Published online May 28, 2007. doi: 10.3748/wjg.v13.i20.2811
Revised: March 3, 2007
Accepted: March 19, 2007
Published online: May 28, 2007
AIM: To investigate the anti-cancer mechanisms of Korean mistletoe lectin (Viscum album coloratum agglutinin, VCA) using a human colon cancer cell line (COLO).
METHODS: Cytotoxic effects of VCA on COLO cells were determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay in vitro and tumor-killing effects in vivo. To study the mechanisms involved, the expression of various pro-caspases, anti-apoptotic proteins, and death receptors was determined by western blot. To determine which death receptor is involved in VCA-induced apoptosis of COLO cells, cytotoxicity was examined by MTT assay after treatment with agonists or antagonists of death receptors.
RESULTS: VCA killed COLO cells in a time- and dose-dependent manner and induced complete regression of tumors in nude mice transplanted with COLO cells. Treatment of COLO cells with VCA activated caspase-2, -3, -8, and -9 and decreased expression of anti-apoptotic molecules including receptor interacting protein, nuclear factor-κB, X-linked inhibitor of apoptosis protein, and Akt/protein kinase B. We then examined the involvement of death receptors in VCA-induced apoptosis. Only tumor necrosis factor receptor 1, among the death receptors examined, was involved in apoptosis of COLO cells, evidenced by inhibition of VCA-induced apoptosis and decreased activation of caspases, particularly caspase-8, by tumor necrosis factor receptor 1 antagonizing antibody.
CONCLUSION: VCA-induced apoptotic COLO cell death is due to the activation of caspases and inhibition of anti-apoptotic proteins, in part through the tumor necrosis factor receptor 1 signaling pathway.
- Citation: Khil LY, Kim W, Lyu S, Park WB, Yoon JW, Jun HS. Mechanisms involved in Korean mistletoe lectin-induced apoptosis of cancer cells. World J Gastroenterol 2007; 13(20): 2811-2818
- URL: https://www.wjgnet.com/1007-9327/full/v13/i20/2811.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i20.2811
Extracts from European white berry mistletoe (Viscum album L, Family Viscaceae) have been used in adjuvant chemotherapy[1]. Lectins were identified as one of the therapeutically active molecules in mistletoe extracts[2-4]. European mistletoe lectins (Viscum album agglutinins, VAAs) showed cytotoxic effects on tumor cells by apoptotic cell death[3]. Korean mistletoe (Viscum album var. coloratum Kom.), a subspecies of European mistletoe, has been used as a medicinal herb and has also been shown to be cytotoxic against tumor cells[5]. It was reported that a lectin isolated from Korean mistletoe (Viscum album var. coloratum agglutinin, VCA) was different from VAAs in molecular weight, N-terminal amino acid sequence, and structure. VCA has a molecular mass of 60 kDa, consisting of a 31 kDa A chain and a 34 kDa B chain, and binds preferentially to galactose and N-acetyl-D-galactosamine[6-9]. VCA showed strong cytotoxic activity against human and murine tumor cells[10]. VCA inhibited telomerase activity, resulting in DNA fragmentation and tumor cell apoptosis[11-13].
There are two major pathways for apoptosis: the death receptor-induced pathway and the mitochondria-apoptosome-mediated pathway. The death receptor-induced apoptotic pathway includes ligands such as Fas, tumor necrosis factor (TNF), and death receptor (DR)3 and their receptors and downstream molecules such as caspases[14,15]. The mitochondria-apoptosome-mediated pathway includes apoptotic stimuli induced by radiation or chemotherapy. The caspase cascade is activated by the release of cytochrome c, which is initiated by the formation of apoptosomes[15]. Cross-talk between these two apoptotic pathways also exists[15,16]. Both VAAs and VCA were shown to induce apoptosis of tumor cells through the mitochondria-mediated pathway[17,18]. However, it is not known whether the death receptor-mediated apoptosis pathway is also involved in the killing of tumor cells by VCA.
In this study, it was found that VCA treatment showed a strong killing effect on COLO 320HSR (COLO) cells both in vitro and in vivo. VCA treatment of COLO cells resulted in the activation of caspase-2, -3, -8, and -9, induced the degradation of Akt/protein kinase B (PKB) and poly(ADP) ribose polymerase, and decreased the expression of anti-apoptotic proteins such as receptor-interacting protein (RIP) and X-linked inhibitor of apoptosis (XIAP). Antagonizing anti-tumor necrosis factor receptor (TNFR)1 antibody treatment partially inhibited the activation of caspases and apoptosis induced by VCA. These results suggest that TNFR1, a component of the death receptor-mediated apoptosis pathway, may be involved, in part, in activating caspases, resulting in the apoptosis of colon cancer cells by VCA.
VCA was extracted from Viscum album L. coloratum and the biochemical properties were characterized as described elsewhere[6,7]. Anti-phospho-Akt/PKB (Ser-473), anti-Akt/PKB, anti-caspase-8, anti-caspase-9, and anti-XIAP antibodies were purchased from New England Biolabs (Beverly, CA). Anti-caspase-2, anti-RIP, anti-FasL, anti-Fas, and anti-caspase-3 antibodies were purchased from BD Pharmingen (San Diego, CA). Anti-nuclear factor (NF)-κB, and anti-TNFR2 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-β-actin antibody was purchased from Sigma Chemicals (St. Louis, MO). Anti-DR3 and anti-TNFR1 antibodies were purchased from Stressgen Biotechnologies Corp. (Victoria, BC, Canada). Activating anti-Fas antibody (clone CH11) was purchased from Upstate Biotechnology (Charlottesville, VA). Antagonizing anti-TNFR1 antibody and DR3/Fc chimeric protein were purchased from R & D Systems (Minneapolis, MN).
The COLO 320HSR colon cancer cell line (COLO), human epidermoidal cancer cell line (A253), and human diploid cell line from normal embryonic lung tissue (WI-38) were obtained from American Type Culture Collection (Rockville, MD). COLO cells and A253 cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and gentamycin (50 μg/mL). WI-38 cells were cultured in Minimum Eagle's essential medium containing 2 mmol/L L-glutamine, Earle's balanced salts, 0.1 mmol/L non-essential amino acids, 1 mmol/L sodium pyruvate and 10% FBS with antibiotics as above. Cells were cultured at 37°C in a humidified atmosphere containing 5% CO2.
3- (4, 5-Dimethylthiazol-2-yl) -2, 5-diphenyltetrazolium bromide (MTT; Sigma Chemicals) was used to measure the cytotoxic effect of VCA. Cells (2 × 104/well) were seeded and cultured in 96-well plates for 24 h and treated with various reagents. After treatment, 20 μL MTT (5 mg/mL) was added and cells were incubated for 4 h at 37°C. After removing the supernatant, the produced formazan crystals were dissolved in dimethyl sulfoxide, and optical density was measured at A570 nm and at A670 nm as reference with a microplate reader (Thermo Labsystems, Helsinki, Finland). The viability of the cells was calculated as: % viability = (absorbance of treated cells)/(absorbance of untreated cells) × 100.
COLO cells were cultured with or without VCA for 9 h, harvested, washed twice with PBS, and fixed with cold 70% ethanol at 4°C overnight. Fixed cells were stored in absolute alcohol until staining with propidium iodide. Cells were washed twice with PBS and incubated with a propidium iodide mixture containing 100 µg/mL RNase A (Sigma Chemicals) and 50 µg/mL propidium iodide (Sigma Chemicals) in PBS at 37°C for 30 min in the dark. The numbers of cells in various phases of the cell cycle or undergoing apoptosis were determined by flow cytometry.
Male CD1 nu/nu nude mice (5 wk old) were purchased from Charles River (Wilmington, MA). Mice were adapted for 1 wk before experiments and were allowed food and water ad libitum during the experiments. COLO cells (1 × 107 cells/mouse) were subcutaneously injected into the neck of nude mice. After 5 wk, the tumor-bearing mice were injected with VCA (10 μg/kg in PBS; 30 μL/mouse) around the tumor mass every 2 d for 5 wk. The use and care of the animals in this study were approved by the Animal Care Committee, Faculty of Medicine, University of Calgary.
Cells cultured under various experimental conditions were washed twice with ice-cold PBS and lysed in lysis buffer containing 50 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 1 mmol/L EDTA, 0.5 mmol/L dithiothreitol, 1% NP-40, 0.05 mmol/L phenylmethylsulfonyl fluoride, phosphatase inhibitor cocktail, and proteinase inhibitor cocktail (Sigma Chemicals). After incubation for 30 min on ice, cell lysates were centrifuged at 18 300 ×g at 4°C for 20 min. Protein concentrations were determined using a protein assay kit (Bio-Rad, Richmond, CA). Samples of cell lysate containing 50 μg of total protein were separated by 10%-12% SDS-PAGE and transferred onto a polyvinylidene fluoride membrane (Amersham Pharmacia Biotech, Buckinghamshire, UK). After blocking with 5% skim milk in Tris-buffered saline (50 mmol/L Tris-HCl, pH 7.6, 150 mmol/L NaCl, 0.05% Tween-20), the membranes were incubated overnight at 4°C with various primary antibodies and further incubated with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz). The membranes were visualized by an enhanced chemiluminescence detection kit (Amersham Pharmacia Biotech).
The statistical significance of differences between groups was analyzed by Student’s t-test. A level of P < 0.05 was accepted as significant.
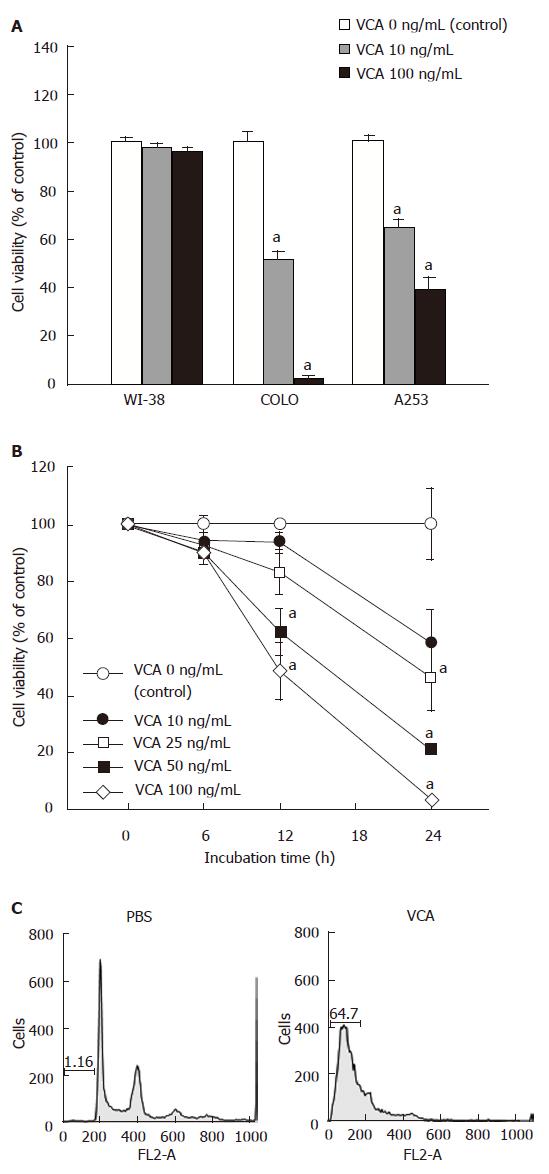
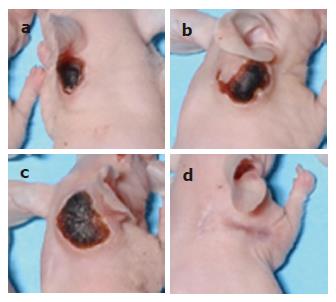
To determine the cytotoxic effects of VCA on cancer cells, COLO (colon cancer), A253 (epidermal cancer), and WI-38 (normal) cells were treated with VCA for 24 h and cell viability was examined by MTT assay. VCA showed a strong killing effect on COLO cells, significantly less on A253 cells, and no effect on WI-38 cells (Figure 1A). COLO cells were treated with various doses of VCA for various times and cell viability was examined by MTT assay. A dose- and time-dependent killing effect could be seen between 10 ng/mL and 100 ng/mL VCA, with 100 ng/mL showing the maximum effect (Figure 1B). Propidium iodide staining and FACS analysis of VCA-treated COLO cells showed apoptosis in 64.7% of the cells (Figure 1C). To determine whether VCA shows a tumor-killing effect on colon cancer cells in vivo, COLO cells were subcutaneously injected into the neck of 6-wk-old male CD1 nu/nu mice. Five weeks later when tumors developed, VCA or PBS, as a control, was injected into the tumor every 2 d for 5 wk. We found that treatment with VCA for 5 wk resulted in the killing of tumors (Figure 2A). All of the tumors treated with VCA disappeared and were replaced by scar tissue, and this scar tissue healed in mice kept for an additional 2 mo without further treatment (Figure 2B). However, tumors remained in PBS-treated mice (data not shown).
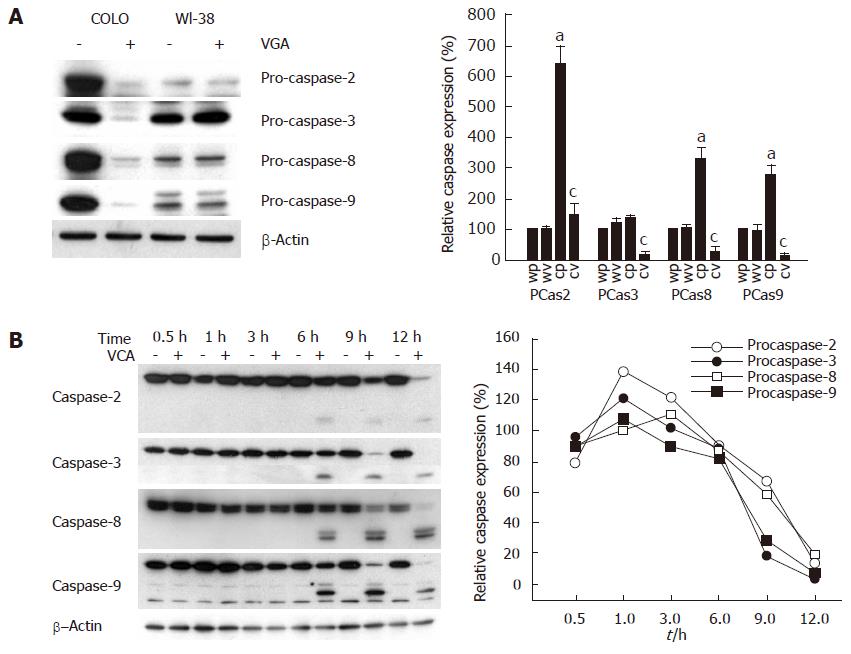
To examine the apoptosis of COLO cells by VCA, COLO cells were treated with VCA for 12 h and the amount of procaspase was examined by western blot. Treatment of COLO cells with VCA decreased the amount of procaspases-2, -3, -8, and -9 as compared with untreated COLO cells, whereas VCA treatment of control WI38 cells did not change the amount of these procaspases (Figure 3A), indicating that VCA treatment activates caspases specifically in COLO tumor cells. To determine the time-dependent activation of caspases, COLO cells were treated with VCA and harvested at 0.5, 1, 3, 6, 9, and 12 h after treatment and western blots were performed using antibodies specific for each procaspase. It was found that cleaved products from procaspase-2, -3, -8, and -9 were detected from 6 h after VCA treatment and the corresponding procaspase bands were almost completely absent at 12 h after treatment (Figure 3B). These results indicate that VCA treatment activated caspase-2, -3, -8, and -9 in a time-dependent manner.
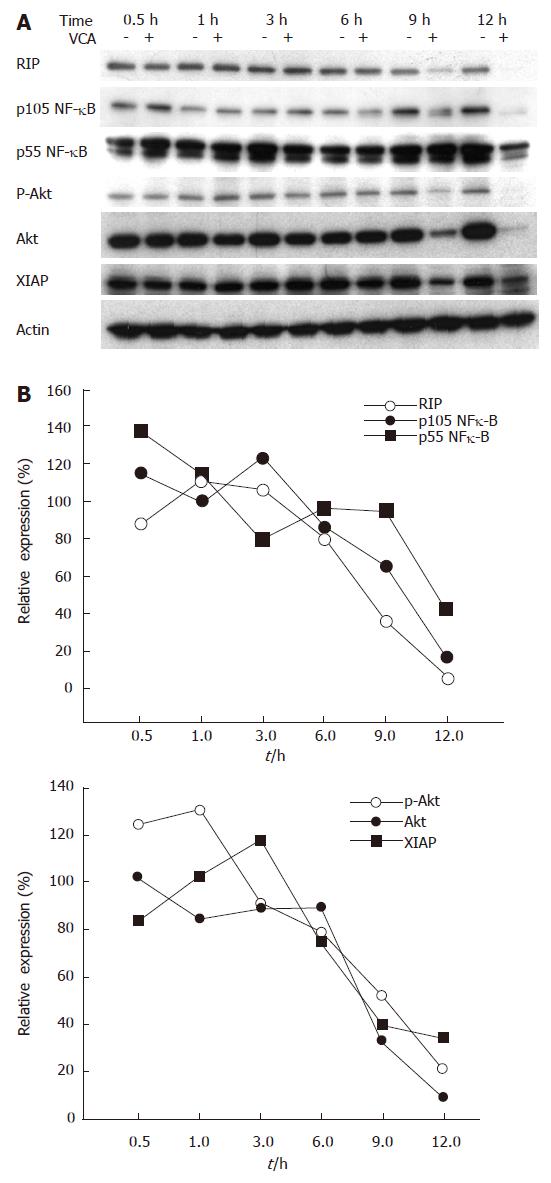
To further determine the mechanisms of the apoptotic pathway involved in VCA-induced killing of COLO cells, we examined the expression of adaptor molecules of death receptors and their downstream molecules, including RIP, Fas-associated death domain (FADD), NF-κB, Akt/PKB, and XIAP, by western blot. The expression of RIP and FADD started to decrease after 6 h of VCA treatment and was correlated with a decreased expression of NF-κB, Akt/PKB, and XIAP in COLO cells (Figure 4).
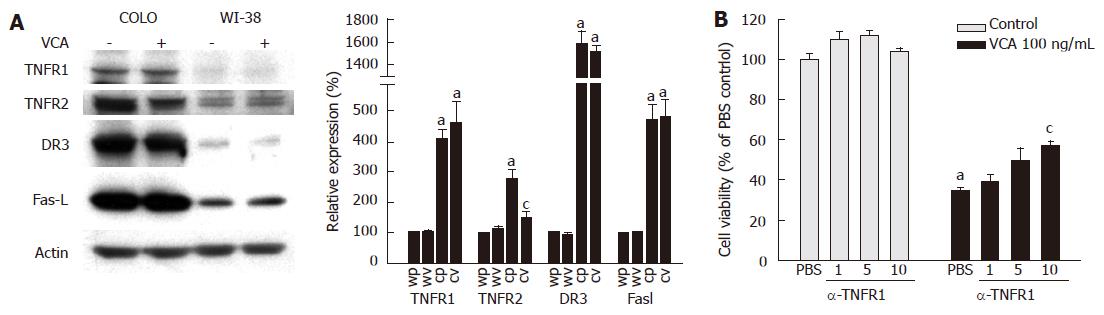
To determine whether death receptors are involved in VCA-induced apoptosis of COLO cells, we examined the expression of the death receptors TNFR1, TNFR2, DR3, Fas, and FasL in COLO and WI-38 cells by western blot. The expression of TNFR1, TNFR2, DR3, and FasL was higher in COLO cells as compared with WI-38 cells. Fas expression was not detected in either cell type (data not shown). Treatment of COLO cells with VCA significantly decreased the expression of TNFR2, but not TNFR1, DR3, or FasL. Treatment of WI-38 cells with VCA did not change the expression of these molecules (Figure 5A).
To determine which death receptor is involved in VCA-induced apoptosis of COLO cells, the killing effect of VCA on COLO cells was examined in the presence or absence of agonists or antagonists of death receptors. Treatment of COLO cells with activating anti-Fas antibody did not induce cell death or affect the killing effect of VCA, which was expected as Fas is not expressed in COLO cells (data not shown). In addition, anti-Fas antibody did not affect the killing effects of VCA on COLO cells. Treatment with DR3-Fc chimeric protein, which inhibits the activation of DR3, did not affect the cytotoxic effect of VCA on COLO cells (data not shown). Treatment with antagonizing anti-TNFR1 antibody, which blocks TNF-α-induced apoptosis, inhibited the killing effect of VCA on COLO cells (Figure 5B).
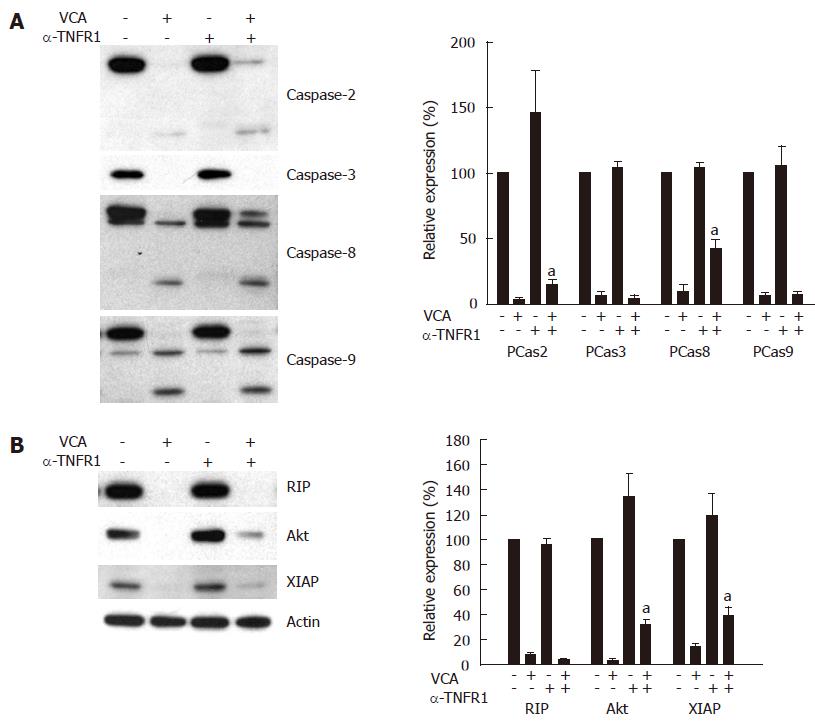
To determine whether blocking TNFR1 inhibits VCA-induced activation of caspase-2, -3, -8, and -9, resulting in the inhibition of apoptotic death of COLO cells, the expression of the respective procaspases in VCA-treated COLO cells was determined in the presence or absence of antagonizing anti-TNFR1 antibody by western blot. Anti-TNFR1 antibody significantly inhibited the activation of caspase-8 and caspase-2, as shown by the inhibition of the decrease of their respective procaspase, but not caspase -3 or -9 (Figure 6A). VCA treatment of COLO cells decreased the expression of anti-apoptotic molecules, such as Akt/PKB and XIAP, and treatment with antagonizing anti-TNFR1 antibody significantly inhibited this decrease (Figure 6B).
It was previously reported that Viscum album var. coloratum agglutinin (VCA) had cytotoxic effects on cancer cells such as hepatocarcinoma and HL-60 leukemia cells by inducing apoptosis[12,18]. Consistent with this, it was found that VCA selectively killed colon cancer cells (COLO 320HSR) in vitro and in vivo, but not normal human diploid cells (WI-38). However, the detailed molecular mechanisms involved in the killing of tumor cells by VCA have not been clearly elucidated. In this study, it was found that VCA-induced apoptosis of colon cancer cells is due to the activation of caspases and inhibition of anti-apoptotic proteins partly through the TNFR1 signaling pathway.
Apoptotic morphological and biochemical changes, including chromatin condensation, degradation of chromosomal DNA, and membrane blebbing[19-21], occur after a cascade of cell signaling and caspase-mediated events that regulate pro-apoptotic and anti-apoptotic proteins[14,22,23]. Apoptosis is triggered by two major pathways: the death-receptor-induced apoptotic pathway and the mitochondria-apoptosome-mediated apoptotic pathway[14]. Both of these pathways lead to caspase activation and cleavage of specific cellular substrates[14,15]. Inhibitors of apoptotic proteins such as XIAP, cellular inhibitor of apoptosis proteins, phosphatidylinositol 3-kinase, Akt/PKB, NF-κB, and heat shock proteins can interact with caspases and inhibit apoptosis[15]. We examined the activation of caspases in VCA-treated COLO cells and found that caspase-2, -3, -8, and -9 were activated as compared with PBS-treated COLO cells.
While the activation of caspases is critical for the induction of apoptosis[23,24]. The degradation of anti-apoptotic proteins such as RIP, Akt/PKB, and XIAP can accelerate the death of cancer cells. Consistent with previous work[14], the present study found that treatment of COLO cells with VCA significantly decreased the amount of RIP, Akt/PKB, and XIAP. The cytotoxic effects of VCA on COLO cells was more rapid and intense in serum-free media (data not shown), probably due to the absence of survival signals mediated by the Akt/PKB pathway by growth factors in serum-containing media. These results support the hypothesis that degradation of anti-apoptotic proteins by VCA treatment contributes to the induction of apoptosis in COLO cells.
It was previously reported that the activation of caspase-8 by VAAs, European mistletoe lectins, was not mediated by death receptors[17]. Transfection of a dominant-negative FADD into BJAB cells did not affect the induction of apoptosis by VAAs, suggesting that VAAs trigger a mitochondria-mediated apoptotic pathway[17]. In our study, it was found that VCA treatment activated caspase-2, -3 and -8, which are mediated by death receptors[15,16,25], and caspase-2 and -9, which are mediated by the mitochondrial pathway[25-27]. Therefore, VCA appears to induce activation of caspases by both mitochondria-mediated and death receptor-mediated pathways. VCA, from Korean mistletoe, and mistletoe lectin (ML) II, an isotype of VAAs from European mistletoe, have the same sugar-binding characteristics; however, the structures of these lectins are different. Variations in VAAs (MLI, ML II, and ML III) result from different degrees of glycosylation[28], whereas variations in VCA result from amino acid sequence variation in the A and B chains, which are 10%-27% different from ML[29]. These differences in amino acid sequence may result in different cytotoxic mechanisms between VCA and VAAs.
COLO cells were found to express a high level of the death receptors TNFR1, TNFR2, and DR3, but did not express Fas. The expression of FasL was also high in COLO cells, probably as a mechanism to escape immune attack[30]. The expression of TNFR2 was decreased in COLO cells by VCA treatment, suggesting that VCA may inhibit survival signals through TNFR2, since TNFR2 mediates both survival and apoptotic signals[25,31]. However, this should be addressed further. To determine if any of these death receptors is involved in VCA-induced apoptosis of COLO cells, we activated or inhibited their signaling pathways in VCA-treated COLO cells using antibodies. Activating anti-Fas antibody did not affect the viability of COLO cells, as expected by the absence of Fas in these cells. Since DR3 is expressed on COLO cells, VCA may bind to DR3 and trigger apoptotic signals, resulting in cell death. Thus, DR3-Fc chimeric protein, which binds to the DR3 ligand and inhibits DR3-mediated apoptosis, was used to determine whether VCA-mediated apoptosis could be inhibited by blocking the interaction between VCA and DR3. DR3-Fc chimeric protein did not affect the cytotoxic effect of VCA on COLO cells.
TNF-α induced apoptosis of COLO cells sensitized with actinomycin D (data not shown)[32], indicating a role for TNFR in apoptosis of COLO cells. In our study, antagonizing anti-TNFR1 inhibited the cytotoxicity of VCA in COLO cells, suggesting that the TNFR1 pathway is involved in VCA-induced cell death. This antibody also inhibited VCA-induced apoptosis and activation of caspase-8 and -2, confirming the involvement of TNFR1 in VCA-induced apoptosis. This finding is supported by previous reports that TNFR1 induces both receptor-mediated (activation of caspase -3 and -8) and mitochondria-mediated (activation of caspase -2 and -9) apoptosis[16,25]. The exact mechanism by which VCA activates TNFR1 is not known; however, VCA was co-precipitated when TNFR1 was immunoprecipitated with TNFR1 antibody in VCA-treated COLO cells (unpublished data). Thus, we speculate that VCA might bind to TNFR1 and trigger cell death. This mechanism needs further investigation. Based on the results obtained in this study, we suggest that VCA may induce the activation of the TNFR1 signal transduction pathway, which contributes, in part, to the activation of caspases and degradation of anti-apoptotic molecules, resulting in the death of colon cancer cells.
We thank Dr. Ann Kyle for editorial assistance.
S- Editor Liu Y L- Editor Alpini GD E- Editor Zhou T
| 1. | Steuer-Vogt MK, Bonkowsky V, Ambrosch P, Scholz M, Neiss A, Strutz J, Hennig M, Lenarz T, Arnold W. The effect of an adjuvant mistletoe treatment programme in resected head and neck cancer patients: a randomised controlled clinical trial. Eur J Cancer. 2001;37:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Holtskog R, Sandvig K, Olsnes S. Characterization of a toxic lectin in Iscador, a mistletoe preparation with alleged cancerostatic properties. Oncology. 1988;45:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Stauder H, Kreuser ED. Mistletoe extracts standardised in terms of mistletoe lectins (ML I) in oncology: current state of clinical research. Onkologie. 2002;25:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Mengs U, Göthel D, Leng-Peschlow E. Mistletoe extracts standardized to mistletoe lectins in oncology: review on current status of preclinical research. Anticancer Res. 2002;22:1399-1407. [PubMed] |
| 5. | Khwaja TA, Varven JC, Pentecost S, Pande H. Isolation of biologically active alkaloids from Korean mistletoe Viscum album, coloratum. Experientia. 1980;36:599-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Lyu SY, Park SM, Choung BY, Park WB. Comparative study of Korean (Viscum album var. coloratum) and European mistletoes (Viscum album). Arch Pharm Res. 2000;23:592-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Park WB, Han SK, Lee MH, Han KH. Isolation and characterization of lectins from stem and leaves of Korean mistletoe (Viscum album var.coloratum) by affinity chromatography. Arch Pharm Res. 1997;20:306-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Hajto T, Hostanska K, Frei K, Rordorf C, Gabius HJ. Increased secretion of tumor necrosis factors alpha, interleukin 1, and interleukin 6 by human mononuclear cells exposed to beta-galactoside-specific lectin from clinically applied mistletoe extract. Cancer Res. 1990;50:3322-3326. [PubMed] |
| 9. | Peumans WJ, Verhaert P, Pfüller U, Van Damme EJ. Isolation and partial characterization of a small chitin-binding lectin from mistletoe (Viscum album). FEBS Lett. 1996;396:261-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Yoon TJ, Yoo YC, Kang TB, Shimazaki K, Song SK, Lee KH, Kim SH, Park CH, Azuma I, Kim JB. Lectins isolated from Korean mistletoe (Viscum album coloratum) induce apoptosis in tumor cells. Cancer Lett. 1999;136:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Kim MS, Lee J, Lee KM, Yang SH, Choi S, Chung SY, Kim TY, Jeong WH, Park R. Involvement of hydrogen peroxide in mistletoe lectin-II-induced apoptosis of myeloleukemic U937 cells. Life Sci. 2003;73:1231-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Lyu SY, Park WB, Choi KH, Kim WH. Involvement of caspase-3 in apoptosis induced by Viscum album var. coloratum agglutinin in HL-60 cells. Biosci Biotechnol Biochem. 2001;65:534-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Choi SH, Lyu SY, Park WB. Mistletoe lectin induces apoptosis and telomerase inhibition in human A253 cancer cells through dephosphorylation of Akt. Arch Pharm Res. 2004;27:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Debatin KM. Apoptosis pathways in cancer and cancer therapy. Cancer Immunol Immunother. 2004;53:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 336] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 15. | Kim R, Tanabe K, Uchida Y, Emi M, Inoue H, Toge T. Current status of the molecular mechanisms of anticancer drug-induced apoptosis. The contribution of molecular-level analysis to cancer chemotherapy. Cancer Chemother Pharmacol. 2002;50:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 193] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 16. | Nuñez G, Benedict MA, Hu Y, Inohara N. Caspases: the proteases of the apoptotic pathway. Oncogene. 1998;17:3237-3245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 766] [Cited by in RCA: 775] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 17. | Bantel H, Engels IH, Voelter W, Schulze-Osthoff K, Wesselborg S. Mistletoe lectin activates caspase-8/FLICE independently of death receptor signaling and enhances anticancer drug-induced apoptosis. Cancer Res. 1999;59:2083-2090. [PubMed] |
| 18. | Lyu SY, Choi SH, Park WB. Korean mistletoe lectin-induced apoptosis in hepatocarcinoma cells is associated with inhibition of telomerase via mitochondrial controlled pathway independent of p53. Arch Pharm Res. 2002;25:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6842] [Cited by in RCA: 6815] [Article Influence: 252.4] [Reference Citation Analysis (0)] |
| 20. | Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899-1911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2769] [Cited by in RCA: 2760] [Article Influence: 106.2] [Reference Citation Analysis (0)] |
| 21. | Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9960] [Cited by in RCA: 10006] [Article Influence: 188.8] [Reference Citation Analysis (0)] |
| 22. | Evan G, Littlewood T. A matter of life and cell death. Science. 1998;281:1317-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1079] [Cited by in RCA: 1047] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 23. | Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5130] [Cited by in RCA: 5089] [Article Influence: 188.5] [Reference Citation Analysis (0)] |
| 24. | Kumar S, Vaux DL. Apoptosis. A cinderella caspase takes center stage. Science. 2002;297:1290-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Gaur U, Aggarwal BB. Regulation of proliferation, survival and apoptosis by members of the TNF superfamily. Biochem Pharmacol. 2003;66:1403-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 404] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 26. | Robertson JD, Enoksson M, Suomela M, Zhivotovsky B, Orrenius S. Caspase-2 acts upstream of mitochondria to promote cytochrome c release during etoposide-induced apoptosis. J Biol Chem. 2002;277:29803-29809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 315] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 27. | Guo Y, Srinivasula SM, Druilhe A, Fernandes-Alnemri T, Alnemri ES. Caspase-2 induces apoptosis by releasing proapoptotic proteins from mitochondria. J Biol Chem. 2002;277:13430-13437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 376] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 28. | Zimmerman R, Pfuller U. Glycosylation pattern of mistletoe lectins. COST 98: Effects of antinutrients on the nutritional value of legume diets. Luxembourg: European Commission 1998; 55-62. |
| 29. | Park CH, Lee DW, Kang TB, Lee KH, Yoon TJ, Kim JB, Do MS, Song SK. cDNA cloning and sequence analysis of the lectin genes of the Korean mistletoe (Viscum album coloratum). Mol Cells. 2001;12:215-220. [PubMed] |
| 30. | Houston A, Bennett MW, O'Sullivan GC, Shanahan F, O'Connell J. Fas ligand mediates immune privilege and not inflammation in human colon cancer, irrespective of TGF-beta expression. Br J Cancer. 2003;89:1345-1351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Gupta S. A decision between life and death during TNF-alpha-induced signaling. J Clin Immunol. 2002;22:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 142] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Powell CB, Mutch DG, Massad LS, Kao MS, Collins JL. Common expression of a tumor necrosis factor resistance mechanism among gynecological malignancies. Cancer Immunol Immunother. 1990;32:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |