Published online Jun 7, 2007. doi: 10.3748/wjg.v13.i21.2923
Revised: February 4, 2007
Accepted: February 14, 2007
Published online: June 7, 2007
AIM: To characterize the immune responses including local and systemic immunity induced by infection with H pylori, especially with CagA+ H pylori strains and the underlying immunopathogenesis.
METHODS: A total of 711 patients with different gastric lesions were recruited to determine the presence of H pylori infection and cytotoxin associated protein A (CagA), the presence of T helper (Th) cells and regulatory T (Treg) cells in peripheral blood mononuclear cells (PBMCs), expression of plasma cytokines, and RNA and protein expression of IFN-γ and IL-4 in gastric biopsies and PBMCs were determined by rapid urease test, urea [14C] breath test, immunoblotting test, flow cytometry , real time RT-PCR and immunohistochemistry.
RESULTS: Of the patients, 629 (88.47%) were infected with H pylori; 506 (71.16%) with CagA+ and 123 (17.30%) with CagA- strains. Among patients infected with CagA+ H pylori strains, Th1-mediated cellular immunity was associated with earlier stages of gastric carcinogenesis, while Th2-mediated humoral immunity dominated the advanced stages and was negatively associated with an abundance of Treg cells. However, there was no such tendency in Th1/Th2 polarization in patients infected with CagA- H pylori strains and those without H pylori infection.
CONCLUSION: Polarization of Th cell immune responses occurs in patients with CagA+ H pylori infection, which is associated with the stage and severity of gastric pathology during the progression of gastric carcinogenesis. This finding provides further evidence for a causal role of CagA+ H pylori infection in the immunopathogenesis of gastric cancer.
-
Citation: Wang SK, Zhu HF, He BS, Zhang ZY, Chen ZT, Wang ZZ, Wu GL. CagA+
H pylori infection is associated with polarization of T helper cell immune responses in gastric carcinogenesis. World J Gastroenterol 2007; 13(21): 2923-2931 - URL: https://www.wjgnet.com/1007-9327/full/v13/i21/2923.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i21.2923
In 1994, epidemiological surveys by WHO showed that more than half of the world’s population was infected with H pylori and H pylori infection was closely related to chronic gastritis, gastric ulcer and gastric adenocarcinoma. These results led to the conclusion that H pylori is a first class risk factor (a definite carcinogen) for gastric adenocarcinomas[1]. It has been generally accepted that H pylori infection is involved in all stages of gastric carcinogenesis which progresses from chronic gastritis (CG) to gastric atrophy (GA), intestinal metaplasia (IM), dysplasia(DP) and ultimately gastric cancer (GC)[2-4]. However, the functional interaction of H pylori infection with distinct members of the immune compartment, especially T cell immune responses, in gastric carcinogenesis has not been fully elucidated. T helper (Th) cells can be divided into two subsets, Th1 cells and Th2 cells. Th1 cells mediate cellular immunity mainly by producing interferon (IFN)-γ, interleukin (IL)-2, IL-12, and tumor necrosis factor (TNF)-β, while Th2 cells primarily mediate humoral immunity by secreting IL-4, IL-5, IL-6, IL-10 and IL-13. Previous studies have reported the differential expression of cytokines between H pylori positive and H pylori negative patients[5-7] or between gastritis and gastric cancer patients[8]. However, these studies did not examine the cellular role of H pylori infection in the immune responses during the progression of gastric pathology. Under normal homeostasis, the cytokines produced by one Th subset reciprocally inhibit the development of the other to keep the balance of Th1 and Th2. Moreover, regulatory T (Treg) cells, which are a low abundance cell subset, help mediate the balance of Th1 and Th2, Treg cells inhibit the proliferation of CD4+ CD25-T lymphocytes, CD8+ T lymphocytes, immune memory cells, and antigen presenting cells (APCs) by recognizing inner and outer antigens. These immune responses result in the decrease of various cytokine secretion and the weakening of cellular immune function in vitro. The aim of the present study was to characterize the immune responses including local and systemic immunity induced by infection with H pylori, especially with CagA+ H pylori strains and the underlying immunopathogenesis, by analyzing the populations of T cells present in a range of progressive gastric pathologies during gastric carcinogenesis.
Candidates with gastric discomfort, who had an endoscopy during October 2004 to May 2006, were recruited into this study based upon three clinical screening tests for H pylori infection. These tests were rapid urease test (RUT) (Lizhu Company, Zhuhai, China) of gastric tissue, urea [14C] breath test (UBT) (Syncor Medicine Company Ltd., Shanghai, China) and the immunoblotting test (Yuangu company Ltd., Shanghai, China). The RUT and UBT tests have been reported to have sensitivity of 80%-99% and 95%-99% and specificity of 92%-100% and 77%-99%, respectively[9,10]. The immunoblotting test was designed to detect four major antigens including vacuolating cytotoxin (VacA, 95K), the cytotoxin associated protein A (CagA, 128K), urease A, urease B, and has been shown to be high sensitive and specific[11]. Patients were considered to be H pylori-positive when more than two tests were positive and to be negative when all three tests were negative, while those with two negative results were excluded from this study. In addition, two gastric biopsies taken from the antrum during the upper endoscopy were used for histological examination, real-time reverse transcriptase polymerase chain reaction (RT-PCR) and immunohistochemistry. Histological examination was performed by experienced pathologists who were blinded to the patients’ clinical diagnosis according to the updated Sydney system[11]. Patients with serious diseases or immune diseases were excluded from the study. None of the current study participants received surgery, radiotherapy, chemotherapy, or any other medical interventions before this study and all provided written informed consent after consultation. All the protocols and patient inclusion and exclusion criteria were approved by the Committee for Human Use and Institutional Review Board of Nanjing Medical University affiliated Nanjing first hospital for human subject studies.
Heparinized venous blood (5 mL) taken from each patient was used to detect the expression of IFN-γ and IL-4. Treg cells were identified with a FACSCalibur flow cytometer (FCM, BDIS Biosciences, Franklin Lakes, USA). All reagents for FCM were provided by Caltag laboratories (Burlingame CA, USA). Cells in the blood were stimulated as described by Morita et al[12]. Briefly, heparinized venous blood was incubated with a combination of 25 ng/mL phorbol 12-myristate 13-acetate (PMA) (Sigma, Saint Louis MO, USA) and 1 μg/mL of the calcium ionophore, ionomycin (Sigma), for 5 h. After cells were cultured in RPMI 1640 for 1 h, 10 μg/mL Brefeldin A (Sigma) was added to enhance flow cytometric analysis of intracellular cytokine staining. After stimulation, the cells were incubated with peridinin chlorophyll (PerCP) mouse anti-human CD3 (CD3-PC) and fluorescein isothiocyanate (FITC) mouse anti-human CD8 (CD8-FITC) for 15-20 min. After fixation and permeabilization, corresponding antibodies [mouse IgG1-phycoerythrin (PE), PE conjugated mouse anti-human IFN-γ (IFN-γ-PE), Rat IgG1-PE, PE-conjugated Rat anti-human IL-4 monoclonal antibody (IL-4-PE)] were added and incubated for 15 min. The cells were then detected by FCM. Data from at least 50 000 cells in one sample were acquired and analyzed by Cell Quest software (BDIS Biosciences). As CD4 expression is known to be down-regulated after stimulation with PMA[13], the CD4+ lymphocytes were analyzed indirectly by gating the CD3+ CD8-lymphocytes. For the detection of Treg cells, whole blood (100 μL) that was incubated with 5 μL CD3-PC, 5 μL CD4-FITC, and 5 μL mouse IgG1-PE for 15-20 min and was used as a control tube, while whole blood (100 μL) that was incubated with 5 μL CD3-TC, 5 μL CD4-FITC and 5 μL CD25-PE,and lysed with 2 mL red blood cell (RBC)-lysis buffer for 5-10 min was used as the detection tube. The precipitates were analyzed by Cell Quest software of FCM.
Enzyme-linked immunosorbent assay (ELISA) kits for quantitative detection of soluble human IL-4 and IFN-γ were purchased from Bender MedsystemsTM (Vienna, Austria), and of IL-2, 6, 10, and 12 from R&D Systems Inc. (Minneapolis, USA). The assays were performed in accordance with the manufacturers’ instructions.
To analyze IFN-γ and IL-4 mRNA, total RNA was extracted with TRIZOL Reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) from PBMCs and gastric biopsy specimens according to the manufacturer’s recommendations and then reverse transcribed into cDNA by PTC-200 DNA Engine (Bio-Rad, Hercules, CA,USA).
PCR primers for human IFN-γ, IL-4 and β-action were designed by Takara Biotechnology Company (Dalian, China, Table 1). The amount of the PCR product was monitored by the SYBR Premix Ex TaqTM (Takara Biotechnology Company) on a Lightcycler system (Roche Molecular Biochemicals, Indianapolis, USA). The PCR mixture contained 20 μL reaction solution, 2.0 μL cDNA, 10 μL 2 × SYBR Premix Ex TaqTM and 250 nM of the primer. PCR amplification was performed according to the temperature profile: 95°C for 10 s, followed by 45 cycles of 95°C for 5 s, annealing and extension at 62°C for 25 s. Data analysis was performed by the Light cycler software. All data were normalized by β-actin. The up-or down-regulation (F) of cytokines were calculated by the formula F = 2-∆∆Ct[14].
| Target | Sequences (5’→3’) | Length of amplicon (bp) |
| IFN-γ | Forward: CTTTAAAGATGACCAGAGCATCCAA | 189 (372-560 NM000619.2) |
| Reverse: GGCGACAGTTCAGCCATCAC | ||
| IL-4 | Forward: GACTGTGCTCCGGCAGTTCTA | 182 (589-770 NM000589) |
| Reverse: CCAACGTACTCTGGTTGGCTTC | ||
| β-actin | Forward: ATTGCCGACAGGATGCAGA | 89 (991-1079 CR609136.1) |
| Reverse: GAGTACTTGCGCTCAGGAGGA |
Immunohistochemical staining was performed on 8-μm-thick frozen sections mounted on glass slides as described previously[15]. Briefly, sections were fixed in 2% paraformaldehyde, air dried, and frozen at -20°C for at least 1 h. After permeabilization and blocking, the sections were incubated with the cytokine-specific monoclonal antibodies (MAbs) (Caltag Laboratories, South San Francisco, CA, USA) at 4°C overnight. They were then treated with 1% normal goat serum and subsequently incubated with 1:300 biotinylated goat anti-mouse IgG1 (Caltag Laboratories) and avidin-biotin horseradish peroxidase complex (Vector Laboratories Inc. Burlingame, CA, USA). The sections were developed with 3, 3-diaminobenzidine (Vector Laboratories Inc.) and counterstained with Mayer’s hematoxylin (Histolab, Goteborg, Sweden). After dehydration, they were mounted with Mountex (Histolab). The tissue sections were analyzed by PAS-9000 Pathological Report system (Logene-IBiotech, Wuxi, China). The positively stained mononuclear cells (MNCs) and polymorphonuclear cells (PMNs) per high power were counted. Only cells with a distinct cytoplasmic staining were included. For each sample, numbers of IL-4 and IFN-γ positive cells from 200 PMNs and MNCs were counted from five randomly selected fields and averaged. The positive rate was used to grade the expression levels: negative: 0%; +: 1%-25%; ++: 26%-50%; +++: 51%-100%.
