groups of genes with differential biological functions. Exp Hematol. 2000;28:1441-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509-2514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2861] [Cited by in RCA: 3045] [Article Influence: 112.8] [Reference Citation Analysis (0)] |
| 23. | Kozarsky KF, Jooss K, Donahee M, Strauss JF, Wilson JM. Effective treatment of familial hypercholesterolaemia in the mouse model using adenovirus-mediated transfer of the VLDL receptor gene. Nat Genet. 1996;13:54-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 131] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Jokinen EV, Landschulz KT, Wyne KL, Ho YK, Frykman PK, Hobbs HH. Regulation of the very low density lipoprotein receptor by thyroid hormone in rat skeletal muscle. J Biol Chem. 1994;269:26411-26418. [PubMed] |
| 25. | Zanlungo S, Amigo L, Mendoza H, Miquel JF, Vío C, Glick JM, Rodríguez A, Kozarsky K, Quiñones V, Rigotti A. Sterol carrier protein 2 gene transfer changes lipid metabolism and enterohepatic sterol circulation in mice. Gastroenterology. 2000;119:1708-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Amigo L, Mendoza H, Castro J, Quiñones V, Miquel JF, Zanlungo S. Relevance of Niemann-Pick type C1 protein expression in controlling plasma cholesterol and biliary lipid secretion in mice. Hepatology. 2002;36:819-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Rigotti A, Trigatti BL, Penman M, Rayburn H, Herz J, Krieger M. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc Natl Acad Sci USA. 1997;94:12610-12615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 710] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 28. | Nervi FO, Del Pozo R, Covarrubias CF, Ronco BO. The effect of progesterone on the regulatory mechanisms of biliary cholesterol secretion in the rat. Hepatology. 1983;3:360-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Nervi F, Marinović I, Rigotti A, Ulloa N. Regulation of biliary cholesterol secretion. Functional relationship between the canalicular and sinusoidal cholesterol secretory pathways in the rat. J Clin Invest. 1988;82:1818-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Kozarsky KF, Donahee MH, Rigotti A, Iqbal SN, Edelman ER, Krieger M. Overexpression of the HDL receptor SR-BI alters plasma HDL and bile cholesterol levels. Nature. 1997;387:414-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 559] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 31. | Ye X, Robinson MB, Pabin C, Quinn T, Jawad A, Wilson JM, Batshaw ML. Adenovirus-mediated in vivo gene transfer rapidly protects ornithine transcarbamylase-deficient mice from an ammonium challenge. Pediatr Res. 1997;41:527-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5:781-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 699] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 33. | Yao PM, Tabas I. Free cholesterol loading of macrophages induces apoptosis involving the fas pathway. J Biol Chem. 2000;275:23807-23813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 149] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Jones BA, Gores GJ. Physiology and pathophysiology of apoptosis in epithelial cells of the liver, pancreas, and intestine. Am J Physiol. 1997;273:G1174-G1188. [PubMed] |
| 35. | Higuchi H, Gores GJ. Bile acid regulation of hepatic physiology: IV. Bile acids and death receptors. Am J Physiol Gastrointest Liver Physiol. 2003;284:G734-G738. [PubMed] |
| 36. | Higuchi H, Gores GJ. Mechanisms of liver injury: an overview. Curr Mol Med. 2003;3:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Philchenkov A. Caspases: potential targets for regulating cell death. J Cell Mol Med. 2004;8:432-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 159] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 38. | Ribeiro PS, Cortez-Pinto H, Solá S, Castro RE, Ramalho RM, Baptista A, Moura MC, Camilo ME, Rodrigues CM. Hepatocyte apoptosis, expression of death receptors, and activation of NF-kappaB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am J Gastroenterol. 2004;99:1708-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 304] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 39. | Yajun Z, Hongshan C, Baoxi S, Dengbing Y, Jianhua S, Xinshun G, Li Y, Yi C. Translocation of Bax in rat hepatocytes cultured with ferric nitrilotriacetate. Life Sci. 2005;76:2763-2772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Obeng EA, Boise LH. Caspase-12 and caspase-4 are not required for caspase-dependent endoplasmic reticulum stress-induced apoptosis. J Biol Chem. 2005;280:29578-29587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 41. | Kroemer G, Martin SJ. Caspase-independent cell death. Nat Med. 2005;11:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 537] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 42. | Alpy F, Latchumanan VK, Kedinger V, Janoshazi A, Thiele C, Wendling C, Rio MC, Tomasetto C. Functional characterization of the MENTAL domain. J Biol Chem. 2005;280:17945-17952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | Foghsgaard L, Wissing D, Mauch D, Lademann U, Bastholm L, Boes M, Elling F, Leist M, Jäättelä M. Cathepsin B acts as a dominant execution protease in tumor cell apoptosis induced by tumor necrosis factor. J Cell Biol. 2001;153:999-1010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 488] [Cited by in RCA: 484] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 44. | Liscum L, Munn NJ. Intracellular cholesterol transport. Biochim Biophys Acta. 1999;1438:19-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 254] [Article Influence: 9.8] [Reference Citation Analysis (0)] |