Published online Jun 7, 2007. doi: 10.3748/wjg.v13.i21.2992
Revised: March 1, 2007
Accepted: March 8, 2007
Published online: June 7, 2007
AIM: To investigate the dynamic changes and signi-ficance of platelet activating factor receptor (PAF-R) mRNA and protein in pancreatic tissues of rats with severe acute pancreatitis (SAP) and effects of BN52021 (Ginkgolide B).
METHODS: Wistar male rats were randomly assigned to the negative control group (NC group), SAP model group (SAP group), and BN52051-remedy group (BN group), and each of the groups was divided into 6 subgroups at different time points after operation (1 h, 2 h, 3 h, 6 h, 12 h, and 24 h) (n = 10 in each). PT-PCR and Western blot methods were used to detect PAF-RmRNA and protein expression in pancreatic tissues of rats respectively. Pathological examination of pancreatic tissues was performed and the serum amylase change was detected.
RESULTS: Serum amylase and pathological results showed the that SAP model was successfully prepared, BN52021 was able to decrease serum amylase, and the pathological ratings in BN group at 3 h, 6 h, and 12 h significantly decreased compared with those in the SAP group (8.85 ± 0.39 vs 5.95 ± 0.19, 9.15 ± 0.55 vs 5.55 ± 0.36, 10.10 ± 0.65 vs 6.72 ± 0.30, P < 0.05). The result of PAF-mRNA showed dynamic changes in SAP and BN groups, which increased gradually in early stage, reached a peak at 3 h (0.71 ± 0.14 vs 0.54 ± 0.14, 0.69 ± 0.13 vs 0.59 ± 0.04, P < 0.05), and decreased gradually later. There were significant differences at each time point except 1 h and 2 h, when compared with those in the NC group (0.71 ± 0.14 or 0.69 ± 0.13 vs 0.47 ± 0.10, 0.38 ± 0.08 or 0.59 ± 0.04 vs 0.47 ± 0.09, 0.25 ± 0.07 or 0.29 ± 0.05 vs 0.46 ± 0.10, 0.20 ± 0.06 or 0.20 ± 0.04 vs 0.43 ± 0.09, P < 0.05), whereas there was no significant difference between BN and SAP groups at each time point. The result of PAF-R protein showed that the change of PAF-R protein in the SAP group and the BN group was consistent with that of PAF-R mRNA. There were significant differences at each time point except 1 h, when compared with those in the NC group (0.90 ± 0.02 or 0.80 ± 0.05 vs 0.48 ± 0.02, 1.69 ± 0.06 or 1.58 ± 0.02 vs 0.48 ± 0.03, 1.12 ± 0.10 or 0.98 ± 0.03 vs 0.49 ± 0.09, 1.04 ± 0.14 or 0.87 ± 0.02 vs 0.52 ± 0.08, 0.97 ± 0.16 or 0.90 ± 0.05 vs 0.49 ± 0.10, P < 0.05), whereas there was no significant difference between the BN group and the SAP group.
CONCLUSION: PAF-R plays an important role in occurrence and development of SAP. BN52021 exerts biological effects through competitively inhibiting the binding of increased both PAF and PAF-R expression rather than through decreasing PAF-R expression in pancreatic tissues.
- Citation: Xia SH, Hu CX, Zhao ZL, Xia GD, Di Y. Significance of platelet activating factor receptor expression in pancreatic tissues of rats with severe acute pancreatitis and effects of BN52021. World J Gastroenterol 2007; 13(21): 2992-2998
- URL: https://www.wjgnet.com/1007-9327/full/v13/i21/2992.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i21.2992
Severe acute pancreatitis (SAP) is a serious condition which has an acute onset and a high death rate. So far, as pathogenesis of SAP has not been clarified, there is no clinically effective therapeutic strategy for it. Therefore, research on its pathogenesis and treatment is quite important. In recent years, people are concerned with the significance of the signal transduction pathway of platelet activating factor (PAF) in the pathogenesis. PAF is an endogenous active substance produced by multiple cells,which has extensively biological effects. Through binding with platelet activating factor receptor (PAF-R), PAF may, through G-protein transduction, activate phospholipase C, phospholipase A2, adenylate cyclase, tyrosine protein kinase, etc.,leading to occurrence and development of SAP[1,2]. BN52021 (Ginkgolide B) is a terpinoid of gingko extract[3]. It is a potent antagonist of PAF, and may inhibit platelet aggregation, antagonize inflammation and shock, and protect blood vessels of heart and brain[4]. BN52021 has shown significant effects in treatment of experimental SAP[5]. The present study was aimed to investigate the role of PAF-R and BN52021 in occurrence and development of SAP, and its mechanism of action. The dynamic change of PAF-R mRNA and protein in pancreatic tissues of rats with SAP and the effects of BN52021 were detected using PT-PCR and Western blot analysis.
BN52021 and sodium taurocholate were purchased from Sigma (St. Louis, MO, USA), amylase kit from Beijing Kemei Reagent Co. (Beijing, China), trizol and diethyl pyrocarbonate (DEPC) from Invitrogen (Carlsbad, CA, USA), RT kit from MBI Fermentas (Ontario, Canada), DNTPs and RNA enzyme inhibitor from TaKaRa Dalian Co., Ltd. (Dalian, China), DNA Tag enzyme from Promega (Madison, WI, USA), primary antibody of PAF-R rabbit-anti-rat serum and the enhanced chemiluminescence (ECL) system from Santa Cruz Biotechnology (Santa Cruz, CA, USA), secondary antibody of sheep-anti-rabbit from Beijing Dingguo Biotechnology Co., Ltd. (Beijing, China), polyvinylidene fluoride (PVDF film) from Millipore Corp. (Bedford, MA, USA), prestained marker from Beijing Tianwei Time Biotechnology Co., Ltd. (Beijing, China), β-actin from Beijing Zhongshan Biotechnology Co., Ltd. (Beijing, China), DYY-12 electrophoresis system and electric trans-blot SD from Beijing Liuyi Instrument Factory (Beijing, China), Type-2720 PCR apparatus from ABI (Foster, CA, USA), and gel scanning and imaging system and vertical electrophoresis system from Bio-Rad (Hercules, CA, USA).
One hundred and eighty Wistar male rats (Laboratory Animal Center of PLA Academy of Military Medical Sciences, Beijing, China), aged 6-8 wk, and weighing 200-220 g (Grade II, Certificate SCXK 2002-001) are used for this study. All rats were maintained in an environment of controlled "temperature (22-25°C)", humidity (55%-58%), and lighting (12 h light/12 h dark), with free access to tap water and regular chow diet. They were randomly assigned to the negative control group (NC group, n = 60), SAP model group (SAP group, n = 60), and BN52051-remedy group (BN group, n = 60), and each of the above groups was divided into 6 subgroups at different time points after operation (1 h, 2 h, 3 h, 6 h, 12 h, and 24 h) (n = 10). SAP model was established based on the method by Aho, et al[6]. Wistar male rats were weighed, marked and fasted for 24 h before the operation, with free access to water. The rats were anesthetized with abdominal injection of 0.4% pentobarbital sodium (40 mg/kg), and fixed in dorsal decubitus. A 2-cm cut was made at the center of the upper belly, entering the abdominal cavity to look for rat’s duodenum and pancreaticobiliary duct. The hepatic end of the pancreaticobiliary duct was clipped with a non-invasive vascular clip, pancreaticobiliary duct retrograde centesis was performed with a obtuse needle through duodenum seromuscular layer. Then 5% sodium taurocholate (0.1 mL/100 g) was injected in the retrograde direction of pancreaticobiliary duct with a micro-syringe at an injection rate of 0.20 mL/min. After injection of the drug, the port of pancreaticobiliary duct entering duodenum was clipped with a non-invasive vascular clip and observed for 10 min. After confirming there was no active bleeding in the abdominal cavity, we closed the abdomen in two layers, and covered the cutting wound with sterile gauze. In the NC group, we stirred duodenum and touch pancreas several times after opening the abdomen. In the BN group, BN52021 (5 mg/kg: dissolved with DMSO) was injected intravenously 15 min after the operation. In the NC and the SAP groups, the same volume of physiological saline (0.9% NaCl) was injected through a femoral vein.
Rats in each group were anaesthetized at all time points after the operation (1 h, 2 h, 3 h, 6 h, 12 h, and 24 h), veinous blood was collected from the right atrium after a 10-min water bath at 37°C, and then centrifuged for 10 min at 3000 g/min. The supernatant was placed into a sterilized EP tube, and stored in a refrigerator at -20°C for determination of serum amylase. Meanwhile, a portion of pancreatic tissues was placed in liquid nitrogen overnight, and frozen in a refrigerator at -80°C. A portion of pancreatic tissues was fixed with 40 g/L neutral buffer formaldehyde, embedded with paraffin wax, cut into slices and examined with HE staining, and pathological observation and scoring were made.
Serum amylase was detected with fully automatic biochemical apparatus and amylase kit.
Pancreatic tissue samples were observed pathologically and scored (Table 1): randomly select 10 visual fields under a high-power microscope (HE stain, × 400), and conduct grading and scoring as shown in Table 1.
| Pathological | Pathological | Scores |
| grading | change | |
| Edema | Inter-lobule local edema, widened pleura | 1 |
| Inter-lobule diffuse edema, widened intra-lobule | ||
| clearance | 2 | |
| Increased intra-lobule clearance, alveolus swollen, | ||
| and separated | 3 | |
| Inflammatory | White cells < 20/visual field under high-power | |
| microscope | 1 | |
| White cells 20-50/visual field under | ||
| high-power microscope | 2 | |
| White cells > 30/visual field under high-power | ||
| microscope, or micro-abscess occurs | 3 | |
| Hemorrhage | Parenchymal hemorrhage < 20% | 1 |
| Parenchymal hemorrhage 20%-50% | 2 | |
| Parenchymal hemorrhage > 50% | 3 | |
| Necrosis | Necrosis area < 20% | 1 |
| Necrosis area 20%-50% | 2 | |
| Necrosis area > 50% | 3 |
PAF-R and β-actin primer series are provided by Invitrogen (Carlsbad, CA, USA): PAF-R: F (Forward primer): 5'-CCGCTGTGGATTGTCTATTA-3', R (Reverse primer): 5'-AGGAGG TGATGAAGATGTGG-3' (377 bp)[7]; β-actin: F: 5’-TCC TAGCACCATGAAGATC-3’, R: 5'-AAACGCAGCTCAGTAACAG-3' (190 bp)[8].
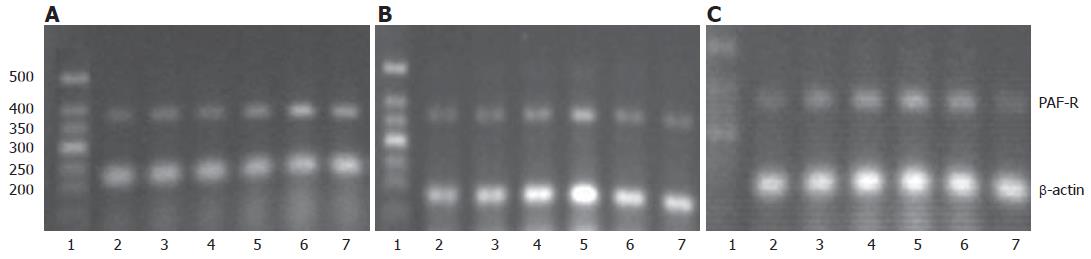
Total RNA from pancreatic tissue in each group was extracted first. Afterward, intergrity of the total RNA was checked with agarose electrophoresis, its concentration and purity were determined with an UV spectrophotometer, and the concentration of the total sample RNA was calculated. RNA 5 μg and Oligo DT15 1.25 μg were placed into a water bath at 70°C for 5 min, rapidly put into ice for 5 min, and centrifuged just for 15 s. 5 × M-MLV reverse transcription buffer 5 mL, DNTPS 0.05 μmol, RNA enzyme inhibitor 40 U, M-MLV and reverse transcriptase 1 μL were added, then diluted to 25 μL with deionized water treated by DEPC. The whole mixture was placed at 42°C for 60 min. Reverse transcription was conducted and reverse transcriptase was deactivated at 95°C for 5 min. Then the mixture was placed at 4°C for 5 min and preserved at -20°C.The PCR reaction system included: reverse transcription product 50 μL:3 μL, DNTPS 0.01 μmol, 10 × PCR buffer 5 μL, MgCl2 (Magnesium Chloride) 0.075 mol, PAF-R specific sense strand and anti-sense strand 50 pmol each, and β-actin sense strand and anti-sense strand 50 pmol each. Reaction conditions were as follows: pre-denaturizing at 94°C for 3 min, denaturizing at 94°C for 45 s, annealing at 58°C for 45 s, and extending at 72°C for 45 s, 38 cycles in total; extending at 72°C for 7 min, and preserving at 4°C. The PCR product was included in the PAF-R gene sequence of rat spleen (gi: 470384), which was performed by Beijing Boya Biotechnology Co., Ltd.. The PCR product was analyzed with gel electrophoresis by 2% agarose, EB staining observation, and a gel imaging system scanning. Gray level of PCR product treated by PAF-R and β-actin was analyzed using the Quantity-One Software, and the change of PAF-R mRNA expression was evaluated semi-quantitatively.
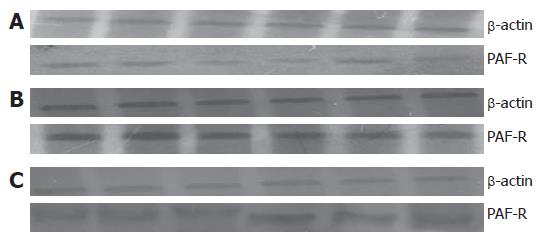
The prepared total protein was added to the gel-loading buffer in the ratio of 1:2 and was boil in 100°C water for 5 min. Electrophoresis with a vertical plate was conducted: 25 μL of the sample was added to two parallel gels, one for staining, the other for transferring membrane. The electrophoresis voltage 80 V was used for 10 min for concentration gel and 120 V for 60 min for isolation of gel. The gel and the membrane were placed between 6 filter papers and 2 foam pads, put into the electric trans-blot containing buffer, and then was added into ice-water mixture to pre-cool for 10 min. Trans-blot was switched on for 4 h at a current of 1 mA/cm2, staining for the trans-blotted gel was performed, and whether the trans-blotting was complete was checked. The labeled PVDF membrane was put in 0.05% Tween-20 buffer (TBST) and closed for 1 h. PAF-R antibody (diluted in 1:200) was added and was placed at 4°C overnight. Afterward, the PVDF membrane was washed with 0.05% TBST for 3 times and each lasted 5 min. The washed PVDF membrane was added into IgG (diluted in 1:200) that was labeled by horseradish peroxidase and incubatedt at 4°C overnight. Subsequently, PVDF membrane was immersed in 3 mL mixture of A and B ECL working solution for 1 min and then taken out with forceps and wrapped well with a charged nylon membrane. After exposure in a dark room for imaging, the PVDF membrane was placed in the desorption solvent and shaken at 50°C for 30 min. After being closed, β-actin primary antibody (diluted in 1:200) was added and preserved at 4°C overnight. The above procedures were repeated and the IgG (diluted in 1:200) labeled with horseradish peroxidase was added. Finally, ECL coloration and imaging were performed. The image was scanned into computer and was analyzed with the Gel-imaging System. The optical density value and the the ratio of the value of the former and the latter were calculated.
All the experiments were repeated for 3 times and their average values were used as the final value. All values were expressed with mean ± SD. Comparison of the difference between paired group was performed using t test and one-way ANOVA was used to analyze the differences of multiple samples. P < 0.05 was considered statistically significant. The statistical analysis was performed using SPSS 11.5 statistical software (SPSS, Chicago, IL, USA).
We showed that the serum amylase in the SAP group and the BN group was more significantly increased at each time phase point than in the NC group (P < 0.05); however, in the BN group it became significantly lower at 3 h, 6 h, and 24 h than in the SAP group (P < 0.05) (Table 2).
The pathological results showed that there was no obvious abnormality in abdominal cavity at each time phase point, and the pancreatic structure was normal in the NC group. In the SAP group, hemorrhagic ascites occurred, and necrosis focused in prancreas, a number of saponifying spots were found in mesentery and greater omentum, inflammatory cells infiltrated in pancreatic stroma and glandular lobule, and diffuse bleeding and piecemeal necrosis occurred. With time elapsed later, the pathological changes were exacerbated. In the BN group, the pathological changes were less serious than in the SAP group. The scores in the SAP and the BN groups were significantly higher at each time phase point than in the NC group (P < 0.05); however, the scores in the BN group were significantly lower at 3 h, 6 h, and 24 h than in the SAP group (P < 0.05) (Table 3).
PAF-R mRNA expression was changed dynamically in SAP and BN groups. It increased gradually in early stage, reached a peak at 3 h (P < 0.05), and then decreased gradually. There were significant differences at each time point except 1 h and 2 h, when compared with those in NC group (all P < 0.05), whereas there were no significant differences between the BN and SAP groups at each time point (Table 4, Figure 1).
The changes of PAF-R protein in SAP and BN groups were consistent with those of PAF-R mRNA. There were significant differences at each time point except 1h, when compared with those in the NC group (all P < 0.05), whereas there was no significant difference between BN group and SAP group (Table 5, Figure 2).
At present, the four generally accepted mechanisms associated with SAP are theories of self-digestion of pancreas by pancreatic enzyme, microcirculation disturbance of pancreas, over-activation of leucocytes, and migration of intestinal bacteria in pancreas[9]. The four mechanisms may interact with each other. All the four theories concentrate on the important roles of cytokine and inflammatory mediator in the pathogenesis of SAP and the influence of PAF on SAP has been proved[10]; however, the actual mechanism still needs further investi-gations.
To date, PAF is the strongest platelet aggregation agonist and active vascular lipid transmitter known[11,12]. It may activate and aggregate neutrophils, and release free oxygen radicals. It may act on endothelial cells of blood vessel and increase permeability of micro-blood vessels significantly, the effect of which is 1000-10 000 times as great as that of histamine[13]. It may also exert effects through a lot of inflammatory mediators, such as amines (histamine, 5-HT, catecholamine, etc.), arachidonic acids metabolites, as well as other activated fluid and cellular substances (free oxygen radicals, lysozyme, cytokines, etc.). Current studies have shown that PAF may transmit signals and exert its biological effects through the signal transduction system consisting of PAF-R, G-protein, and membrane effector enzyme[14].
PAF-R was found in the ligand-binding experiment of [3H]-PAF and [3H]-WEB2086, an antagonist of PAF-R[15]. It is a G-protein coupled receptor and it has been proved that PAF-R exists in many cells of human beings and animals, such as platelet, neutrophil, differentiated cell in leukemia, T or B lymphocyte, monocyte, macrophage, Kupffer cell, and smooth muscle cell[16]. Flickinger et al[17] found that PAF-R exists in capillaries of rat pancreatic tissues. Our study found that PAF-R also exists in nucleus and cytoplasm of rat pancreatic islets[18]. Therefore, if inflammation occurs in pancreatic tissues, the PAF released from pancreatic cells will increase. A large number of PAF will activate the signal transduction pathway of PAF and PAF-R through bounding with PAF-R in endothelial cells and pancreatic islet cells of pancreatic blood vessel. It will interact with other inflammatory cytokines, resulting in network effects and exacerbating injury to pancreatic tissue. It is important to investigate the dynamic changes of PAF-R in SAP and intervention.
This study showed that dynamic changes occurred in PAF-R mRNA and protein in the SAP model group, with similar trend. They increased gradually in the early stage, reached a peak at 3 h, and then decreased gradually. There were significant differences in the middle and late stages of disease course compared with those in the NC group. This suggested that PAF-R played a role in the pathogenesis of SAP and a significant role in occurrence and development of SAP. At 3 h, infiltration by substantial inflammatory cells can be seen in the pathological slices of pancreatic tissues of rats with SAP, including cells with PAF-R expression, such as leucocyte, macrophage, and monocyte. The results showed that the blood PAF level of SAP increased gradually in the early stage; meanwhile, other inflammatory factors also increased, such as sPLA2 and TNF-α[19]. PLA2 is a significant effector of PAF. PLA2 may decompose phospholipid of cell membrane, release PAF, and form a vicious cycle of the following fashion: PAF synthesis → activation of signal transduction pathway → increased PAF synthesis, to release substantial inflammatory mediators to induce increased expression of PAF-R[20,21]. TNFα may activate NF-κB to induce increased expression of PAF-R in MonoMac-1[22]. A previous study incubated B-lymphocytes with different levels of PAF for 24 h and the results showed PAF-R number substantially increased in such lymphocytes with dose-dependence and without any change of receptor affinity. However, it was reported that, in the early stage, PAF expression decreased in the form of PAF-R mRNA expression in renal mesangial cells when it was incubated, the mechanism of which is still unclear[23].
The pathological results showed that large area necrosis occurred at 6 h in rat pancreatic tissue of SAP. The cells with expression of PAF-R were damaged, and thus PAF-R expression decreased, which may be a key reason for decreased PAF-R expression in the middle and late stages of SAP course. Studies on human monocytes showed that PAF was able to activate PKC. PKC was able to deactivate PAF-R through phosphorylation, leading to decreased PAF-R expression and inhabitation of PAF effects[24]. It was suggested that there was a negative feedback mechanism between deactivated PAF-R and PAF. Low-level endotoxin was able to down-regulate PAF-R expression in human macrophage through inhibiting combination of NF-κB and DNA[12]. Endotoxin produced due to infection in the middle and late stages of SAP course was also able to down-regulate PAF-R expression through NF-κB[25,26].
The expression of PAF-R in rat pancreatic tissues with SAP increased in the early stage and then decreased in the middle and late stages, being consistent with the pathological change of pancreatic tissues. However, there are many influencing factors that need further investigations. It is also suggested that diagnosis and treatment of SAP in the early stage is quite significant, and that further investigation, as well as use of specific PAF-R antagonist in the early stage, is particularly significant. BN52021 is a specific PAF-R antagonist[27,28]. It is able to antagonize binding of PAF and its receptor (PAF-R) competitively, and thus PAF is unable to activate effector enzyme through G-protein transduction to block signal transduction of PAF-R[29]. The results of our early studies showed that Ginkgolide B was able to decrease the PAF level in blood, thus decreasing biological effects of PAF.
The serum amylase and pathological results of this study showed that BN52021 decreased serum amylase and pathological scores of SAP to some extent, thus exerting some therapeutic effects; however, it showed no significant effect for PAF-R expression in SAP course. It is obvious that BN52021 decreases the biological effects of PAF possibly through inhibiting binding of increased PAF with PAF-R with increased expression, other than through decreasing PAF-R expression. It inhibits PAF signal transduction and release and self-activation of pancreatic enzyme, and finally plays a therapeutic role in SAP.
To sum up, many factors induce the increased PAF-R expression in pancreatic tissues in the early stage of SAP, and the increased plasma PAF may bind PAF-R at many sites, which expands its biological effects of PAF, increases inflammatory network effects to a large extent, and exacerbates the injury of pancreatic and non-pancreatic tissues. Regarding treatment of rats with SAP, BN52021 exerts biological effects through competitively inhibiting the binding of increased both PAF and PAF-R expression rather than through decreasing PAF-R expression in pancreatic tissues.
BN52021 (ginkgolide B) is a specific antagonist to platelet activating factor receptor (PAF-R). In recent years, studies at home and abroad have showed that it has significant physiological activities, such as platelet aggregration inhibition, anti-inflammation, anti-shock, etc. BN52021 has significant effects in treatment of animals with severe acute pancreatitis (SAP). But the exact pathogenesis of BN52021 on SAP is unknown. This study was aimed at dynamically investigating the changes and significance of the expression of PAF-R mRNA and its protein in pancreatic tissues and effects of BN52021 in rats with SAP.
To explore molecule mechanism of BN52021 on SAP.
BN52021 has remarkable curative effect in SAP. But the mechanism of BN52021 needs further studies. This study explored the significance of PAF-R in the molecule mechanism of BN52021 on SAP.
The results may provide theoretic and experimental evidences for the study and application of BN52021, and new approachs for the treatment of SAP.
Platelet activating factor receptor (PAF-R) is a G-protein coupled receptor and it exists in multiple cells of human beings and animals, such as platelet, neutrophil, differentiated cell in leukemia, smooth muscle cell, etc. PAF is bound with PAF-R to activate the signal transduction pathway between PAF and PAF-R, and interact with other inflammatory cytokines, forming network effects, and exacerbating injury to various tissues. BN52021, code of ginkgolide B, one of the effective components of Chinese medicine Ginkgo Biloba leaf and a strong antagonist against the inflammatory medium of PAF, can not only block the signal transduction of PAF but also decrease the blood content of PAF to exert its biological effects. It has significant physiological activities, such as platelet aggregation inhibition, anti-inflammation, anti-shock, etc.
In this experimental study, the authors analyzed the effect of BN52021 (Ginkgolide B), a terpinoid of gingko extract, on the severity of acute pancreatitis in rats. The authors tried to find a causal link between the effect of BN52021 on tissue injury and the level of expression of PAF receptor (PAF-R) in pancreatic tissues. The data show that BN52021 attenuates amylase activity in serum as well as tissue damage but does not influence the inflammation-induced PAF-R expression.
S- Editor Wang J L- Editor Ma JY E- Editor Ma WH
| 1. | Miike S, Kurasawa K, Saito Y, Iwamoto I. Platelet-activating factor activates mitogen-activated protein kinases through the activation of phosphatidylinositol 3-kinase and tyrosine kinase in human eosinophils. J Leukoc Biol. 2000;67:117-126. [PubMed] |
| 2. | Xia GD, Xia SH. Recent development of platelet activating factor receptor. Shijie Huaren Xiaohua Zazhi. 2005;13:381-384. |
| 3. | Mauri P, Simonetti P, Gardana C, Minoggio M, Morazzoni P, Bombardelli E, Pietta P. Liquid chromatography/atmospheric pressure chemical ionization mass spectrometry of terpene lactones in plasma of volunteers dosed with Ginkgo biloba L. extracts. Rapid Commun Mass Spectrom. 2001;15:929-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Chao W, Olson MS. Platelet-activating factor: receptors and signal transduction. Biochem J. 1993;292:617-629. [PubMed] |
| 5. | McKenna DJ, Jones K, Hughes K. Efficacy, safety, and use of ginkgo biloba in clinical and preclinical applications. Altern Ther Health Med. 2001;7:70-86, 88-90. [PubMed] |
| 6. | Aho HJ, Koskensalo SM, Nevalainen TJ. Experimental pancreatitis in the rat. Sodium taurocholate-induced acute haemorrhagic pancreatitis. Scand J Gastroenterol. 1980;15:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 258] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Diserbo M, Cand F, Ziade M, Verdetti J. Stimulation of platelet-activating factor (PAF) receptors increases inositol phosphate production and cytosolic free Ca2+ concentrations in N1E-115 neuroblastoma cells. Cell Calcium. 1995;17:442-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Collins LC, Roberts AM. Effects of platelet-activating factor on arteriolar and venular tone in rat trachea. Microvasc Res. 1997;53:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Liu LR, Xia SH. Role of platelet-activating factor in the pathogenesis of acute pancreatitis. World J Gastroenterol. 2006;12:539-545. [PubMed] |
| 10. | Di Y, Xia SH, Tong CQ. Effect of Ginkgolide B on plasma levels of cytokines in severe acute pancreatitis in rats. Shijie Huaren Xiaohua Zazhi. 2006;14:2169-2173. |
| 11. | Sugano T, Narahara H, Nasu K, Arima K, Fujisawa K, Miyakawa I. Effects of platelet-activating factor on cytokine production by human uterine cervical fibroblasts. Mol Hum Reprod. 2001;7:475-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Boccellino M, Biancone L, Cantaluppi V, Ye RD, Camussi G. Effect of platelet-activating factor receptor expression on CHO cell motility. J Cell Physiol. 2000;183:254-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Seale JP, Nourshargh S, Hellewell PG, Williams TJ. Mechanism of action of platelet activating factor in the pulmonary circulation: an investigation using a novel isotopic system in rabbit isolated lung. Br J Pharmacol. 1991;104:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Wang X, Sun Z, Börjesson A, Haraldsen P, Aldman M, Deng X, Leveau P, Andersson R. Treatment with lexipafant ameliorates the severity of pancreatic microvascular endothelial barrier dysfunction in rats with acute hemorrhagic pancreatitis. Int J Pancreatol. 1999;25:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Korth R, Benveniste J. BN 52021 displaces [3H]paf-acether from, and inhibits its binding to intact human platelets. Eur J Pharmacol. 1987;142:331-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Marrache AM, Gobeil F, Bernier SG, Stankova J, Rola-Pleszczynski M, Choufani S, Bkaily G, Bourdeau A, Sirois MG, Vazquez-Tello A. Proinflammatory gene induction by platelet-activating factor mediated via its cognate nuclear receptor. J Immunol. 2002;169:6474-6481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Flickinger BD, Olson MS. Localization of the platelet-activating factor receptor to rat pancreatic microvascular endothelial cells. Am J Pathol. 1999;154:1353-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Zhao ZL, Xia SH, Chen H. Location of platelet-activating factor receptor in rat pancreas tissue. ZhonghuaXiahua Zazhi. 2006;14:54-55. |
| 19. | Xia SH, Quan JM, Qiao AJ, Zhang WL, Ren WY, Guo P, Zhao XY. Curative effect of sandostatin on severe acute pancreatitis with doubling dosage. Shijie Huaren Xiaohua Zazhi. 2002;10:1157-1161. |
| 20. | Baker RR, Chang HY. Alkylglycerophosphate acetyltransferase and lyso platelet activating factor acetyltransferase, two key enzymes in the synthesis of platelet activating factor, are found in neuronal nuclei isolated from cerebral cortex. Biochim Biophys Acta. 1996;1302:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Schievella AR, Regier MK, Smith WL, Lin LL. Calcium-mediated translocation of cytosolic phospholipase A2 to the nuclear envelope and endoplasmic reticulum. J Biol Chem. 1995;270:30749-30754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 366] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 22. | Dagenais P, Thivierge M, Stankova J, Rola-Pleszczynski M. Modulation of platelet-activating factor receptor (PAFR) gene expression via NF kappa B in MonoMac-1 cells. Inflamm Res. 1997;46 Suppl 2:S161-S162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Nakao A, Watanabe T, Bitoh H, Imaki H, Suzuki T, Asano K, Taniguchi S, Nosaka K, Shimizu T, Kurokawa K. cAMP mediates homologous downregulation of PAF receptor mRNA expression in mesangial cells. Am J Physiol. 1997;273:F445-F450. [PubMed] |
| 24. | Thivierge M, Parent JL, Stankova J, Rola-Pleszczynski M. Modulation of human platelet-activating factor receptor gene expression by protein kinase C activation. J Immunol. 1996;157:4681-4687. [PubMed] |
| 25. | Hourton D, Stengel D, Chapman MJ, Ninio E. Oxidized low density lipoproteins downregulate LPS-induced platelet-activating factor receptor expression in human monocyte-derived macrophages: implications for LPS-induced nuclear factor-kappaB binding activity. Eur J Biochem. 2001;268:4489-4496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Gray KD, Simovic MO, Blackwell TS, Christman JW, May AK, Parman KS, Chapman WC, Stain SC. Activation of nuclear factor kappa B and severe hepatic necrosis may mediate systemic inflammation in choline-deficient/ethionine-supplemented diet-induced pancreatitis. Pancreas. 2006;33:260-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Hosford D, Braquet P. Antagonists of platelet-activating factor: chemistry, pharmacology and clinical applications. Prog Med Chem. 1990;27:325-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Burgos RA, Hidalgo MA, Matthei SM, Hermosilla R, Folch H, Hancke JL. Determination of specific receptor sites for platelet activating factor in bovine neutrophils. Am J Vet Res. 2004;65:628-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Honda Z, Takano T, Gotoh Y, Nishida E, Ito K, Shimizu T. Transfected platelet-activating factor receptor activates mitogen-activated protein (MAP) kinase and MAP kinase kinase in Chinese hamster ovary cells. J Biol Chem. 1994;269:2307-2315. [PubMed] |