Published online Jun 7, 2007. doi: 10.3748/wjg.v13.i21.2918
Revised: December 30, 2006
Accepted: January 14, 2007
Published online: June 7, 2007
AIM: To investigate the ultrastructure of oval cells in children with chronic hepatitis B, with special emphasis on their location in areas of collagen fibroplasia.
METHODS: Morphological investigations were conducted on biopsy material obtained from 40 children, aged 3-16 years with chronic hepatitis B. The stage of fibrosis was assessed histologically using the arbitrary semiquantitative numerical scoring system proposed by Ishak et al. The material for ultrastructural investigation was fixed in glutaraldehyde and paraformaldehyde and processed for transmission–electron microscopic analysis.
RESULTS: Ultrastructural examination of biopsy specimens obtained from children with chronic hepatitis B showed the presence of two types of oval cells, the hepatic progenitor cells and intermediate hepatic-like cells. These cells were present in the parenchyma and were seen most commonly in areas of intense periportal fibrosis (at least stage 2 according to Ishak et al) and in the vicinity of the limiting plate of the lobule. The activated nonparenchymal hepatic cells, i.e. transformed hepatic stellate cells and Kupffer cells were seen in close proximity to the intermediate hepatic-like cells.
CONCLUSION: We found a distinct relationship between the prevalence of oval cells (hepatic progenitor cells and intermediate hepatocyte-like cells) and fibrosis stage in pediatric patients with chronic hepatitis B.
- Citation: Sobaniec-Lotowska ME, Lotowska JM, Lebensztejn DM. Ultrastructure of oval cells in children with chronic hepatitis B, with special emphasis on the stage of liver fibrosis: The first pediatric study. World J Gastroenterol 2007; 13(21): 2918-2922
- URL: https://www.wjgnet.com/1007-9327/full/v13/i21/2918.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i21.2918
Several authors have suggested that oval cells (syn. liver progenitor/oval cells) play an essential role in the development of liver regeneration and carcinogenesis. These cells have been investigated extensively, using both experimental material[1-6] as well as human biopsy specimens, obtained mainly from adult patients[7-12].
It has been proposed that the major approach to compensate for the loss of liver mass (e.g. after viral infection, hepatectomy, etc) involves the proliferation and differentiation in to a fully mature liver of primary, low-differentiated, multipotential cells of bone marrow origin, known as the progenitor cells or stem cells. Although, it is still a matter of dispute, these cells are believed to act as precursor cells of intrahepatic progenitor cells, i.e. oval cells[2,6,7,12-15].
It is believed that oval cells constitute a heterogenic cell population which account for 1%-3% of the normal liver cell pool. These cells are located in the portal and periportal spaces, with the nucleus serving as the common morphological feature shared by these cells. The oval cells are not easy to recognize. They have many features in common, both structural and functional, with hepatoblasts of the embryonic and fetal period[12,16,17]. The oval cells are thought to constitute a reserve compartment that is activated only when hepatocytes fail to proliferate[18].
The most common pathological process involving oval cells occurs in chronic hepatitis, especially during the phase of acute necrosis, when the oval cells are found in the areas of regeneration/proliferation, as well as during the phase of fibrosis and structural reorganization of hepatic parenchyma (liver cirrhosis)[7,4,10,11,19-21].
The oval cells also share some features with the cells that appear in the mature organ in the process of hepatocarcinogenesis, giving rise to hepatocellular carcinoma and cholangio-cellular type neoplasms[8,9,12,16]. Parent et al[8] reporting on hepatic progenitor cells in liver pathology, attached special emphasis to their role in carcinogenesis seen in human chronic liver diseases.
Several authors agree that the oval cells have a bipotent nature (and can therefore be called bipotent small epithelial cells, bipotent oval cells, bipotent liver progenitor cells), i.e. these cells exhibit a two-directional differentiating ability, which during regeneration and hepatocarcinogenesis, constitutes a major source of precursor cells both for hepatocytes and for epithelial cells of bile ductules[2,6,8,11,12,19,20].
Among the numerous reports on the role of oval cells in various liver pathologies, the one published by Novikoff et al[13] deserves special attention. Using an experimental model of carcinogenesis, the authors identified a population of non-differentiated cells in the liver, termed small blast-like cells (syn. small non-epithelial cells). These cells give rise to two morphologically and phenotypically different groups of oval cells. One group contains non-polarized basic ductal blast-like cells. The other comprises two types of polarized transitional epithelial cells-the oval/bile ductule epithelial cells and hepatocytes.
Roskams et al[22], have identified three categories of oval cells in humans, which ultrustructurally and phenotypically do not differ much from those described earlier by Novikoff et al[13]. However, they treated the putative progenitor cells as categoryIoval cells and not as a separate group. Under category II, these researchers included intermediate bile-duct like cells, and in category III-the intermediate hepatocyte-like cells[22]. As noted in the experimental model, the “progenitor cells” exhibit immunoreactivity for a panel of bile ductular cell markers, including rat oval cell marker OV6, cytokeratin 7, cytokeratin 19 and chromogranin A[22].
There is no agreement with regard to the morpho-genesis and role of oval cells in different liver pathologies, which is reflected in a number of names used to describe this cell population. Moreover, we have found no reports on this subject in children with chronic viral infections.
Therefore, the objective of the current study was the ultrastructural assessment of oval cells in children with chronic hepatitis B, especially in the areas of collagen fibroplasia of varying intensity. The current study is a continuation of our morphological research on liver fibrosis in pediatric patients with chronic inflammation of the liver, including chronic hepatitis B[23-26].
Histological and ultrastructural assessment was made of liver specimens of children with biopsy proven chronic hepatitis B (HBs/+/, HBe/+/ and HBV DNA/+/), before administration of antiviral treatment. The study was carried out in the Department of Clinical Pathomor-phology, Medical University of Bialystok. Retrospective evaluation of the stage of liver fibrosis was made on material obtained by needle biopsy in 40 children, aged 3-16 years (mean age 8.5 years; 25 boys and 15 girls). Patients with autoimmune hepatitis, liver cirrhosis (including incomplete cirrhosis) and HCV co-infection were excluded from the study. None of the children were treated with antiviral or immunomodulating drugs during the 12-mo-period before enrolment in the study.
The liver biopsy specimens were fixed in 10% buffered formalin and embedded in paraffin. Histological stains used in the analysis included hematoxylin and eosin, Azan method, Masson’s trichrome, Masson’s-Goldner and reticulum stain according to Gomori. Fibrosis stage (S) was assessed in a blind fashion by a single pathologist using the semiquantitative scoring system proposed by Ishak et al[27]. In the group of 40 children, we identified 10 patients with advanced fibrosis, 10 with mild and 20 with moderate liver fibrosis. The material was also subjected to ultrastructural analysis.
For ultrastructural examination, fresh liver blocks (1 mm3) were fixed in a solution containing 2.5 glutaraldehyde, 2% paraformaldehyde, and 0.1 mol/L cacodylate buffer at pH 7.4. The specimens were post fixed in 2% OsO4, dehydrated in ethanol and propylene oxide, embedded in Epon 812 and sectioned on an ultramicrotom (Reichert) to obtain semithin sections (0.5-1 μm thick) which were stained with 1% methylene blue in 1% sodium borate and examined under a light microscope. Ultrathin sections prepared from selected specimens were double stained with uranyl acetate and lead citrate, and examined using an Opton 900 PC transmission electron microscope (Zeiss, Oberkochen, West Germany). Assessment of oval cells was made by an investigator who was blinded to the clinical information. The study was approved by the Local Ethical Committee at the Medical University of Białystok.
In the group of children (10) with Ishak’s fibrosis stage (S) 0-1, ultrastructural analyses revealed either no oval cells or only sporadic presence of these cells. The cells were found in one patient with S-0 and in 3 patients with S-1 (i.e. in 4 cases out of 10). Oval cells were more common in patients with S-2 fibrosis (7 cases out of 10). In patients with advanced liver fibrosis (S-3 or 4), the number of oval cells, although still not very high, showed a two to three-fold increase in all cases (10 patients).
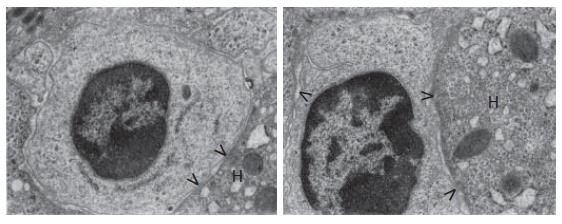
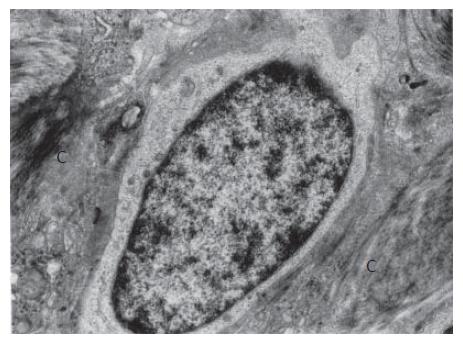
The oval cells were seen mainly in areas of periportal and portal fibrosis, especially in areas close to the limiting plate of the lobule, where they were squeezed in the intercellular spaces, being enclosed by hepatocytes, and in the vicinity to bile ductules (Figure 1, Figure 2, Figure 3).
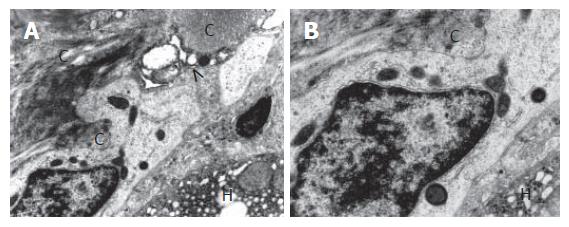
At times the cells were observed in dilated perisinu-soidal spaces of Disse, usually accompanied with collagen fiber bundles (Figure 4A and B). Activated nonparenchymal hepatocytes were seen in the vicinity of the intermediate hepatocyte-like cells. These cells transformed into hepatic stellate cells, i.e. transitional Ito-fibroblast/myofibroblast cells and Kupffer cells. Sometimes the activated nonparenchymal cells were found to adhere to the oval cells (Figures 3 and 4).
Ultrastructural examination allowed us to distinguish two types of oval cells:I-hepatic progenitor cells (HPCs) and II-intermediate hepatocyte-like cells (IHCs). In patients with S-4 fibrosis, intermediate bile duct-like cells were found, but because of their sporadic occurrence they are not discussed any further.
Hepatic progenitor cells were small (usually not exceeding 5 microns) and oval or nearly oval in shape. They had a large nucleus containing dense and highly clumped heterochromatin, accumulated distinctly under the nuclear envelope, and less abundant euchromatin (Figure 1). The cytoplasm was relatively scarce and slightly brighter than in the surrounding hepatocytes. As a result, the nucleus to the cytoplasm ratio was very high. The number of cytoplasmic structures was very small and these were only minimally differentiated (Figure 1). The cytoplasm contained tonofilaments. Some of the progenitor cells had intercellular junctions (point desmosomes), which helped connect these cells to the adjacent fully mature hepatocytes (Figure 1).
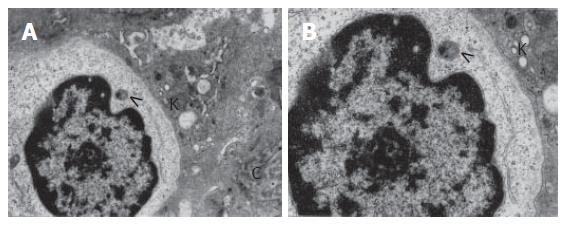
Intermediate hepatocyte-like cells varied in size, and were twice as large as the hepatic progenitor cells, whereas their diameter did not exceed one-half of the diameter of the mature hepatocytes. The nuclei were less abundant in heterochromatin compared to the progenitor cell nuclei, and occasionally contained nucleoli (Figures 4A and B). Frequently, these nuclei resembled the nuclei of mature hepatocytes.
The IHC cytoplasm showed much lower electron density compared to that of HPCs and contained better developed cell organelles, mainly mitochondria and elements of the endoplasmic reticulum, in which channels of the granular endoplasmic reticulum prevailed (Figures 2-4). The organelles accumulated in the vicinity of one of the nuclear poles (ultrastructural polarization) or were irregularly scattered throughout the cytoplasm. Among the intracellular organelles, small structures were seen that could correspond to newly formed peroxisomes (Figures 4A and B). Occasionally, the cells showed apical alterations in the form of well developed or newly formed capillary bile canaliculus.
Our study of the ultrastructure of liver specimens obtained from children with chronic hepatitis B showed the presence of small cells with an oval nucleus in the parenchyma, especially in areas of intense periportal fibrosis (at least stage 3 according to classification of Ishak et al[27]).
These cells corresponded to two types of submicro-scopic oval cells, previously described by Roskams et al[22], i.e. the hepatic progenitor cells and intermediate hepatocyte-like cells. Intermediate bile duct-like cells were seen sporadically, which may be related to the absence of regenerative nodules that are characteristic of liver cirrhosis.
In the vicinity of some intermediate hepatocyte-like cells, especially those cells lying close to collagen fiber bundles we observed activated nonparenchymal hepatocytes-transformed hepatic stellate cells, i.e. transitional Ito-fibroblast/myofibroblasts and Kupffer cells.
To the best of our knowledge, this is the first report on the electron microscopic study of oval cells in children with chronic viral infection of the liver associated with pronounced hepatic fibrosis.
In the present study, the ultrastructural appearance of the oval cells in children with chronic hepatitis B was very similar to that observed by other authors in adult patients, including those suffering from chronic viral hepatitis B and hepatitis C[10,11,20,22], as well as in various experimental models of liver damage[2,6,13,14,20]. These cells did not exhibit any significant morphological specificity related to the type and duration of liver damage and the patient’s age.
It is worth noting, that our results regarding the location of oval cells are consistent with the observations made with the light microscope by Fotiadu et al[7] in chronic hepatitis B and chronic hepatitis C in adults. These authors performed a semiquantitative evaluation of the liver progenitor cells stained for cytokeratin 7, and found an increase in the number of oval cells parallel to the grade and stage of the disease in both types of hepatitis. These workers suggested that the proliferating liver progenitor cells may play a role in hepatic regeneration that occurs in the setting of viral hepatitis[7].
Xiao et al[10,11] conducted some very interesting ultrastructural and immunohistochemical studies on the hepatic progenitor cells in liver cirrhosis. These researchers found a small number of progenitor cells mainly at the sites of intensive collagen fibrosis-on the margins of regenerative nodules, across the fibrous span and within the proliferating bile ductules. The cells exhibited immunoreactivity to cytokeratin 7 and albumin[10,11].
In our pediatric patient population also, the oval cells, both the hepatic progenitor cells and the intermediate hepatocyte-like cells, were observed mainly in the areas of intense liver fibrosis, i.e. at least in patients with stage S-2.
It is believed that the proliferation of oval cells, their gradual migration in the organ and differentiation into hepatocytes or cholangiocytes is controlled by the nonparenchymal cells, especially the activated hepatic stellate cells, and by a number of growth factors and cytokines[1,19,28,29].
It should be mentioned that activated Ito cells, also found in the vicinity of the oval cells in our study, assume phenotypic features of fibroblasts by producing desmine, alpha-actin and specific laminin chains and play a key role in the morphogenesis of collagen fibroplasia[28,29].
On the other hand, in the course of chronic inflamma-tory diseases it is the oval cells that by synthesizing and releasing numerous growth factors (transforming growth factor alpha, transforming growth factor beta, acid fibroblast growth factor, insulin-like growth factor, stem cell factor) and cytokines may exert a significant effect on the environment and stimulate (especially through the release of transforming growth factor beta) hepatic extracellular matrix synthesis[4,19,20,31,32].
Finally, oval cells form a compartment of the so called “cell reserve”, which are activated when the regenerative/proliferative properties of hepatocytes are inhibited. Therefore, some researchers treat these cells as a highly effective “third” protective system, especially in relation to the process of hepatocyte regeneration, which may open the way to cell-based therapy for liver diseases[12,19].
In conclusion, our study shows that there is a substantial correlation between the prevalence of oval hepatic progenitor cells and intermediate hepatocyte-like cells, and the stage of fibrosis in pediatric patients with chronic hepatitis B. Since the activated nonparenchymal cells were observed in the vicinity of the oval cells, it can be assumed that an interaction between these cells, especially between the transformed Ito cells, and the growth factors and cytokines secreted by them may play an essential role in the development of fibrosis in chronic hepatitis B. The present ultrastructural study provides interesting material for studies on the morphogenesis and differentiation of oval cells, and fibrosis progression in chronic hepatitis B in children.
S- Editor Liu Y L- Editor Anand BS E- Editor Chen GJ
| 1. | Bustos M, Sangro B, Alzuguren P, Gil AG, Ruiz J, Beraza N, Qian C, Garcia-Pardo A, Prieto J. Liver damage using suicide genes. A model for oval cell activation. Am J Pathol. 2000;157:549-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | He ZP, Tan WQ, Tang YF, Zhang HJ, Feng MF. Activation, isolation, identification and in vitro proliferation of oval cells from adult rat livers. Cell Prolif. 2004;37:177-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Kofman AV, Morgan G, Kirschenbaum A, Osbeck J, Hussain M, Swenson S, Theise ND. Dose- and time-dependent oval cell reaction in acetaminophen-induced murine liver injury. Hepatology. 2005;41:1252-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Matsusaka S, Toyosaka A, Nakasho K, Tsujimura T, Sugihara A, Takanashi T, Uematsu K, Terada N, Okamoto E. The role of oval cells in rat hepatocyte transplantation. Transplantation. 2000;70:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Menthena A, Deb N, Oertel M, Grozdanov PN, Sandhu J, Shah S, Guha C, Shafritz DA, Dabeva MD. Bone marrow progenitors are not the source of expanding oval cells in injured liver. Stem Cells. 2004;22:1049-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Yoon BI, Choi YK, Kim DY. Differentiation processes of oval cells into hepatocytes: proposals based on morphological and phenotypical traits in carcinogen-treated hamster liver. J Comp Pathol. 2004;131:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Fotiadu A, Tzioufa V, Vrettou E, Koufogiannis D, Papadimitriou CS, Hytiroglou P. Progenitor cell activation in chronic viralhepatitis. Liver Int. 2004;24:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Parent R, Marion MJ, Furio L, Trépo C, Petit MA. Origin and characterization of a human bipotent liver progenitor cell line. Gastroenterology. 2004;126:1147-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 191] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 9. | Uenishi T, Hirohashi K, Shuto T, Tsukamoto T, Yamamoto T, Ogawa M, Kubo S, Tanaka H, Kinoshita H. Primary liver cancer with dual expression of hepatocyte and bile duct epithelial markers. Hepatogastroenterology. 2002;49:1092-1094. [PubMed] |
| 10. | Xiao JC, Ruck P, Adam A, Wang TX, Kaiserling E. Small epithelial cells in human liver cirrhosis exhibit features of hepatic stem-like cells: immunohistochemical, electron microscopic and immunoelectron microscopic findings. Histopathology. 2003;42:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Xiao JC, Jin XL, Ruck P, Adam A, Kaiserling E. Hepatic progenitor cells in human liver cirrhosis: immunohistochemical, electron microscopic and immunofluorencence confocal microscopic findings. World J Gastroenterol. 2004;10:1208-1211. [PubMed] |
| 12. | Guettier C. Which stem cells for adult liver? Ann Pathol. 2005;25:33-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Novikoff PM, Yam A, Oikawa I. Blast-like cell compartment in carcinogen-induced proliferating bile ductules. Am J Pathol. 1996;148:1473-1492. [PubMed] |
| 14. | Sell S. Electron microscopic identification of putative liver stem cells and intermediate hepatocytes following periportal necrosis induced in rats by allyl alcohol. Stem Cells. 1997;15:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Housset C. Biliary epithelium, hepatocytes and oval cells. Rev Prat. 2000;50:2106-2111. [PubMed] |
| 17. | Grompe M. The role of bone marrow stem cells in liver regeneration. Semin Liver Dis. 2003;23:363-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Brooling JT, Campbell JS, Mitchell C, Yeoh GC, Fausto N. Differential regulation of rodent hepatocyte and oval cell proliferation by interferon gamma. Hepatology. 2005;41:906-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Schaff Z, Nagy P. Novel factors playing a role in the pathomechanism of diffuse liver diseases: apoptosis and hepatic stem cells. Orv Hetil. 2004;145:1787-1793. [PubMed] |
| 20. | Sun C, Jin XL, Xiao JC. Oval cells in hepatitis B virus-positive and hepatitis C virus-positive liver cirrhosis: histological and ultrastructural study. Histopathology. 2006;48:546-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Tan J, Hytiroglou P, Wieczorek R, Park YN, Thung SN, Arias B, Theise ND. Immunohistochemical evidence for hepatic progenitor cells in liver diseases. Liver. 2002;22:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Roskams T, De Vos R, Van Eyken P, Myazaki H, Van Damme B, Desmet V. Hepatic OV-6 expression in human liver disease and rat experiments: evidence for hepatic progenitor cells in man. J Hepatol. 1998;29:455-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 209] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 23. | Lebensztejn DM, Sobaniec-Lotowska M, Kaczmarski M, Werpachowska I, Sienkiewicz J. Serum concentration of transforming growth factor (TGF)-beta 1 does not predict advanced liver fibrosis in children with chronic hepatitis B. Hepatogastroenterology. 2004;51:229-233. [PubMed] |
| 24. | Lebensztejn DM, Sobaniec-Łotowska ME, Bauer M, Kaczmarski M, Voelker M, Schuppan D. Serum fibrosis markers as predictors of an antifibrotic effect of interferon alfa in children with chronic hepatitis B. Eur J Gastroenterol Hepatol. 2005;17:843-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Lebensztejn DM, Sobaniec-Lotowska ME, Kaczmarski M, Voelker M, Schuppan D. Matrix-derived serum markers in monitoring liver fibrosis in children with chronic hepatitis B treated with interferon alpha. World J Gastroenterol. 2006;12:3338-3343. [PubMed] |
| 26. | Maria Elzbieta SL, Marek LD. Histological outcome of chronic hepatitis B in children treated with interferon alpha. World J Gastroenterol. 2005;11:7179-7182. [PubMed] |
| 27. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3521] [Cited by in RCA: 3784] [Article Influence: 126.1] [Reference Citation Analysis (1)] |
| 28. | Martinez-Hernandez A, Amenta PS. The extracellular matrix in hepatic regeneration. FASEB J. 1995;9:1401-1410. [PubMed] |
| 29. | Suh H, Song MJ, Park YN. Behavior of isolated rat oval cells in porous collagen scaffold. Tissue Eng. 2003;9:411-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Friedman SL. Mechanisms of disease: Mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol. 2004;1:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 387] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 31. | Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1126] [Cited by in RCA: 1205] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 32. | Michalopoulos GK, DeFrances M. Liver regeneration. Adv Biochem Eng Biotechnol. 2005;93:101-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |