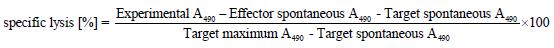INTRODUCTION
Induction of tumor-specific immunity is an attractive approach to cancer therapy because of the possibility to harness the body’s own defense mechanisms to destroy metastatic tumors and to provide long-term protection against tumor recurrence. The conceptual framework for immunotherapy depends on the presence of tumor-specific antigens and the ability to induce a cytotoxic immune response that recognizes tumor cells presenting antigens. Cytotoxic T lymphocytes (CTLs) recognize major histocompatibility complex (MHC) classImolecules complexed to peptides derived from cellular proteins presented on the cell surface in combination with costimulatory molecules[1]. However, immunotherapeutic application using tumor-associated antigens as a vaccine component is limited to patients with a defined cancer because only few antigens have been identified to date[2]. Some studies circumvent this limitation by utilizing tumor-cell lysates, which probably include both known and unknown antigens[3,4]. The tumor-cell lysate is a very attractive antigen source for the development of versatile cancer immunotherapy. In fact, several studies demonstrated that dendritic cells pulsed with tumor-cell lysates could offer the potential advantage of augmenting a broader T cell-immune response against uncharacterized tumors. However, this method is rather laborious and time-consuming, as the dendritic cells (DC) have to be prepared from the patient’s blood for ex vivo pulsing with tumor cell lysates and are then reinfused[3-5].
The possibility of eliciting antitumor immunity by in situ vaccination by unmasking tumor antigens for appropriate presentation in a cytokine environment stimulating cell-mediated immunity would abrogate the need to obtain and culture a patient’s autologous tumor cells for manipulation in vitro, including transduction with cytokine genes, irradiation, and subsequent vaccination.
Using the fusogenic membrane protein G from vesicular stomatitis virus (VSV-G), which triggers cell fusion at pH 5.5, Linardakis et al recently demonstrated in a syngeneic murine B16 melanoma model that FMG expression can enhance the efficacy of a weak allogeneic vaccine[5]. Fusogenic membrane glycoproteins (FMG) were introduced as a new class of therapeutic genes for cancer gene therapy by Bateman et al[6], who demonstrated that FMG expression alone resulted in a significantly greater tumor growth control than suicide prodrug systems. For cancer gene therapy, glycoproteins from human immunodeficiency virus (HIV-1)[7], gibbon ape leukemia virus (GALV)[8,9], and measles virus (MV)[10] have been evaluated.
Intratumoral expression of viral fusogenic glyco-proteins leads to syncytia formation of infected cells with adjacent cells, thereby increasing the dispersion of viruses throughout the tumor, lateral spread of the transgene, virus release and enhanced immunogenicity of tumor cells. In measles virus, the hemagglutinin (H) protein mediates attachment to its receptor on the target cell[11,12] and thus triggers conformational changes in the virus fusion (F) glycoprotein[13]. This leads to a biologically active fusogenic form of the F protein that interacts with the host cell membrane, causing virus-cell or cell-cell fusion[14]. Both H and F proteins are necessary for fusion to occur.
In this study, we assessed whether the intratumoral expression of measles virus fusogenic membrane proteins alone or in combination with local cytokine expression can serve as an in situ tumor vaccination strategy for colorectal cancer in two syngeneic bilateral subcutaneous colorectal cancer models in C57BL/6 and BALB/c mice. We evaluated the following five cytokines encoded by replication-defective adenovirus vectors: IL-2, which acts as a growth factor for T, B and natural killer (NK) cells and regulates T cell survival by promoting activation-induced cell death[15]; IL-12, which stimulates proliferation of T as well as NK cells[16]; IL-18, which regulates Th1 and Th2 immune responses[17] and stimulates IFN-γ production from immune cells[18]; IL-21, which has immunostimulatory effects on T and NK as well as dendritic cells[19] and promotes the proliferation of some B cells[19,20]; and GM-CSF, which acts mainly on CD4+ and CD8+ T cells and dendritic cells[21] but can also promote humoral immune responses[22,23].
In several clinical studies, systemic administration of cytokines has been evaluated for the treatment of cancer. High-dose cytokine therapy has proven to be effective in some cases, but there has been a considerable range of adverse side effects limiting the applicability[24,25]. In our study, the cytokines were expressed intratumorally, resulting in local high cytokine concentration and therefore reduced systemic side effects[26].
We monitored the anti-neoplastic effects of the directly vector-inoculated tumor and effects on the growth of the contralateral untreated tumor in a bilateral subcutaneous syngeneic colon cancer model in mice. In addition we analyzed the induction of a tumor-specific cytotoxic T lymphocyte (CTL) response. Our data indicate that intratumoral expression of MV-FMG particularly in combination with cytokine expression can serve as an in situ tumor vaccination strategy for colorectal cancer.
MATERIALS AND METHODS
Cells and cell culture
The human colon adenocarcinoma cell line HT-29 (ATCC HTB-38)[27] was purchased from the American Type Culture Collection (Manassas, VA), and the murine colon adenocarcinoma Colon26 cell line from CLS (Heidelberg, Germany). The murine colon adenocarcinoma cell line MC38 was a gift from Steven A. Rosenberg, NCI, NIH, Bethesda, MD. The human embryonic kidney cell line 293 was purchased from Microbix Biosystems (Toronto, ON).
Viruses
The adenovirus vector Ad.MV-H/F (previously named as Ad CMV F&H[28]), which carries a bicistronic expression cassette H/IRES/F encoding measles virus H and F, was kindly provided by Matthias Dobbelstein, Department of Molecular Oncology, University of Göttingen, Germany. The adenovirus vector Ad.IL-2 encoding human IL-2, which is cross-active in mice[29], has been described previously[30].
The adenovirus vectors Ad.IL-12, Ad.IL-18, Ad.IL-21 and Ad.GM-CSF encoding the murine cytokines IL-12, IL-18, IL-21, and GM-CSF, respectively, were generated using the AdEasy-1 system[31]. The cDNA for mIL12 (pNGVL3-mIL12[32]; kindly obtained from Alexander Rakhmilevich, Department of Human Oncology, University of Wisconsin-Madison, Madison, WI), mIL-18 (pCR3.1:IL-18[33]; kindly provided by Camille Locht, Laboratoire de Microbiologie Génétique et Moléculaire, Institut Pasteur de Lille, Lille, France), mIL-21 (pORF9-mIL21, InvivoGen, San Diego, CA), and mGM-CSF (pGT60mGM-CSF, InvivoGen) were cloned into the adenovirus transfer vector pAd.Track[31].
All adenovirus vectors used in this study were E1 and E3-deleted and produced in 293 cells. All viruses were purified with the Vivapure AdenoPACK 100 kit (Vivascience, Hannover, Germany). The adenovirus particle concentration in purified preparations was determined by spectrophotometry as described previously[34] and expressed as viral particles (VP)/mL. With the used ion-exchange column purification kit we obtained constant particle-to-PFU ratios of about 30:1. The vector Ad.MV-H/F was produced in the presence of the synthetic fusion inhibitory peptide Z-D-Phe-Phe-Gly-OH (10 μmol/L; Bachem AG, Bubendorf, Switzerland)[35]. The functionality of the cytokine encoding adenovirus vectors were determined using cytokine specific ELISA kits (Biosource International, Camarillo, CA and R&D Systems, Minneapolis, MN). For this, 500 000 293 cells were transduced at an MOI of 30 VP/cell in 1 mL with the vectors. Twenty-four hours after transduction with Ad.IL-2, Ad.IL-12, Ad.IL-18, Ad.IL-21, or Ad.GM-CSF we detected in the supernatants 150, 500, 180, 130, and 500 pg of the respective cytokines.
Quantification of syncytia formation by confocal laser scanning microscopy and flow cytometric analysis
To detect cell fusion, the opposing fusion partners were cytosolically stained with CellTracker Green CMFDA or CellTracker Orange CMTMR (Invitrogen, Molecular Probes, Eugene, OR) according to the manufacturer's instructions and seeded in an equal ratio onto culture slides (BD Biosciences Pharmingen, San Diego, CA). Next morning, 95%-100% confluent cell monolayers were transduced with Ad.MV-H/F at a multiplicity of infection (MOI) of 1000 VP/cell (MC38 and Colon26) and 200 VP/cell (HT-29), respectively. The chosen MOI for all cell lines resulted with Ad5.GFP in -100% transduction efficiency.
For flow cytometric analysis, 24 h after viral infection cells were detached by trypsin treatment, washed once with PBS and analyzed (FACSCalibur flow cytometer, Becton Dickinson Immunocytometry Systems, Mansfield, MA). For confocal laser scanning microscopy, 36 h after transduction, cells were washed and fixed with 2% paraformaldehyde. Slides were mounted and covered with thin cover slips before analyzing with the confocal laser scanning microscope TCS SP2 + DMIRE2 (Leica, Bensheim, Germany). Dual fluorescence, indicating membrane fusion, was quantified using the ImageJ (Version 1.36b, NIH, Bethesda, MA) software with the colocalization plug-in.
Animal studies
Six to eight week-old female C57BL/6 and BALB/c mice were obtained from Janvier (Le Genest-St-Isle, France). For the syngeneic bilateral subcutaneous syngeneic tumor model, C57BL/6 or BALB/c mice received subcutaneously 1 × 105 MC38 or Colon26 cells, respectively, in 100 μL into the right hind flank and 1 × 104 cells in 100 μL into the left hind flank. Animals were randomly assigned to treatment groups (n = 5 for each tumor model) when the tumor on the right hind flank reached a volume of some 200 mm3 and the tumor on the left side was palpable.
Animals treated just with the Ad.MV-H/F or the cytokine encoding adenovirus vectors received 6 × 109 VP in 100 μL PBS on d 0 and 2 into the right tumor. When Ad.MV-H/F was administered in combination with the cytokine encoding adenoviral vectors, 3 × 109 VP of each vector in 100 μL PBS was injected on d 0 and 2 into the right tumor. At least once a week, minimum and maximum perpendicular tumor axes were measured using vernier calipers, and tumor volume was calculated using the simplified formula of a rotational ellipse (l×w2× 0.5). The skin thickness of 0.4 mm was subtracted from the measurements. Animals were maintained under specific pathogen-free conditions. To generate effector cells, mice were sacrificed and spleens were harvested and weighed 28 d after virus inoculation.
Immunohistochemistry
For sectioning, tumors were embedded in Jung tissue-freezing medium (Leica Instruments, Nussloch, Germany) as described previously[36]. A Leica CM1900 (Leica Instruments, Wetzlar, Germany) cryostat was used to prepare ten micron cryosections. Sections were transferred to microscope slides, followed by acetone fixation at room temperature for 2 min. After three times washing with phosphate buffered saline (PBS), sections were immunostained with rat anti-mouse CD11b (M1/70.15.11.5) fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies (Miltenyi Biotec Inc., Auburn, CA). Digital images were taken with a high-resolution still camera (Olympus DP50, Tokyo, Japan) attached to a fluorescence microscope (Olympus BX51, Tokyo, Japan).
CTL assay
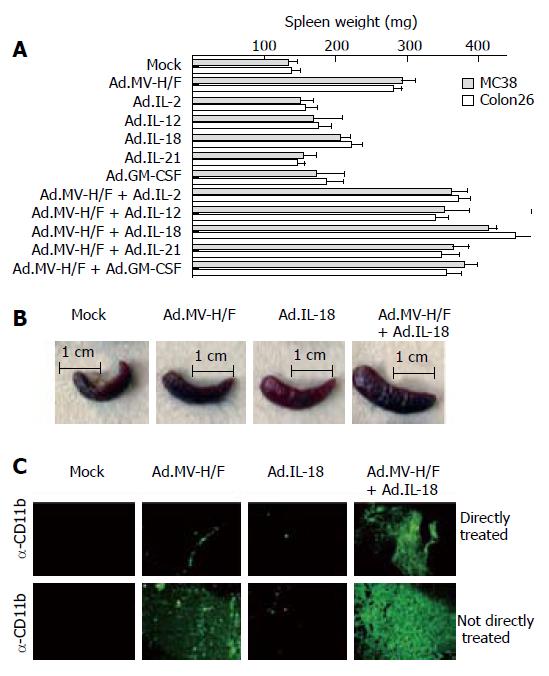
We analyzed the cytotoxic T lymphocyte (CTL) response to tumor cells, using the lactate dehydrogenase (LDH) based CytoTox 96 (Promega) assay according to the manufacturer’s instructions. In brief, target cells (MC38 or Colon26 cells) were plated at a density of 5000 cells per well in round-bottom 96-well plates. Target cells were then mixed with effector cells for 4 h at the indicated ratios. LDH release was determined measuring absorbance at 490 nm with a plate reader, and the specific lysis was calculated from triplicate samples as follows:
Math 1
Statistical analysis
The statistical software package SPSS 13 (SPSS Inc., Chicago, IL) was used for data analysis with indicated tests. P < 0.05 was considered significant.
RESULTS
MV-FMG expression induces cell-cell fusion and the formation of multinucleated syncytia of colorectal cells
First, using flow cytometry and confocal laser scanning microscopy, we analyzed whether transduction of confluent MC38 and Colon26 cell monolayers with the measles virus H and F encoding adenovirus Ad.MV-H/F results in dye colocalization, indicative of cell-cell fusion. The human colon cancer cell line HT-29 served as a positive control.
As shown in Figure 1A, flow cytometric analyses, 24 h after transduction with Ad.MV-H/F, revealed in the murine colon carcinoma cell lines a slight cell-cell fusion, whereas in the HT29 cells we observed extensive cell-cell fusion. We confirmed these data qualitatively by confocal laser scanning microscopy 36 h after transduction with Ad.MV-H/F (Figure 1B).
Figure 1 Quantification of cell-cell fusion by flow cytometry and laser scanning confocal microscopy.
A: Twenty-four hours later cells were analyzed for dye colocalization by flow cytometry. As a control we used the synthetic fusion inhibitory peptide (FIP); B: In addition we analyzed the cells 36 h after transduction with Ad.MV-H/F by confocal laser scanning microscopy. Transduction of Colon26 cells with Ad.MV-H/F resulted in similar cell-cell fusion as in MC38 cells (data not shown). One representative experiment out of three is shown.
Regression of the vector-treated tumor by intratumoral expression of MV-FMG was enhanced by local cytokine expression
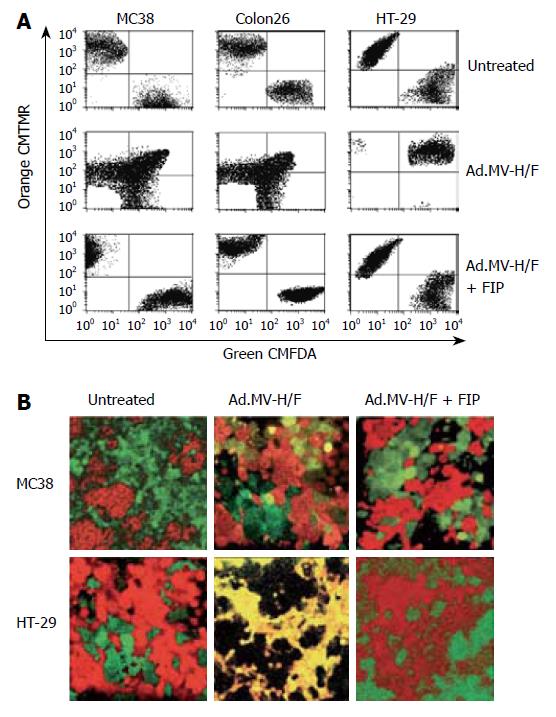
We evaluated whether the combination of MV-FMG expression and cytokine (IL-2, IL-12, IL-18, IL-21, or GM-CSF) expression results in an enhanced in vivo treatment efficacy of the directly vector-treated tumor and the contralateral tumor, when compared to single agent treatment of the treatment components, in a syngeneic bilateral subcutaneous MC38 colorectal tumor model in C57BL/6 mice. As shown in Figure 2, intratumoral inoculation of Ad.MV-H/F alone resulted in a 51% reduction of the treated tumor at d 28 (P < 0.005, ANOVA with Tukey’s HSD). Administration of IL-12, IL-18 or IL-21 encoding vector resulted in an about 10% to 47% reduction of directly treated tumor (P < 0.01, ANOVA with Tukey’s HSD). Treatment with Ad.IL-2 and Ad.GM-CSF produced a 10% reduction of the directly treated tumor (P = NS, ANOVA with Tukey’s HSD). Intratumoral administration of Ad.MV-H/F in combination with the IL-2, IL-12, IL-18, IL-21, or GM-CSF encoding vectors resulted in a 87% to 98% reduction of directly treated tumor, respectively (P < 0.01, ANOVA with Tukey’s HSD).
Figure 2 Local and immune-mediated tumor control in a syngeneic bilateral subcutaneous colon cancer model.
A: The volume of the tumor on the right flank was measured at d 28 and presented as box-and-whisker plots, showing minimum, 25th percentile, median, 75th percentile, and maximum tumor volume; B: The volume of the tumor on the left flank, which did not receive direct viral vector injections, was measured at d 28 and the volume reduction relative to mock treated animals is presented as bar graphs (mean ± SD). Data of C57BL/6 are presented in white and the data of BALB/c are presented in orange.
To assess whether our results are unique to MC38 cells and C57BL/6 mice (H-2b), we repeated the syngeneic bilateral tumor model with Colon26 cells in BALB/c mice (H-2d), which have contrasting susceptibilities to certain intracellular pathogens[37,38]. The experimental design was identical to that described above for MC38 cells in C57BL/6 mice. As shown in Figure 2, the results are qualitatively similar to that obtained with MC38 cells.
The efficacy of MV-FMG expression as a tumor vaccine is enhanced by intratumoral cytokine expression
To determine whether intratumoral expression of measles virus H and F can serve as an in situ tumor vaccination, we monitored the tumor growth of the not directly vector-treated tumor on the left flank (Figure 2). Intratumoral expression of measles virus H/F resulted in a 54% reduction of the contralateral left tumor when compared to mock treated animals, respectively (P < 0.001, ANOVA with Tukey’s HSD). Intratumoral treatment of animals with IL-2, IL-12, IL-18, IL-21, or GM-CSF encoding vectors resulted in a 8%, 20%, 46%, 32%, or 21% reduction of the not directly vector-treated tumor, respectively (P≤ 0.05, ANOVA with Tukey’s HSD; P = NS for Ad.IL-2). The combination of Ad.MV-H/F with cytokine encoding adenoviral vectors resulted in an about 85% reduction of the not directly vector-treated tumor (P < 0.001, ANOVA with Tukey’s HSD).
Intratumoral cytokine expression increased measles virus H and F expression induced splenomegaly
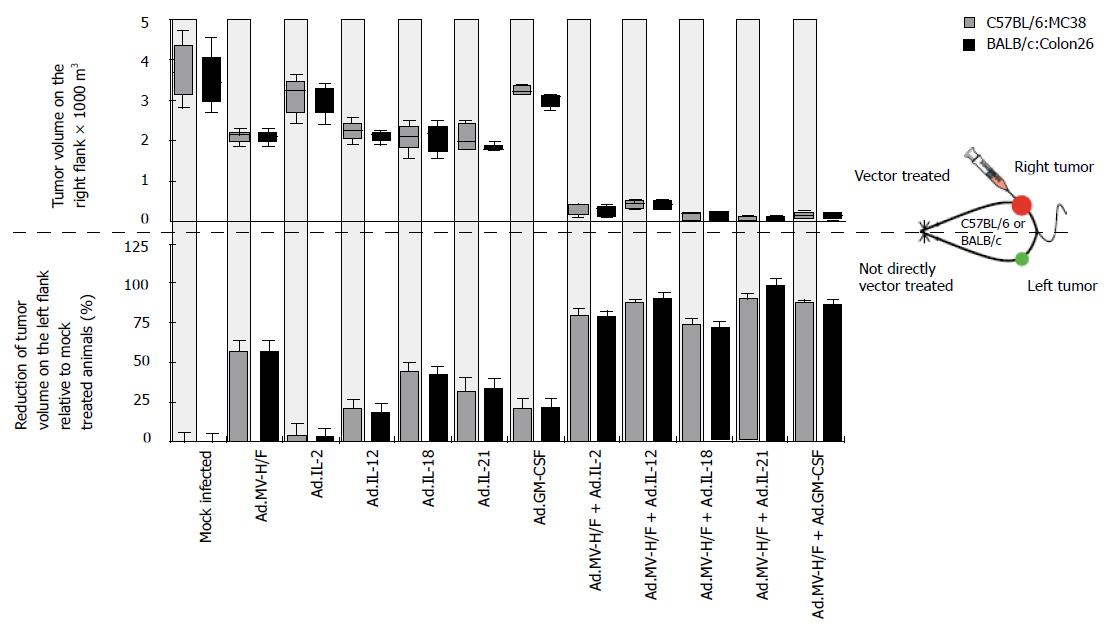
To analyze whether the observed growth regression of the not directly vector-treated tumor was immune mediated, we determined on d 29 the spleen weight of the animals (Figure 3A). We observed in both tumor models in animals treated with Ad.MV-H/F about 230% increased median spleen weight when compared to mock infected animals. When compared to mock infected animals, treatment with cytokine encoding adenoviral vectors resulted in about 30% increased spleen weight (P = NS, ANOVA with Tukey's HSD; P < 0.05 for Ad.IL-18). The combination of intratumoral Ad.MV-H/F inoculation with the cytokine encoding vectors resulted in a -300% increased spleen weight. The treatment combination of intratumoral MV-FMG and IL-18 expression resulted in the most pronounced splenomegaly. The spleens of representative mice of different treatment groups are shown in Figure 3B.
Figure 3 Effect of indicated treatments on the spleen weight and infiltration of tumors with macrophages.
Treatment was carried out as described in Figure 2. A: At d 29 animals were euthanized and spleen weight was determined (mean ± SD); B: The spleens of representative mice of different treatment groups are shown; C: In addition, fourteen days after initiation of therapy continous serial sections of the directly and not directly vector treated tumors of spare animals were prepared and individually immunostained for indicated cells. Representative slides are shown; original magnification × 400. Similar data were obtained in the Colon26 tumor model (data not shown).
Local and distant anti-neoplastic effects are associated with the tumor infiltration of macrophages
Shown by immunohistochemistry (Figure 3C), the combination therapy consisting of Ad.MV-H/F and Ad.IL-18 resulted in a strongly enhanced infiltration of macrophages into the not directly vector-treated tumors, when compared to mock or single vector-treated animals.
Intratumoral cytokine expression enhanced measles virus H and F expression-induced tumor cell-specific cytotoxic T cell response
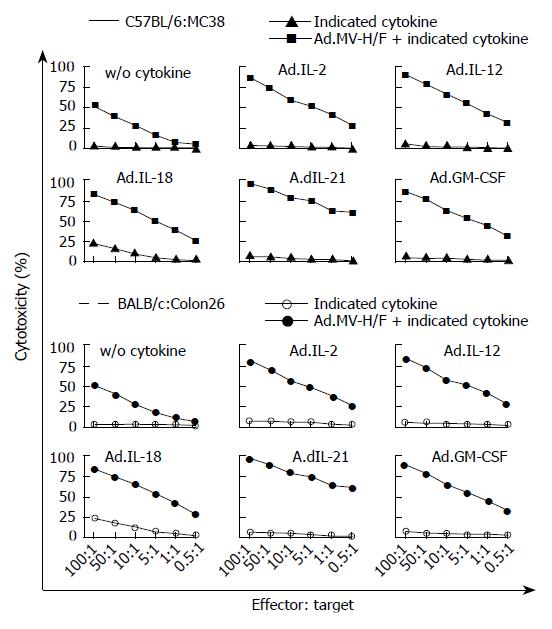
To analyze whether the observed effects on tumor growth regression of the not directly vector-treated tumors were mediated by tumor-specific lymphocyte response, we performed an LDH based cytotoxicity assay. As shown in Figure 4, the effector splenocytes derived from untreated mice without tumor did not lyse target tumor cells (MC38 or Colon26 cells). Splenocytes derived from mock treated tumor bearing animals did not lyse target tumor cells. A slight lysis of target cells was observed for splenocytes of animals treated with Ad.IL-2, Ad.IL-12, Ad.IL-21, or Ad.GM-CSF, while Ad.IL-18 treated animals had the highest CTL activity resulting in 25% cell lysis at an effector to target ratio of 100:1. Splenocytes of animals treated with Ad.MV-H/F showed a cytotoxicity of about 51% at a ratio of 100:1. The combination with the interleukin encoding adenoviruses resulted in a median cytotoxicity of 76% at an effector to target ratio of 100:1, whereas the highest cytotoxicity was observed with the splenocytes from Ad.MV-H/F and Ad.IL-21 treated animals.
Figure 4 T cell mediated tumor regression by expression of MV-FMG alone or in combination with cytokines.
Data of all animals were expressed as the percentage of specific release of three independent experiments (mean ± SD).
DISCUSSION
The intratumoral expression of viral fusogenic membrane proteins is a promising approach for cancer gene therapy, since their expression in tumor cells is directly cytotoxic and associated with a local bystander effect[6] but can also induce an anti-tumor immunity[39,40]. Whether the expression of measles virus H and F in murine cells results in cell-cell fusion remains controversial. There have been reports that no cell-cell fusion occurs in murine cells upon expression of MV-FMG[41], while others demonstrated cell-cell fusion upon measles virus FMG expression in highly confluent murine cell monolayers[42,43].
In this study, we demonstrated dye colocalization by flow cytometry and confocal laser scanning microscopy in murine cells upon MV-FMG expression, indicating cell-cell fusion[44]. Fused murine cells were smaller and cell-cell fusion occurred to a lesser extent than in human cells, but fusion was clearly due to measles virus H and F expression since cell-cell fusion could be blocked by adding a measles virus-specific fusion inhibitory peptide[35].
The key findings of the colorectal cancer models in C57BL/6 and BALB/c mice can be summarized as the following. First, intratumoral expression of measles virus H and F by the adenovirus vector Ad.MV-H/F resulted, despite the limited intratumoral spread and transduction efficiency of the replication-defective adenovirus vectors, in tumor regression of the directly vector-treated tumors, confirming previous studies[6]. Due to the host specificity of adenovirus, generally human adenovirus will not infect murine cells productively[45]. Thus a trans-complementation of the replication-defective vectors for replication[46] to improve tumor transduction efficiency[47] is not possible in this model. Second, we confirmed that FMG expression can serve as a tumor vaccination platform[5,39], since we observed regression of the not directly vector-treated tumor. Third, intratumoral expression of IL-12, IL-18 and IL-21 resulted in reduction of both the directly vector-treated and the contralateral untreated tumor. However, in both models the intratumoral expression of IL-2 did not result in a regression of the contralateral tumor. There have been several studies examining the tumor therapy potential of interleukins, mostly IL-2 and IL-12, administered as recombinant proteins or expressed from DNA plasmid vectors in tumor vaccination trials mostly in combination with chemotherapy[48,49]. Fourth, intratumoral expression of MV-FMG in combination with the cytokines IL-2, IL-12, IL-18, IL-21 or GM-CSF, encoded by adenovirus vectors, resulted in a significantly improved treatment efficacy of the directly vector-inoculated tumors, but also of the contralateral, not vector-treated tumors, when compared to single agent therapy. Fifth, treatment of animals with the combination of Ad.MV-H/F and or cytokine expression induced tumor-specific cytotoxic T lymphocyte responses and a massive increased spleen weight. This suggests that the cytoreductive effects of MV-FMG expression alone and in combination with intratumoral cytokine expression on the not directly vector-treated tumors were immune mediated.
A conceivable mechanism for the induction of tumor-specific immunity by expression of measles virus fusogenic membrane proteins and cytokines are the xenogenization of tumor cells by presentation of viral antigens on the cell surface in conjunction with major histocompatibility complex classImolecules leading to cytotoxic T lymphocyte (CTL)-mediated tumor cell destruction[50,51]. Furthermore, the expression of FMG has been postulated to result in an efficient presentation of tumor antigens on antigen-presenting cells having taken up debris of apoptotic cells or exosomes of fused cells[52,53]. The impact of MV-FMG expression seen in our study confirms the findings published to date, demonstrating that dendritic cell (DC) maturation and naïve T cell activation are effectively primed upon contact with FMG-transduced, syncytia forming tumor cells[40]. Furthermore, we observed by immunohistochemistry a pronounced tumor infiltration with macrophages of animals that received intratumoral injections of adenovirus vector encoding measles virus H and F in combination with intratumoral IL-18 expression. Previously, Shimura et al[54] demonstrated that tumor-associated macrophages are inversely correlated with tumor progression in human prostate cancer, since macrophages provide important antigen-presenting functions[55].
In summary, our data demonstrate that the intratumoral expression of measles virus fusogenic membrane glycoproteins in combination with intratumoral cytokine expression gives the best results with regard to the anti-neoplastic effects on the directly vector-treated tumor, and also with regard to the induction of an anti-tumor immunity affecting an untreated tumor. To further improve the treatment efficacy, it should be advantageous to use an oncolytic vector expressing the FMG and cytokines, most likely resulting in a more efficient liberation of potential tumor-associated antigens[56].
ACKNOWLEDGMENTS
The authors are grateful to Malcolm Brenner (St. Jude Children’s Research Hospital, Memphis, TN) for providing via Jay Ramsey the Ad.IL-2 vector, Alexander Rakhmilevich (Department of Human Oncology, University of Wisconsin-Madison, Madison, WI) for providing the mIL-12 encoding plasmid, Camille Locht (Laboratoire de Microbiologie Génétique et Moléculaire, Institut Pasteur de Lille, Lille, France) for giving the mIL-18 encoding plasmid, Steven A. Rosenberg (Surgery Branch, NCI, National Institutes of Health, Bethesda, MD) for the MC38 cells. Furthermore the authors would like to thank Matthias Dobbelstein (Molecular Oncology, University of Göttingen) and German P Horn (Philipps University Marburg, Germany) for providing the Ad CMV F&H and for helpful advice, Klaus Überla for providing support, and Cathrin Walter (West German Cancer Center University of Duisburg-Essen, Essen, Germany) for critical review of this manuscript.
S- Editor Zhu LH L- Editor Lutze M E- Editor Lu W