One-way analysis of variance (ANOVA) and Pearson Correlation was performed to determine the difference and association by using Statistical Product and Service Solutions (SPSS, version 11.5, Chicago, IL, USA). P values of less than 0.05 were considered statistically significant.
Among 711 patients, 61 had normal gastric mucosa (normal mucosa, NM), 268 suffered from CG, 114 from GA, 104 from IM, 71 from dysplasia and 93 from GC (Table 2). Overall, 629 (88.47%) were infected with H pylori; 506 (71.16%) with CagA+ H pylori and 123 with CagA- H pylori (Table 2). Following the progression of gastric lesions, the prevalence of CagA+ H pylori infection in NM, CG, GA, IM, DP and GC groups increased and the rate of CagA+ H pylori was positively associated with the severity of gastric pathologies (r = 0.896, P = 0.016), while the prevalence of CagA-H pylori infection was not significantly associated with the severity of gastric pathologies (r = -0.794, P = 0.059).
| Pathological diagnosis | Gender | Age | H pylori positive | H pylori negative | |||
| Male | Female | (yr, mean ± SD) | Overall (%) | CagA+ (%) | CagA- (%) | ||
| Normal mucosa (n = 61) | 31 | 30 | 44.62 ± 12.41 | 48 (78.69) | 37 (60.65) | 11 (18.03) | 13 (21.31) |
| Chronic gastritis (n = 268) | 121 | 147 | 44.53 ± 14.37 | 232 (86.57) | 170 (63.43) | 62 (23.13) | 36 (13.43) |
| Gastric atrophy (n = 114) | 73 | 41 | 51.60 ± 12.39 | 104 (91.23) | 88 (77.19) | 16 (14.03) | 10 (8.77) |
| Intestinal metaplasia (n = 104) | 47 | 57 | 50.69 ± 12.81 | 95 (91.35) | 80 (76.92) | 15 (14.42) | 9 (8.65) |
| Dysplasia (n = 71) | 45 | 26 | 54.23 ± 12.25 | 65 (91.55) | 57 (80.28) | 8 (11.26) | 6 (8.45) |
| Gastric cancer (n = 93) | 70 | 23 | 64.70 ± 11.33 | 85 (91.40) | 74 (79.57) | 11 (11.83) | 8 (8.60) |
| Total (n = 711) | 387 | 324 | 50.18 ± 14.65 | 629 (88.47) | 506 (71.16) | 123 (17.30) | 82 (11.53) |
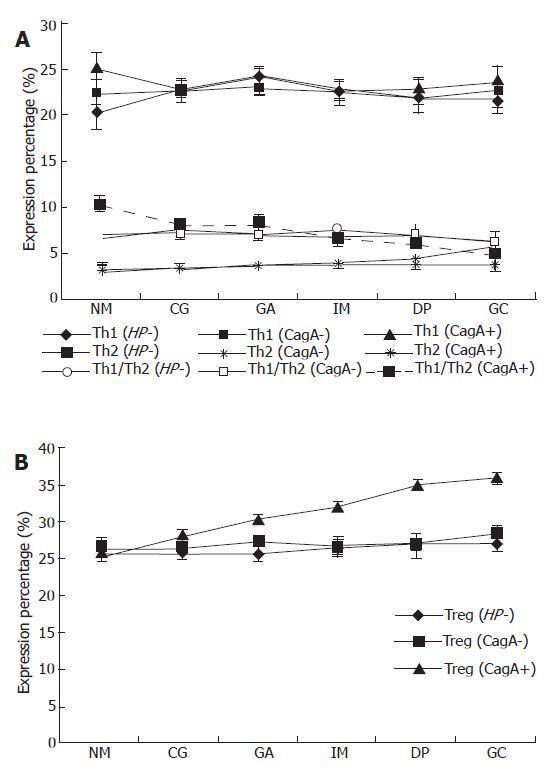
Because the data of Th1/Th2 did not follow a Gaussian distribution, analysis of variance (ANOVA) was performed after the data were transferred by logarithm. As shown in Figure 1, there was no significant difference in IFN-γ among the different gastric pathologies, while there was an increasing tendency of IL-4 expression (P = 0.022) following the progression of gastric pathologies, leading to a gradual decrease in Th1/Th2 ratio in CagA+ H pylori infected patients. However, there was no such a tendency both in patients with CagA- H pylori infection and those without H pylori infection for both IFN-γ and IL-4, indicating that there was no significant difference in Th1/Th2 ratios in these patients. As for Treg cells, there was an increasing trend according to the progression of gastric pathologies, which was strongly correlated with CagA+ H pylori infection, but not with CagA- H pylori infection. There was a significant negative association between Th1/Th2 and the expression of Treg cells in gastric patients’ PBMCs in patients with CagA+ H pylori infection (r = -0.321, P < 0.001), however, no such association was found in patients with CagA- H pylori infection and those without H pylori infection.
Following the progression of gastric pathologies associated with CagA+ H pylori infection, the levels of IL-2 and IL-12 gradually decreased, but the levels of IL-6, IL-10 gradually increased, which indicated that there were some changes in the patterns of cellular to humoral immunity. However, there was no significant difference for the key representative cytokines of Th1 and Th2 cells in all the groups (IFN-γ and IL-4, respectively) (Table 3). The patterns of six cytokines in the patients with CagA- H pylori infection and those without H pylori infection were similar to that in CagA+ H pylori infected patients (Tables 4 and 5), indicating there was no correlation between H pylori infection and cytokine levels.
| Pathological diagnosis | IFN-γ | IL-4 | IL-12 | IL-10 | IL-6 | IL-2 |
| Normal mucosa (NM, n = 37) | 27.46 ± 1.05 | 10.86 ± 0.62 | 81.42 ± 1.89 | 66.92 ± 2.15 | 47.96 ± 1.99 | 54.17 ± 1.09 |
| Chronic gastritis (CG, n = 170) | 28.94 ± 0.59 | 11.22 ± 0.45 | 72.49 ± 0.93 | 80.65 ± 0.99 | 63.40 ± 1.57 | 45.95 ± 0.49 |
| Gastric atrophy (GA, n = 88) | 30.48 ± 0.75 | 11.23 ± 0.33 | 53.29 ± 0.79 | 100.80 ± 1.69 | 94.10 ± 2.57 | 39.61 ± 0.69 |
| Intestinal metaplasia (IM, n = 80) | 31.71 ± 1.02 | 12.08 ± 0.59 | 52.18 ± 0.94 | 104.18 ± 1.90 | 112.03 ± 2.64 | 34.07 ± 0.54 |
| Dysplasia (DP, n = 57) | 30.14 ± 1.04 | 10.92 ± 0.39 | 43.30 ± 1.10 | 157.72 ± 2.64 | 156.74 ± 3.66 | 32.71 ± 0.81 |
| Gastric cancer (GC, n = 74) | 31.00 ± 0.84 | 11.03 ± 0.29 | 39.10 ± 0.89 | 196.65 ± 1.83 | 159.32 ± 2.57 | 28.39 ± 0.65 |
| Pathological diagnosis | IFN-γ | IL-4 | IL-12 | IL-10 | IL-6 | IL-2 |
| Normal mucosa (NM, n = 11) | 29.28 ± 0.54 | 11.07 ± 0.89 | 82.17 ± 4.59 | 67.29 ± 3.26 | 48.37 ± 4.01 | 54.11 ± 2.87 |
| Chronic gastritis (CG, n = 62) | 29.19 ± 1.18 | 10.89 ± 0.27 | 64.59 ± 3.10 | 80.25 ± 1.87 | 57.99 ± 2.61 | 53.14 ± 1.25 |
| Gastric atrophy (GA, n = 16) | 30.25 ± 2.01 | 10.67 ± 0.67 | 49.67 ± 3.28 | 91.28 ± 3.19 | 92.56 ± 5.79 | 42.33 ± 2.32 |
| Intestinal metaplasia (IM, n = 15) | 28.91 ± 2.23 | 11.08 ± 0.74 | 48.19 ± 2.99 | 98.11 ± 4.08 | 98.20 ± 5.92 | 40.98 ± 2.38 |
| Dysplasia (DP, n = 8) | 31.08 ± 2.19 | 11.01 ± 1.27 | 42.64 ± 5.49 | 157.09 ± 6.81 | 147.65 ± 6.89 | 34.79 ± 3.05 |
| Gastric cancer (GC, n = 11) | 28.06 ± 2.45 | 12.07 ± 0.91 | 38.97 ± 3.29 | 185.29 ± 9.38 | 154.68 ± 6.78 | 30.29 ± 2.11 |
| Pathological diagnosis | IFN-γ | IL-4 | IL-12 | IL-10 | IL-6 | IL-2 |
| Normal mucosa (NM, n = 13) | 31.83 ± 0.17 | 10.57 ± 0.68 | 80.84 ± 4.81 | 68.56 ± 3.87 | 49.53 ± 3.94 | 53.13 ± 1.48 |
| Chronic gastritis (CG, n = 36) | 29.10 ± 1.27 | 10.37 ± 0.30 | 68.57 ± 2.43 | 79.03 ± 1.64 | 57.66 ± 2.56 | 52.54 ± 1.41 |
| Gastric atrophy (GA, n = 10) | 29.06 ± 1.93 | 10.49 ± 0.58 | 51.14 ± 3.47 | 90.94 ± 3.63 | 91.08 ± 6.11 | 41.39 ± 2.18 |
| Intestinal metaplasia (IM, n = 9) | 28.57 ± 2.72 | 11.75 ± 0.61 | 50.97 ± 1.54 | 97.60 ± 3.53 | 96.35 ± 5.19 | 41.54 ± 0.99 |
| Dysplasia (DP, n = 8) | 31.59 ± 1.29 | 11.46 ± 0.58 | 43.10 ± 5.02 | 153.34 ± 6.12 | 149.92 ± 6.23 | 35.00 ± 2.04 |
| Gastric cancer (GC, n = 6) | 27.04 ± 2.30 | 11.91 ± 1.26 | 40.06 ± 2.08 | 181.36 ± 9.02 | 153.04 ± 6.63 | 30.95 ± 1.91 |
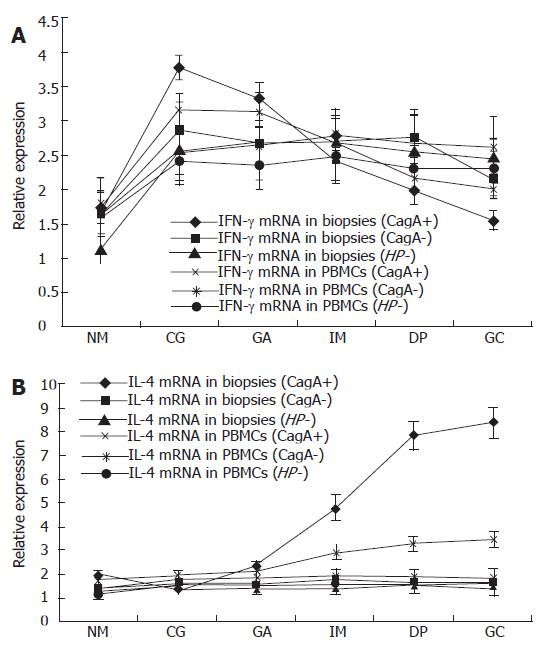
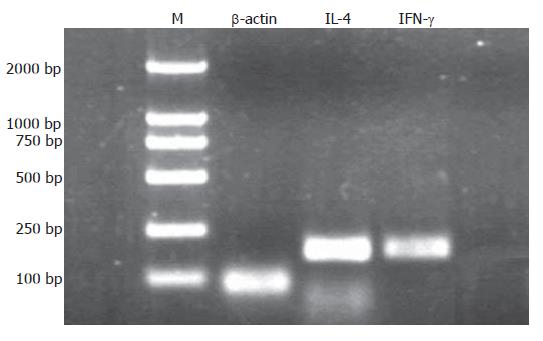
Following the progression of the CagA+ H pylori associated gastric pathologies, the expression of IFN-γ mRNA showed a decreasing tendency (P = 0.006) while the expression of IL-4 mRNA increased (P = 0.006) (Figure 2). To check the specificity of the experiment, the molecular weight of the amplicon was confirmed by agarose gel electrophoresis (Figure 3). The expression of IFN-γ mRNA was significantly higher in CG than in IM, DP, GC (P < 0.001, P < 0.001, and P < 0.001, respectively), and was significantly higher in the GA than in DP and GC groups (P < 0.001, and P < 0.001), but there was no significant difference in the expression among the IM, DP and GC. On the contrary, the expression of IL-4 mRNA was significantly lower in CG than in IM, DP, and GC (P < 0.001, P < 0.001, and P < 0.001, respectively), and in GA than in DP and GC (P < 0.001 and P < 0.001), but there was no significant difference in the expression between DP and GC. In patients with CagA- H pylori infection and those without H pylori infection, there was no significant difference in the expression of IFN-γ and IL-4 mRNAs along the progression of gastric pathologies (Figure 2).
As shown in Figure 2, the expression of IFN-γ mRNA in gastric biopsies of patients with CagA+ H pylori infection decreased gradually along the progression of gastric pathologies from CG to GC; there was a significant difference in the expression of IFN-γ mRNA between CG and DP, GC (P = 0.013, P = 0.001), between GA and DP, GC (P = 0.043, P = 0.004). However, the gastric expression of IFN-γ mRNA was constant from CG to GC in patients with CagA- H pylori infection and those without H pylori infection. On the contrary, there was an increase in gastric IL-4 mRNA expression in patients with CagA+ H pylori infection along the progression of gastric pathologies from CG to GC (P = 0.004). The expression of IL-4 mRNA was significantly lower in CG and GA than in IM, DP and GC. However, the gastric IL-4 mRNA in patients with CagA- H pylori infection and those without H pylori infection was expressed at low levels and remained constant in all the gastric pathologies.
Those results indicated that IFN-γ mRNA expression showed a decreasing tendency while the IL-4 mRNA expression showed an increasing tendency following the progression of the CagA+ H pylori associated pathologies. Pearson correlation analysis indicated that there was a significant positive correlation in IFN-γ mRNA expression (r = 0.201, P < 0.001), and IL-4 mRNA expression (r = 0.212, P < 0.001) between gastric biopsy and PBMCs. Compared with the expression of IFN-γ mRNA and IL-4 mRNA in PBMCs, changes in the biopsy specimens were more conspicuous, especially in IM, DP and GC (Figure 2).
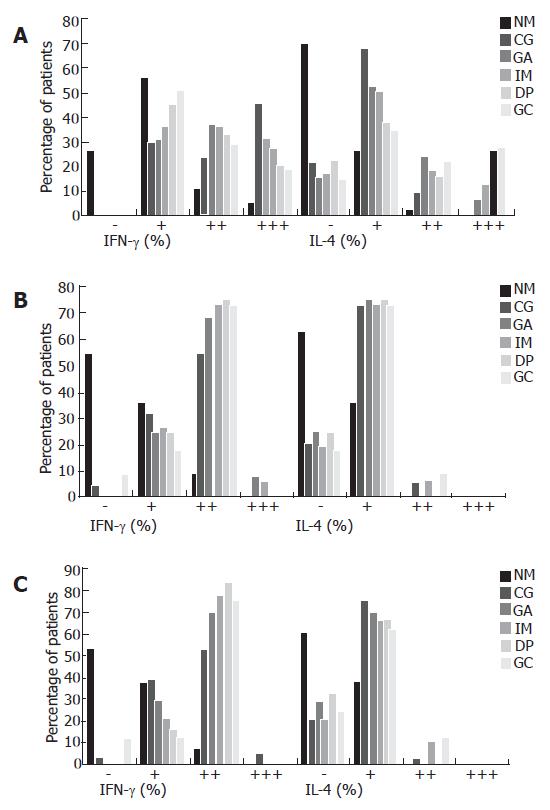
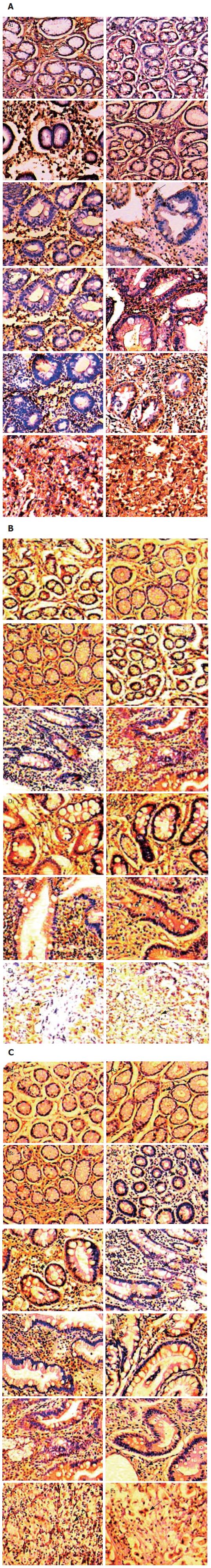
As shown in Figure 4A, gastric IFN-γ expression from CG to GC in patients with CagA+ H pylori infection showed a decreasing tendency with the percentage of cases with IFN-γ expression of “+++” decreasing from 45.9% in CG patients to 18.9% in GC patients. IFN-γ expression with “+++” predominated in CG, while the percentage of cases with IFN-γ expression with “+” increased significantly in GC. On the other hand, the percentage of cases with IL-4 expression with “+” decreased gradually from 68.2% in CG patients to 35.1% in GC patients, but the rate of IL-4 expression with “+++” increased gradually from 0% in CG patients to 28.4% in GC patients. In patients with CagA- H pylori infection and those without H pylori infection, there was no difference between IFN-γ and IL-4 expression among the different gastric pathologies; IFN-γ expression with “++” was always dominant, while IL-4 expression with “+” was always dominant (Figure 4B and C). The representative immunohistochemistry staining results in the gastric biopsies of with CagA+, CagA- H pylori infected patients and uninfected patients with different gastric pathologies are shown in Figure 5A-C.
H pylori is a small, curved, highly motile, Gram-negative bacterium that colonizes the epithelium of the human stomach. Previous studies have shown that H pylori infection is associated with the development of chronic gastritis and gastric cancer[3,4,16] Several factors including virulence among different H pylori strains have been attributed to the diversity in clinical outcome[17]. Until now, very few papers have reported the prevalence of H pylori infection in China, especially the subtypes of the organism[18,19]. Our study showed that the prevalence of H pylori infection in our outpatients is 88.47% (629/711) and the majority (80.45%, 506/629) of the infected subjects is infected with CagA+ H pylori. This confirms that CagA+ H pylori infected subjects are predominant in the Chinese H pylori-infected population[19]. Moreover, we observed that the subtypes of H pylori infection may play different role in the progression of gastric carcinogenesis; CagA+ H pylori infection was positively associated with the severity of gastric pathologies, especially gastric cancer, whereas CagA- H pylori infection was not associated with the severity of gastric pathologies.
Previous epidemiological studies have demonstrated that severe clinical manifestations do not frequently occur in most individuals with H pylori infection and the different clinical manifestations caused by this bacterium could be a consequence of interactions among microorganism characteristics, environmental influences, and the host immune response[20-22]. In the present study, 711 patients with different gastric pathologies were recruited to explore the interaction between bacterial virulence and host immune responses including systemic immunity and local immunity. We observed that in patients with CagA+ H pylori infection, the expression of IFN-γ remained unchanged, while IL-4 increased gradually in PBMCs during the progression of gastric pathologies, resulting in decreases of Th1/Th2 ratios, which was negatively correlated to the increased expression of Treg cells. During the development of gastric pathologies, IFN-γ mRNA expression gradually decreased, while IL-4 mRNA expression gradually increased, which was consistent with Th1/Th2 results in PBMCs. In addition, IL-2 and IL-12 protein expression decreased gradually while IL-6 and IL-10 increased gradually following the progression of gastric pathologies, although IFN-γ and IL-4, the representative cytokines of Th1 and Th2 cells, did not change. However, the ratios of Th1/Th2 in patients with CagA- H pylori infection and those without H pylori infection remained unchanged at both the cellular level and the mRNA level. Therefore, we postulate, based on our observations, that following the progression of the gastric pathologies, Th1 cells change slightly but Th2 cells are gradually increased, resulting in a gradual decrease in the ratio of Th1/Th2. Indeed, our systemic immunity analysis demonstrated that there was a shift from Th1 response to Th2 response during the progression of CagA+ H pylori infection associated gastric pathologies. However, it is noticed that IFN-γ and IL-4 expression in the plasma did not show any tendency of the Th1/Th2 shift, which may be due the fact that the levels of cytokines in plasma are affected by many factors other than H pylori infection[23,24], which may also explain the observation that IFN-γ and IL-4 expression were similar among patients with CagA+, or CagA- H pylori infection and those without H pylori infection.
Real-time RT-PCR and immunohistochemistry analysis were employed in the preset study to compare the local immunity of H pylori infected subjects, including mRNAs and protein expression of IFN-γ and IL-4 in gastric mucosa of patients with H pylori infection, with that in patients without H pylori infection. We observed that IFN-γ mRNA expression decreased gradually, while IL-4 mRNA expression increased during the development of gastric pathologies and the changing magnitude of IFN-γ mRNA and IL-4 mRNA expression in gastric mucosa were more obvious than those in PBMCs. Moreover, during the progression of gastric pathologies associated with CagA+ H pylori infection, IFN-γ expression primarily decreased from grade “+++” in CG patients to grade “+” in GC patients, whereas IL-4 expression increased from “+” in CG patients to “+++” in GC patients. On the contrary, in patients with CagA- H pylori infection and those without H pylori infection, IFN-γ and IL-4 expression remained unchanged during the progression of gastric pathologies; IFN-γ expression remained with grade “++” and IL-4 expression with grade “+”. These findings demonstrate that there is a shift from Th1 mediated immune response to Th2 mediated immune response within gastric mucosa during the progression of CagA+ H pylori infection associated gastric carcinogenesis, which is more significant than the Th1/Th2 shift in the systemic immunity.
In conclusion, there is a shift from Th1 mediated cellular immunity to Th2 mediated hormonal immunity in the immune response during the progression of gastric carcinogenesis in patients with CagA+ H pylori infection, which is negatively associated with the expression of Treg cells. Moreover, among the immune responses associated with CagA+ H pylori infection, local immunity is predominant over systemic immunity and the polarization of Th cells mediated immune response is associated with the chronicity and progression of gastric pathologies, especially gastric cancer. These findings provide clinical evidence for exploring the immunopathogenesis of gastric cancer associated with CagA+ H pylori infection.
Studies have shown that H pylori infection is involved in all stages of gastric carcinogenesis which progresses from chronic gastritis to gastric atrophy, intestinal metaplasia, dysplasia and gastric cancer. However, the functional interaction of H pylori infection with immune components, especially T cell immune responses, has not been fully elucidated.
Previous studies have reported the differential expression of cytokines between H pylori positive and H pylori negative patients or between gastritis and gastric cancer patients, but these studies did not examine the cellular role of H pylori, especially CagA+ H pylori, infection in the immune responses during the progression of gastric pathology.
Among patients infected with CagA+ H pylori infected, Th1-mediated cellular immunity was associated with earlier stages of gastric carcinogenesis, while Th2-mediated humoral immunity dominated the advanced stages and was negatively associated with an abundance of Treg cells. However, there was no such tendency in Th1/Th2 polarization in patients infected with CagA- H pylori strains and those without H pylori infection.
Polarization of Th cell immune responses occurs in patients with CagA+ H pylori infection, which is associated with the stage and severity of gastric pathology during the progression of gastric carcinogenesis, will provide further evidence for a causal role of CagA+ H pylori infection in the immunopathogenesis of gastric cancer.
Immune response: Actions of the body’s immune system that come into play to control infection or disease; T helper cells: can be divided into two subsets, Th1 cells and Th2 cells. Th1 cells mediate cellular immunity mainly by producing interferon (IFN)-γ, interleukin (IL)-2, IL-12, and tumor necrosis factor (TNF)-β, while Th2 cells primarily mediate humoral immunity by secreting IL-4, IL-5, IL-6, IL-10 and IL-13; regulatory T cells: a low abundance cell subset, help mediate the balance of Th1 and Th2, Treg cells inhibit the proliferation of CD4 + CD25-T lymphocytes, CD8 + T lymphocytes, immune memory cells, and antigen presenting cells (APCs) by recognizing inner and outer antigens; Immunopathogenesis: the process of development of a disease in which an immune response or the products of an immune reaction are involved.
This is an excellent work that I think has to be accepted as it is. It is well written, well done and reaches original findings.
S- Editor Liu Y L- Editor Ma JY E- Editor Wang HF
| 1. | International Agency for Research on Cancer, World Health Organization. Infection with Helicobacter pylori. In: Schistosomes, liver flukes and Helicobacter pylori. Lyon: IARC Monogr Eval. Carcinog Risks Hum. 1994;60:177-240. |
| 2. | Stolte M, Meining A. The updated Sydney system: classification and grading of gastritis as the basis of diagnosis and treatment. Can J Gastroenterol. 2001;15:591-598. [PubMed] |
| 3. | Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol. 1995;19 Suppl 1:S37-S43. [PubMed] |
| 4. | Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology. 1998;115:642-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 674] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 5. | Lindholm C, Quiding-Järbrink M, Lönroth H, Hamlet A, Svennerholm AM. Local cytokine response in Helicobacter pylori-infected subjects. Infect Immun. 1998;66:5964-5971. [PubMed] |
| 6. | Straubinger RK, Greiter A, McDonough SP, Gerold A, Scanziani E, Soldati S, Dailidiene D, Dailide G, Berg DE, Simpson KW. Quantitative evaluation of inflammatory and immune responses in the early stages of chronic Helicobacter pylori infection. Infect Immun. 2003;71:2693-2703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Dlugovitzky DG, Nogueras MG, Fiorenza G, Raynoldi J, Proske A, Badano A, Ballilengua C. Changes in cytokine levels related to the immunopathogenesis of Helicobacter pylori disease. Immunological and histological effects of triple treatment (omeprazol, azytromycin, and amoxycillin). Inmunologa. 2005;24:11-16. |
| 8. | Ren Z, Pang G, Clancy R, Li LC, Lee CS, Batey R, Borody T, Dunkley M. Shift of the gastric T-cell response in gastric carcinoma. J Gastroenterol Hepatol. 2001;16:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Wong BC, Wong WM, Wang WH, Tang VS, Young J, Lai KC, Yuen ST, Leung SY, Hu WH, Chan CK. An evaluation of invasive and non-invasive tests for the diagnosis of Helicobacter pylori infection in Chinese. Aliment Pharmacol Ther. 2001;15:505-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Gomes AT, Coelho LK, Secaf M, Módena JL, Troncon LE, Oliveira RB. Accuracy of the 14C-urea breath test for the diagnosis of Helicobacter pylori. Sao Paulo Med J. 2002;120:68-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Chmiela M, Wiśniewska M, Bak-Romaniszyn L, Rechciński T, Płaneta-Małecka I, Bielański W, Konturek SJ, Płonka M, Klink M, Rudnicka W. Serological differentiation of Helicobacter pylori CagA(+) and CagA(-) infections. Arch Immunol Ther Exp (Warsz). 2003;51:131-136. [PubMed] |
| 12. | Morita Y, Yamamura M, Kawashima M, Harada S, Tsuji K, Shibuya K, Maruyama K, Makino H. Flow cytometric single-cell analysis of cytokine production by CD4+ T cells in synovial tissue and peripheral blood from patients with rheumatoid arthritis. Arthritis Rheum. 1998;41:1669-1676. [PubMed] [DOI] [Full Text] |
| 13. | Anderson SJ, Coleclough C. Regulation of CD4 and CD8 expression on mouse T cells. Active removal from the cell surface by two mechanisms. J Immunol. 1993;151:5123-5134. [PubMed] |
| 14. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117419] [Cited by in RCA: 133214] [Article Influence: 5550.6] [Reference Citation Analysis (1)] |
| 15. | Andersson J, Abrams J, Björk L, Funa K, Litton M, Agren K, Andersson U. Concomitant in vivo production of 19 different cytokines in human tonsils. Immunology. 1994;83:16-24. [PubMed] |
| 16. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3177] [Article Influence: 132.4] [Reference Citation Analysis (0)] |
| 17. | Blaser MJ. Hypotheses on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology. 1992;102:720-727. [PubMed] |
| 18. | Li YY, Hu PJ, Du GG, Hazell SL. The prevalence of Helicobacter pylori infection in the Peoples Republic of China. Am J Gastroenterol. 1991;86:446-449. [PubMed] |
| 19. | Wong BC, Yin Y, Berg DE, Xia HH, Zhang JZ, Wang WH, Wong WM, Huang XR, Tang VS, Lam SK. Distribution of distinct vacA, cagA and iceA alleles in Helicobacter pylori in Hong Kong. Helicobacter. 2001;6:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Ernst PB, Crowe SE, Reyes VE. The immunopathogenesis of gastroduodenal disease associated with Helicobacter pylori infection. Curr Opin Gastroenterol. 1995;11:512-518 [DOI : 10.1097/00001574-199511000-00009]. |
| 21. | Russo F, Jirillo E, Clemente C, Messa C, Chiloiro M, Riezzo G, Amati L, Caradonna L, Di Leo A. Circulating cytokines and gastrin levels in asymptomatic subjects infected by Helicobacter pylori (H. pylori). Immunopharmacol Immunotoxicol. 2001;23:13-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Labigne A, de Reuse H. Determinants of Helicobacter pylori pathogenicity. Infect Agents Dis. 1996;5:191-202. [PubMed] |
| 23. | Andersen LP, Holck S, Janulaityte-Günther D, Kupcinskas L, Kiudelis G, Jonaitis L, Janciauskas D, Holck P, Bennedsen M, Permin H. Gastric inflammatory markers and interleukins in patients with functional dyspepsia, with and without Helicobacter pylori infection. FEMS Immunol Med Microbiol. 2005;44:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Itoh T, Wakatsuki Y, Yoshida M, Usui T, Matsunaga Y, Kaneko S, Chiba T, Kita T. The vast majority of gastric T cells are polarized to produce T helper 1 type cytokines upon antigenic stimulation despite the absence of Helicobacter pylori infection. J Gastroenterol. 1999;34:560-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |