Published online May 28, 2007. doi: 10.3748/wjg.v13.i20.2798
Revised: March 3, 2007
Accepted: March 8, 2007
Published online: May 28, 2007
AIM: To evaluate the effect of hydroxyapatite nano-particles (Nano HAP) by intravenous injection on the inhibition of implanted hepatic VX2 tumor growth in rabbits and cell p53/c-Myc protein expression.
METHODS: 60 hepatic VX2 tumor-bearing rabbits was randomly divided into five groups. Nano HAP collosol 20 mg/kg, 40 mg/kg, 5-FU solutions 20 mg/mL, mixed liquor of 5-FU solution 20 mg/mL and Nano HAP collosol 20 mg/kg were infused by vein, normal saline conducted as the control. The general state, weight, liver function and gross tumor volume were detected dynamically. The expression of p53 and c-Myc gene protein in tumor tissue was detected by immunohistochemistry methods.
RESULTS: The growth of implanted hepatic VX2 tumors was significantly inhibited in all therapy groups, 3 wk after the injection, the tumor control rates in Nano HAP collosol groups were 25.5% and 32.5% respectively, and the gross tumor volumes were obviously less than that of control group. (24.81 ± 5.17 and 22.73 ± 4.23 vs 33.32 ± 5.26, P < 0.05). The tumor control rate of 5-FU group was 43.7% (18.74 ± 4.40 vs 33.32 ± 5.26, P < 0.05), but the general state of the animals after injection aggravated; and the adverse reaction in the drug combination group obviously decreased. Due to the effect of Nano HAP, the positive expression of tumor associated the mutated p53 and c-Myc in tumor tissue was decreased obviously compared with the control group.
CONCLUSION: Nano HAP has evident inhibitory action on rabbit implanted hepatic VX2 tumor in vivo, which may be the result of decreasing the expression of the mutated p53 and c-myc, and drug combination can obviously decrease the adverse reaction of 5-FU.
- Citation: Hu J, Liu ZS, Tang SL, He YM. Effect of hydroxyapatite nanoparticles on the growth and p53/c-Myc protein expression of implanted hepatic VX2 tumor in rabbits by intravenous injection. World J Gastroenterol 2007; 13(20): 2798-2802
- URL: https://www.wjgnet.com/1007-9327/full/v13/i20/2798.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i20.2798
As the nanometer technique continuous to develop, its application in medical area has became a hot spot of current investigation. Hydroxyapatite (HAP), the molecular formula of which is Ca10(PO4)6(OH)2, is the essential component of inorganic composition in human bone. It has been found to have obvious inhibitory function on growth of many kinds of tumor cells, and its nanoparticle has stronger anti-tumor effect than macromolecule microparticles. In vitro, HAP has been demonstrated to have obvious apoptosis induction and growth inhibition effects[1]. Based on the above, we examined the effect of HAP on growth inhibition of implanted hepatic VX2 tumor in rabbits and cell p53/c-Myc protein expression in tumor tissue in vivo.
New Zealand rabbits, female or male, weighing 2.5-3.5 kg, were obtained from the Laboratory Animal Center of Wuhan University Medical School; tumor bearing rabbits were presented by Tangdu Hospital of the Fourth Military Medical University; Nano HAP and 0.2% CMC-Na were provided by Biomaterial Center of East China University of Science and Technology; 5-FU injection was produced by Shanghai Xudong Haipu Medicine Company Ltd.; rat anti rabbit c-Myc, P53 monoclonal antibody and the immunohistochemistry kit were purchased from Fuzhou Maixin Biologic Product Company. 20 mg/mL and 40 mg/mL solutions were prepared with normal saline and Nano HAP powder, and 20 mg/mL Nano HAP and 20 mg/mL 5-FU were mixed in vol. proportion 1:1, 0.2% CMC-Na was separately added as dispersion stabilizer, then dispersed to suspending-stable Nano HAP sol and mix injection in Sonic V130 ultrasonic cell pulverizer; 5-FU injection was diluted with normal saline to 20 mg/mL.
VX2 tumor fragments were implanted in rabbit thigh muscle, and were taken out when their diameter extended to 1.0-1.5 cm. Having removed the connective tissue,we cut the tumor tissue, like flesh of a fish into small cubes about 1 mm3 in size and implanted it in the left lobe visceral surface of healthy rabbit liver, with a depth exceeding 1 cm. Ultrasound detection was taken 7 d after the implantation, and the tumor growth state was recorded. If the tumor doesn’t have ectopia implantation, colliquation or cystoid degeneration, the animal implantation model is considered successful. The selected 60 tumor bearing rabbits were randomly divided into five groups: A, B, C, D and E. The drug began to be injected through dorsal ear vein 3 wk after the implantation every other day. Group A was given normal saline, 2 mL every time; group B was given Nano HAP sol 20 mg/kg; group C was given Nano HAP sol 40 mg/kg; group D was given 5-FU 20 mg/kg; group E was given the mixed liquor of Nano HAP sol 20 mg/mL and 5 Fu 20 mg/mL, the doses were both 20 mg/kg.
The tumor-bearing rabbits were fed in the standard cleaning level lab, the general state changes of the animals, such as color pattern, food-intake, water-drinking, activity, oral and nasal discharge were followed up daily. The weight of the animals were taken before feeding in the early morning every 3 d, and the liver function (ALT) was detected by extracting venous blood of experimental rabbits every week.
3 wk after the injection, rabbits were intravenously anesthetized and their abdominal cavities were opened, integrate liver were taken out, abdominal and thoracic cavities were examined. tumor volume was calculated by the formula V = 0.5 × a × b2 (a: maximum diameter, b: maximum transverse diameter), growth inhibition rate of tumor (GIR) = ( tumor volume of control group-tumor volume of therapy group)/tumor volume of control group × 100%.
The samples were labeled, numbered and placed in 10% formalin solution for fixation. Then the tissue was embedded in paraffin and serially sectioned (4 μm) for HE staining. The final diagnosis of tumor depended on pathology. Immunohistochemistry staining was made using SABC assay, PBS was used as negative control instead of first antibody, and existing P53 and c-Myc positive liver tumor slides (provided by pathology division of Wuhan University Zhongnan Hospital) were used as positive control.
The mutated p53 positive staining area was located in the nuclei, and c-Myc positive staining was mainly located in the plasma, but part of the nuclei can also be stained. 5 high power lens fields were chosen and stained uniformly to count positive cells. The immunostaining of the mutated p53 and c-Myc had 4 grades according to the staining intensity or positive cell rate: negative (-): no positive staining or positive cell rate < 5%; Weakly positive (+): staining yellow or 6%-20% positive tumor cells; positive (++): staining buffy or 21%-60% tumor cell staining; strongly positive (+++): buffy particles or > 61% tumor cells staining.
All Data were expressed as means ± standard deviation (SD). Means between multi-groups were compared using a one-way ANOVA with LSD test and Student-Newman-Keuls q test. Rates between groups were compared using Fisher’s exact propability test. Statistical analysis was performed using SPSS 12.0.
The general health state of rabbits was fine before drug injection in each groups , and the rabbit hair was smooth and glossy, food intake and activity were normal, weight between groups had no significant difference. The animals in control group began to have symptoms of gradual failure 2 wk after injection, the activity and food intake worsened, stools got loose, oral and nasal discharge increased. The time when two Nano HAP groups had failure symptom were later than the control group, and the weight of the rabbits was also higher than that in control group (P < 0.05) (Table 1). After 3 continuous drug injections, the food intake and activity of the animals decreased obviously, and at 1 wk after injection, the weight of the rabbits was lower than that in control group (P < 0.05), and at 3 wk after injection, the weight of animals was lower than other groups (P < 0.01). The weight in the drug combination group lowered after injection, but had no (do they mean no or do they mean “were significantly different”) significant difference compared with 5-FU group.
| Group | n | Weight (kg) | ||
| Before injection | One week after injection | Three weeks after injection | ||
| A (Control) | 12 | 2.73 ± 0.21 | 2.67 ± 0.21 | 2.32 ± 0.14 |
| B (HAP) | 11 | 2.66 ± 0.15 | 2.59 ± 0.14 | 2.46 ± 0.19ad |
| C (HAP) | 12 | 2.70 ± 0.16 | 2.61 ± 0.14 | 2.52 ± 0.12ad |
| D (5-FU) | 11 | 2.70 ± 0.17 | 2.47 ± 0.17a | 1.95 ± 0.14b |
| E (5-FU + HAP) | 12 | 2.78 ± 0.19 | 2.60 ± 0.18 | 2.23 ± 0.22d |
Differences in the ALT level of each group was not statistically significant. One week after injection , ALT in 5-FU group became significantly higher than other groups (Table 2, P < 0.05), and ALT in drug combination group increased obviously compared with control group (P < 0.05), but lower than that in 5-FU group (P < 0.05). Three weeks after injection, the liver function got worse in control group, displaying an obvious rise in ALT. ALT in two Nano HAP group increased slightly, but obviously lower than that in control group (P < 0.05). ALT in 5-FU group was higher than other groups. ALT in drug combination group increased compared with control group, but lower than that in 5-FU group (P < 0.05).
| Group | n | ALT (U/ L) | ||
| Beforeinjection | One week after injection | Three weeks after injection | ||
| A (Control) | 12 | 43.56 ± 7.24 | 45.33 ± 8.29 | 75.33 ± 12.51 |
| B (Nano HAP) | 11 | 39.29 ± 6.52 | 42.73 ± 10.05d | 59.55 ± 15.40ad |
| C (Nano HAP) | 12 | 40.38 ± 6.88 | 43.75 ± 6.30d | 62.00 ± 14.49ad |
| D (5-FU) | 11 | 42.70 ± 7.08 | 74.64 ± 15.55b | 99.73 ± 14.70b |
| E (5-FU + HAP) | 12 | 42.86 ± 6.79 | 59.67 ± 12.66bc | 86.42 ± 11.54ac |
Before treatment, the tumor size of each group was not statistically significant. Table 3 shows the average tumor volume and tumor growth inhibition rate 3 wk after injection. The tumor growth inhibition rate of each group were 25.5%, 32.5%, 43.7% and 60.9% respectively, and the tumor volume had significant differences compared with that in control group (P < 0.01). The tumor volume of drug combination group also showed significant deference compared with B, C and D group (P < 0.05).
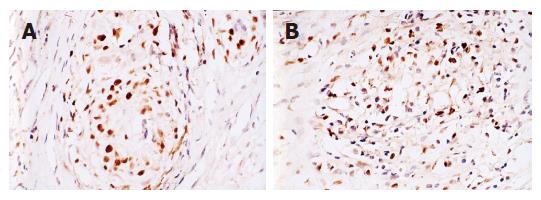
Table 4 shows the result of immunohistochemistry staining: Figure 1 show the results of mutated P53 and c-Myc positive staining. In this result 2 groups that injected HAP combined to B group, and other groups did not change. The mutated P53 and c-Myc both expressed strongly in tumor tissue, and positive cell rates were 83.3% and 75.0%, through the Nano HAP treatment, the positive expression rates of these two protein were 43.5% and 47.8%, lower than the control group, in which expression rate of mutated P53 has statistical significant difference, but there was no significant difference of the two proteins between therapy groups. In tumor tissue of control group, positive expression rate of both mutated P53 and c-Myc was 75.0%, which in Nano HAP group decreased to 39.1%.
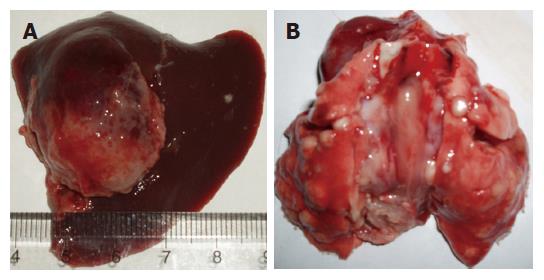
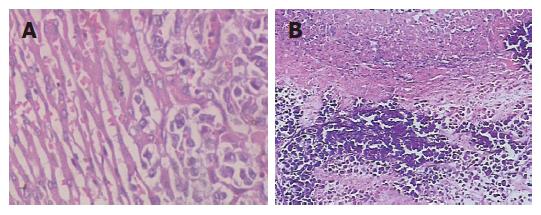
VX2 liver tumor tissue was gray and had clear borderline with liver tissue, it had no envelope, and the section turned like the flesh of fish (Figure 2A). The VX2 liver tumors in control group were much larger than that in therapy group, and grew to the surface of liver, adhering with surrounding tissue, most of them had chest and abdominal wall metastasis, moreover two cases had lung metastasis, showing many lesser tubercles on the surface of lung (Figure 2B), as diagnosed by histology. Few animals in other groups had abdominal cavity and wall metastasis, but did not have lung metastasis. Observed in light microscope after HE staining, tumor cells turned to be round, and fusiform shape or irregular shape, and the size was large, irregular shape, less endochylema, Karyomegaly and dense staining. The cells ranked abnormally, and initial infiltrative hepatic cords distributed at the edge of cancer nest (Figure 3A), some centers of tumors occurred colliquation and necrosis. At the edge of necrotic tumor tissue in HAP group, many microcalcification focuses were observed (Figure 3B), however tumor tissure in control group and normal liver tissue did not have same calcification focus.
Liver cancer is one of the most common malignant tumors in China, and the incidence of disease has continuously increased, its integral & clinical therapy effect is not still ideal due to features of difficulty in early diagnosis, high malignancy and easy relapse etc. Therefore, it is imperative to speed-up the research of biological medicine, to look for a highly efficient, low poison chemotherapy medicine which is one of the main directions of our present study.
HAP has a good issue compatibility both inside and outside the body[2-7], it has been widely used as the biological material for bone damage and oral cavity medicine, simultaneously used as in body medicine carrier[8,9], moreover Nano HAP had a very small excitation on blood vessel, feeding medicine can be made by intravenous injection[5,10]. Nano HAP used for this research is synthesized by sol-coagel method having a good dispersive effect, its particle size with about 50 nm is very uniform, which meets the requirement for Nano meter grade (0.1 nm-100 nm), having advantage of high surface energy etc., that can not exist for the large particle size of HAP. During early out-body experiment Nano HAP has been proved to have obvious inhibition function for multi-kinds of tumors.
This research has proved that Nano HAP had obvious inhibition effect on the growth on the implanted tumor in VX2 liver of rabbit through its intravenous injection into rabbit body. During these experiments the early model was selected for implanted tumor of VX2 liver, different concentrations of Nano HAP have been injected after 3 wk, their inhibition rates of tumors reached separately to 25.3% and 32.5%, which indicated that it could inhibit the growth of tumors. Moreover, liver function on the experimental rabbits has also proved that Nano HAP had a little effect of physiological function on the organism. Inhibition function for tumor growth in 5-FU group was stronger than that in Nano HAP group, but the poisonous side effect has also been indicated obviously, after being continuously injected of 5-FU, the food-intake & activity of animals were obviously decreased; their body weights were obviously lower than that of other groups; the degree of liver function damaged was obviously higher than that of other groups. These results indicated that Nano HAP has no obvious in body adverse reaction.
Through experiment, it was also discovered that not only did Nano HAP have an anti-tumor function by itself, but also it had very strong cooperation effect with other anti-tumor medicines. The inhibition rate of tumor is the highest in the combinative therapy group in experiments. Moreover after having been injected into tumor-bearing rabbit with same dosage of 5-FU, the indication of two groups of animals was quite different. The general situation for animal of combinative therapy group was obviously better than that in 5-FU group. Dynamic supervision on body weight & liver function indicated also that the effect of 5-FU on the animal physiological function of combinative therapy group is obviously less than that in 5-FU group.
In combination with feature of Nano HAP having large surface & high surface energy, it, may be, has played a role of its carrier after being mixed of Nano HAP with 5-FU. This adsorption of carrier[11] could obviously reduce first blood-medicine concentration of 5-FU, and reach to a relaxation effect, it was also reported in the literature that Nano HAP could produce a complex with blood serum protein in biological body[12,13], it was inferred that the Nano HAP-5-FU protein compound could be formed in animal blood after mixing of Nano HAP with 5-FU, therefore not only the poisonous by-effect of high concentration free 5-FU in body could be reduced, but also the blood solubility of Nano HAP could be increased.
The present researches for various Nano HAP anti-tumors mechanism indicate the anti-tumor effect can be reached through effecting the end grain enzyme of cancer cell, cell skeleton, Ca2+ concentration and immunity function. In experiment the expression product in tumor tissue was detected after reaction of gene mutated P53 and c-Myc with Nano HAP. The result indicates that the positive expression rates of two kinds of proteins are separately 43.5%, 47.8% after reaction of Nano HAP, which are obviously lower than that in comparison control group. p53 and c-Myc are the important genes for controlling proliferation and withering of tumors cells, of which c-Myc protein positive expression can lead to directional development of non-function, originalization of cells. Low differentiation of cells with high toxicity is early accident occurring malignant tumor, so c-Myc protein positive expression was mainly distributed at the edge of tumor. Nano HAP can lower positive expression of these two kinds of protein, it was prompted it could prevent the malignant cell proliferation of tumor through effecting control of cancer cell gene, to reach anti-tumor effect. But it has to be further researched carefully for the detail mechanism.
To sum up, not only the Nano HAP has expressed obvious anti-tumor effect in vivo of animal, but also, its poisonous by-effect was low, and it could bring very strong cooperation effect with using other chemotherapy medicine, reduce toxicity of other chemotherapy medicine. It might become new clinical anti-tumor medicine.
S- Editor Liu Y L- Editor Di Mari JF E- Editor Wang HF
| 1. | Liu ZS, Tang SL, Ai ZL. Effects of hydroxyapatite nanoparticles on proliferation and apoptosis of human hepatoma BEL-7402 cells. World J Gastroenterol. 2003;9:1968-1971. [PubMed] |
| 2. | Aoki H, Aoki H, Kutsuno T, Li W, Niwa M. An in vivo study on the reaction of hydroxyapatite-sol injected into blood. J Mater Sci Mater Med. 2000;11:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Tachibana Y, Ninomiya S, Kim YT, Sekikawa M. Tissue response to porous hydroxyapatite ceramic in the human femoral head. J Orthop Sci. 2003;8:549-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Huang J, Best SM, Bonfield W, Brooks RA, Rushton N, Jayasinghe SN, Edirisinghe MJ. In vitro assessment of the biological response to nano-sized hydroxyapatite. J Mater Sci Mater Med. 2004;15:441-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Pezzatini S, Solito R, Morbidelli L, Lamponi S, Boanini E, Bigi A, Ziche M. The effect of hydroxyapatite nanocrystals on microvascular endothelial cell viability and functions. J Biomed Mater Res A. 2006;76:656-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Fu Q, Zhou N, Huang W, Wang D, Zhang L, Li H. Effects of nano HAP on biological and structural properties of glass bone cement. J Biomed Mater Res A. 2005;74:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Wahl DA, Czernuszka JT. Collagen-hydroxyapatite composites for hard tissue repair. Eur Cell Mater. 2006;11:43-56. [PubMed] |
| 8. | Kunieda K, Seki T, Nakatani S, Wakabayashi M, Shiro T, Inoue K, Sougawa M, Kimura R, Harada K. Implantation treatment method of slow release anticancer doxorubicin containing hydroxyapatite (DOX-HAP) complex. A basic study of a new treatment for hepatic cancer. Br J Cancer. 1993;67:668-673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Rauschmann MA, Wichelhaus TA, Stirnal V, Dingeldein E, Zichner L, Schnettler R, Alt V. Nanocrystalline hydroxyapatite and calcium sulphate as biodegradable composite carrier material for local delivery of antibiotics in bone infections. Biomaterials. 2005;26:2677-2684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 212] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 10. | Mizushima Y, Ikoma T, Tanaka J, Hoshi K, Ishihara T, Ogawa Y, Ueno A. Injectable porous hydroxyapatite microparticles as a new carrier for protein and lipophilic drugs. J Control Release. 2006;110:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Zhu S, Zhou K, Huang B, Huang S, Liu F, Li Y, Xue Z, Long Z. Hydroxyapatite nanoparticles: a novel material of gene carrier. ShengWu YiXue GongChengXue ZaZhi. 2005;22:980-984. [PubMed] |
| 12. | Kandori K, Masunari A, Ishikawa T. Study on adsorption mechanism of proteins onto synthetic calcium hydroxyapatites through ionic concentration measurements. Calcif Tissue Int. 2005;76:194-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Niwa M, Li W, Sato T, Daisaku T, Aoki H, Aoki H. The adsorptive properties of hydroxyapatite to albumin, dextran and lipids. Biomed Mater Eng. 1999;9:163-169. [PubMed] |