Published online Jan 14, 2007. doi: 10.3748/wjg.v13.i2.289
Revised: November 2, 2006
Accepted: December 6, 2006
Published online: January 14, 2007
AIM: To evaluate the diagnostic accuracy of histological evaluation of pancreatic tissue samples obtained by a modified method for recovering and processing the endoscopic ultrasound (EUS)-guided fine needle aspiration (FNA) material in the differential diagnosis of pancreatic solid masses.
METHODS: Sixty-two consecutive patients with pancreatic masses were prospectively studied. EUS was performed by the linear scanning Pentax FG-38UX echoendoscope. Three FNAs (22G needle) were carried out during each procedure. The materials obtained with first and second punctures were processed for cytological study. Materials of the third puncture were recovered into 10% formol solution by careful injection of saline solution through the needle, and processed for histological study.
RESULTS: Length of the core specimen obtained for histological analysis was 6.5 ± 5.3 mm (range 1-22 mm). Cytological and histological samples were considered as adequate in 51 (82.3%) and 52 cases (83.9%), respectively. Overall sensitivity of both pancreatic cytology and histology for diagnosis of malignancy was 68.4%. Contrary to cytology, histology was able to diagnose tumours other than adenocarcinomas, and all cases of inflammatory masses. Combination of cytology and histology allowed obtaining an adequate sample in 56 cases (90.3%), with a global sensitivity of 84.21%, specificity of 100% and an overall accuracy of 90.32%. The complication rate was 1.6%.
CONCLUSION: Adequate pancreatic core specimens for histological examination can be obtained by EUS-guided FNA. This technique is mainly useful for the diagnosis of different types of pancreatic tumours and evaluation of benign diseases.
- Citation: Iglesias-Garcia J, Dominguez-Munoz E, Lozano-Leon A, Abdulkader I, Larino-Noia J, Antunez J, Forteza J. Impact of endoscopic ultrasound-guided fine needle biopsy for diagnosis of pancreatic masses. World J Gastroenterol 2007; 13(2): 289-293
- URL: https://www.wjgnet.com/1007-9327/full/v13/i2/289.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i2.289
Differential diagnosis of pancreatic masses is a frequent clinical challenge. Therapeutic decision in this context is mainly based on the ability to establish or exclude malignancy[1]. Although ductal adenocarcinoma is the most frequent cause of pancreatic masses, other neoplasms (e.g. lymphoma, cystic tumours) and benign conditions (e.g. chronic pancreatitis) with different prognoses and treatment options can arise within the pancreas. A histological diagnosis becomes therefore highly relevant for an optimal therapeutic decision[2].
Endoscopic ultrasound (EUS)-guided fine needle aspiration (FNA) has been proved to be a safe and useful method for tissue sampling of intramural and extramural gastrointestinal lesions including the pancreas[3,4]. Cytological study of the materials obtained by FNA allows the evaluation of cellular findings suggestive of malignancy, such as anisonucleosis, nuclear membrane irregularity and nuclear enlargement. Unfortunately, inflammation causes a reactive and regenerative process leading to cellular changes that can be difficult to distinguish from well-differentiated neoplasias. Histological study of tissue samples allows the assessment of tissue architecture and cell morphology, as well as the performance of immunohistochemical analysis[5,6], thus usually providing with a higher diagnostic accuracy than cytology.
Retrieving pancreatic tissue fragments with different EUS-guided techniques has been explored. In this context, needles of different diameters and trucut needles have been used with variable success and complication rates[7-11].
The aim of the present study was to evaluate the diagnostic accuracy of the histological evaluation of pancreatic tissue samples obtained by a modified method for recovering and processing the EUS-guided FNA material in the differential diagnosis of pancreatic solid masses.
Sixty-two consecutive patients (mean age 57 years, range 20 to 83 years, 35 males and 27 females), who underwent an EUS-guided FNA for the evaluation of solid pancreatic masses were prospectively included in the study over a two-years period.
In addition to abdominal ultrasound, all patients had a previous evaluation of the pancreatic mass by CT scan. Lesions were located in the head of the pancreas in 45 cases, in the body in 15 cases, and in the tail in two cases. Once the corresponding signed informed consent was obtained, EUS was performed under conscious sedation by a single operator (JIG). A standard blood coagulation analysis was performed before EUS-guided FNA, and an uncorrectable coagulation profile (prothrombin time < 60%) was considered as a contraindication for the procedure.
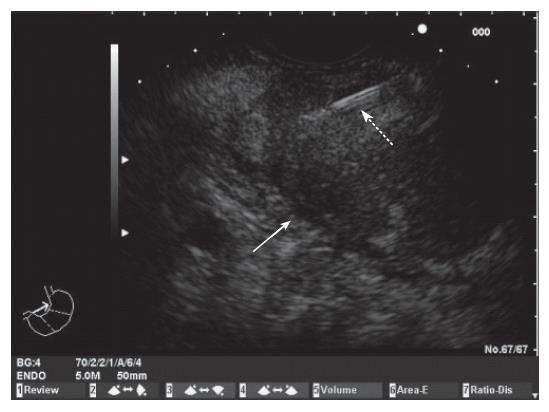
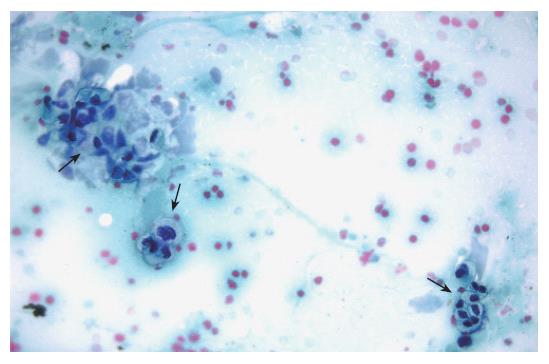
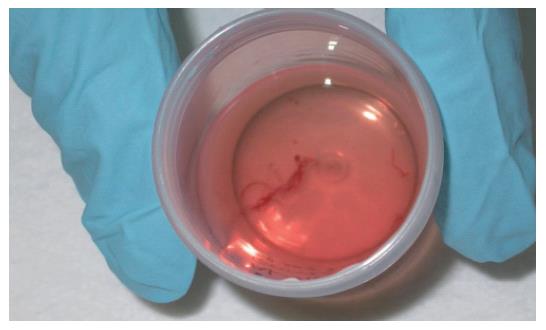
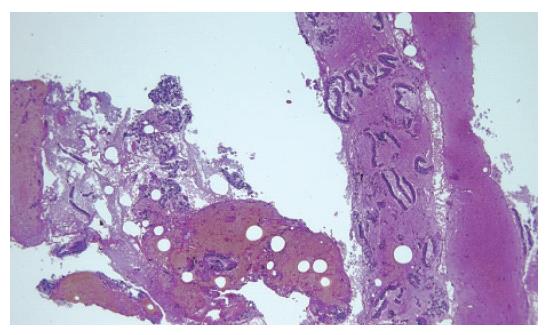
EUS was performed using a convex array echoendoscope (Pentax FG-38UX®), connected to an ultrasound equipment Hitachi-E6000®. FNA was performed with a standard 22-gauge needle (Sonotip II®, Mediglobe, Germany). This needle is equipped with a round nitinol stylet covered by a 118 cm protective metal spiral coil sheath. The needle can be advanced up to 8.5 cm from the spiral sheath. The target lesion was endosonographically visualized and the region was scanned for vessels using colour and pulsed Doppler. FNA was performed from the duodenum or the stomach according to the location of the lesion in the head or the body/tail of the pancreas, respectively. Before puncture, the stylet was withdrawn several millimeters, thereby exposing the sharp needle tip. The needle was then advanced into the target tissue under endosonographic guidance (Figure 1). Once the lesion was penetrated, the stylet was advanced to the original position to “unplug” the needle, and to push out any potentially needle-clogging tissue or body fluids. The stylet was then removed and suction was applied using a 5 mL syringe while moving the needle to and fro within the lesion. Suction was released before removing the needle. This procedure was repeated three times and the material obtained was recovered as follows: (1) The samples obtained after the first and second punctures were expelled on microscope slides by pushing the needle stylet and injecting air through the needle. The material was then spread on the slides, fixed in 96% ethanol and processed for cytological study by Papanicolau staining (Figure 2). Cytology samples were evaluated for cellular preservation, background substance, cellularity, architectural integrity, and cytoplasmic and nuclear details. Cytology diagnoses were categorized into non-diagnostic, negative for malignancy, and positive for malignancy, based on published criteria[12]. (2) Samples obtained after the third puncture were recovered into a tube containing a 10% formol solution by injecting 2 ml of saline solution through the needle (Figure 3). Samples were then embedded in paraffin. Tissue sections of 3 to 4 µm were stained by the classical haematoxylin-eosin technique for morphological evaluation. The sample was considered adequate if a coherent core tissue specimen from the target lesion was obtained (Figure 4).
No pathologist was present in the endoscopy room during the procedure. Samples were initially processed by the endoscopist, who was specifically trained with this aim by pathologists. Thus, no microscopic evaluation of sample adequacy was performed at that time. Two experienced pathologists examined both cytological smears and histological specimens. Cytological and histological findings were compared with the surgical specimen as gold standards in patients who were further operated upon. In non-operated patients, a clinical, morphological (EUS and CT scan) and biochemical evaluation (including serum levels of Ca 19.9) over a minimum follow-up of at least 6 mo was considered as gold standards. The criteria for establishing a benign course of disease were thus a subjective well-being, absence of weight loss, no progression of the disease on imaging studies and no elevation of serum tumour markers.
Sensitivity, specificity, overall accuracy and positive and negative predictive values for malignancy were calculated. Data from histology and cytology are shown as percentages and 95% confidence intervals and compared by the Fisher’s exact test. P < 0.05 was considered as significant.
Pancreatic masses were secondary to a malignant condition in 38 cases (61.3%), and to benign diseases in 24 cases (38.7%). Distribution of patients according to the final diagnosis based on the gold standards is shown in Table 1. A total of 27 patients underwent surgery, including 20 pancreatic adenocarcinomas, one patient with an endocrine tumour, and 6 patients with an inflammatory mass related to a chronic pancreatitis. The remaining 35 patients were followed up for a median of 10 mo (range 6-20 mo).
| Final diagnosis | n | Correctdiagnosisby cytology | Correctdiagnosisby histology | Correct diagnosisby both cytologyand histology |
| Adenocarcinoma | 33 | 24 | 21 | 27 |
| Anaplastic carcinoma | 1 | 1 | 1 | 1 |
| Small cell lung cancer | 1 | 0 | 1 | 1 |
| Squamous cell carcinoma | 1 | 1 | 1 | 1 |
| B cell lymphoma | 1 | 0 | 1 | 1 |
| Endocrine carcinoma | 1 | 0 | 1 | 1 |
| Inflammatory process | 24 | 17 | 24 | 24 |
| Total | 62 | 42 | 55 | 56 |
The length of the core specimen obtained for histological analysis was 6.5 ± 5.3 mm (range 1-22 mm). Cytological and histological samples were considered as adequate in 51 (82.3%, 95% CI, 71.0%-89.8%) and 52 cases (83.9%, 95% CI, 72.8%-91.0%), respectively (not significant). Global sensitivity of both pancreatic cytology and histology for diagnosis of malignancy was 68.4% (52.5%-80.9%).
Diagnostic accuracy of both techniques in cases of adequate sample is shown in Table 2. In this context, histology tended to be more sensitive and accurate, and showed a significantly higher negative predictive value for malignancy than cytology.
| Cytology | Histology | P | |
| Sensitivity | 76.5% (60.0-87.6) | 92.85% (77.3-98.0) | 0.097 |
| Specificity | 100% (81.6-100) | 100% (86.2-100) | NS |
| Negative predictive value | 68.0% (48.4-82.8) | 92.3% (75.9-97.9)a | < 0.05 |
| Positive predictive value | 100% (87.1-100) | 100% (87.1-100) | NS |
| Overall accuracy | 84.3% (72.0-91.8) | 96.1% (87.0-98.9) | 0.05 |
Histological evaluation provided a correct diagnosis in all 24 cases of inflammatory masses, compared with 17 cases correctly classified by cytology (Table 1). Although both techniques were similarly sensitive for the diagnosis of pancreatic adenocarcinomas, histology was the only one able to diagnose other tumours like lymphomas, endocrine tumours and small cell lung cancer metastasis (Table 1). The combination of cytology and histology allowed obtaining an adequate sample in 56 cases (90.3%, 95% CI, 80.4%-95.5%), and a correct diagnosis in all 24 cases of inflammatory masses and 32 cases of pancreatic malignancy (Table 1). Thus, the global sensitivity of EUS-guided FNA was 84.21% (95% CI, 69.6%-92.6%), specificity of 100% (95% CI, 86.2%-100%), and overall accuracy of 90.32% (95% CI, 80.4%-95.5%).
The complication rate of the procedure was 1.6%, and only one case of mild acute pancreatitis that resolved within three days of conservative treatment was observed. No patient died because of the procedure.
Recovery of pancreatic EUS-guided FNA specimen into a 10% formol solution by careful injection of saline through the needle allows obtaining an adequate tissue sample for histological diagnosis of pancreatic masses in most cases. Compared to cytology, histology provides a significantly higher negative predictive value for malignancy, and tends to be more accurate. In addition, tumours other than adenocarcinomas are more easily diagnosed by histology.
Several studies have evaluated the accuracy of cytology after EUS-guided FNA for the diagnostic assessment of pancreatic masses. According to those reports, an adequate cytological specimen can be obtained in 82% to 91% of cases, with a sensitivity for malignancy ranging from 64% to 96%[13-25]. In our series, the sensitivity of cytology for the diagnosis of malignancy was 68.4%, which improved up to 76.5% when only adequate samples were considered, similar to the previous report.
In previous studies showing high diagnostic yields of cytology, 3 to 6 needle passes through the lesion[16-23,26] and on-site evaluation of the FNA sample adequacy by a cytopathologist[10,27-29] was considered essential. We were able to obtain an adequate sample in 90% of cases by performing three passes, two for cytological evaluation and one for histological evaluation.
Compared to cytology, histological evaluation of a tissue sample seems to have several advantages, such as a better distinction between well-differentiated adenocarcinoma and chronic pancreatitis, an appropriate cellular subtyping and architectural analysis for the diagnosis of tumours (i.e. lymphoma), as well as the possibility of using special stains[5,6]. In our series, obtaining a core specimen for histological evaluation allowed us to categorize malignant lesions that, although rare, were impossible to be diagnosed by cytology (i.e., pancreatic lymphomas, small cell lung cancer metastasis and endocrine carcinomas). Histological analyses were also able to properly diagnose benign pancreatic lesions in all patients. However, we had difficulties in acquisition of adequate samples from pancreatic adenocarcinomas, which might be explained by the tissue features of this solid tumour, characterized by infiltrating duct-like and tubular structures embedded in a highly desmoplastic stroma[30].
Different needles and different needle diameters have been evaluated to obtain core tissue specimens for histopathological analysis[7-11]. Binmoeller et al[7] were able to obtain adequate tissue core specimens in 40 out of 45 patients with pancreatic masses using an 18-gauge needle. Despite that, the sensitivity for detection of a malignancy was only 53%[7]. In a more recent retrospective study, Levy et al[9] reported an accuracy of 85% for the diagnosis of different pancreatic and non-pancreatic lesions using a 19-gauge trucut needle, compared to a 60% accuracy achieved by the standard fine needle aspiration technique. Varadarajulu et al[10] compared a 19-gauge trucut needle with the standard 22-gauge needle with fine needle aspiration, and no difference in the diagnostic accuracy between both techniques was found (78% vs 89%). The diagnostic yield of the trucut needle biopsy is strongly limited to lesions located in the head of the pancreas[11]. This is due to the impossibility to reach within the duodenum the degree of deflection of the echoendoscope tip required to bring the target lesion to an adequate position for puncture. Larghi et al[11], despite performing trucut needle biopsy only in lesions accessible for the transgastric approach, were able to obtain materials in only 74% of cases, with an overall diagnostic accuracy of 61%. Contrary to these, the method described in the present study allowed us to achieve a high diagnostic accuracy for pancreatic masses located both in the head and in the body and tail of the pancreas.
Despite the advantages of obtaining tissue core specimens for histological analysis, two cases in our series of malignant pancreatic masses were only detected by cytology. This strongly argues in favour of obtaining specimens for both cytological and histological evaluation. Similar data were reported by other authors[7,9]. In fact, this approach allowed obtaining an adequate sample (either for histology and/or cytology) in 90.3% of cases, with an overall diagnostic accuracy for a malignancy as high as of 90.3%.
EUS-guided biopsy of the pancreas is a safe technique[31,32], with a slightly higher complication rate related to the use of trucut needles[10]. In fact, the risk of pancreatitis and bleeding has been reported to be higher with trucut needles than with the standard FNA needles[10], even though this was not confirmed by other authors[7,11]. A case of mild acute pancreatitis was the only complication observed in the present series after EUS-guided FNA of the pancreas. This low complication rate is similar to that reported previously using a standard 22G needle[21-25].
In conclusion, pancreatic core specimens for histological examination can be obtained by EUS-guided FNA with a 22-gauge needle by careful injection of saline through the needle and by expelling the tissue samples into a tube containing 10% formol solution. The samples obtained by this procedure are highly adequate for histological analyses allowing an appropriate evaluation of pancreatic solid masses. This technique is mainly useful for the diagnosis of different types of pancreatic tumours as well as for the evaluation of benign diseases. Combination with cytology tends to increase the sensitivity of histology for the diagnosis of pancreatic adenocarcinomas.
Differential diagnosis of pancreatic masses is a frequent clinical challenge. Endoscopic ultrasound (EUS)-guided fine needle aspiration (FNA) has been proved to be a safe and useful method for tissue sampling of pancreatic solid masses. Histological study of tissue samples allows the assessment of tissue architecture and cell morphology, as well as the performance of immunohistochemical analysis, thus usually providing with a higher diagnostic accuracy than cytology.
Further research is needed in order to improve the diagnostic yield of EUS-guided biopsy, and to provide with better material from pancreatic lesions. Availability of adequate pancreatic tissue samples may allow performing immunohistochemical studies, molecular analysis, and evaluation of genetic mutations, thus providing the basis for a better knowledge of pancreatic diseases.
Our study demonstrates that a core specimen from pancreatic solid masses can be obtained using a standard 22 gauge needle, thus allowing the histological evaluation of pancreatic lesions. Retrieving pancreatic tissue fragments has been explored using different types of needles (e.g., trucut needles) and different ways of sample processing. In contrast to trucut needles, our technique allows access to lesions located at the head of the pancreas with a low complication rate.
Obtaining samples of pancreatic tissue allows the histological evaluation of pancreatic solid masses, which may be of help for the diagnosis of different pancreatic tumours as well as for the evaluation of benign diseases like chronic pancreatitis.
This paper provides support for the use of this modified method when performing fine needle biopsy of solid pancreatic masses.
S- Editor Wang GP L- Editor Zhu LH E- Editor Liu WF
| 1. | Tamm E, Charnsangavej C. Pancreatic cancer: current concepts in imaging for diagnosis and staging. Cancer J. 2001;7:298-311. [PubMed] |
| 2. | Cohen SJ, Pinover WH, Watson JC, Meropol NJ. Pancreatic cancer. Curr Treat Options Oncol. 2000;1:375-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Rösch T. Endoscopic ultrasonography. Br J Surg. 1997;84:1329-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Hawes RH. Endoscopic ultrasound. Gastrointest Endosc Clin N Am. 2000;10:161-174, viii. [PubMed] |
| 5. | Ribeiro A, Vazquez-Sequeiros E, Wiersema LM, Wang KK, Clain JE, Wiersema MJ. EUS-guided fine-needle aspiration combined with flow cytometry and immunocytochemistry in the diagnosis of lymphoma. Gastrointest Endosc. 2001;53:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 159] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Mesa H, Stelow EB, Stanley MW, Mallery S, Lai R, Bardales RH. Diagnosis of nonprimary pancreatic neoplasms by endoscopic ultrasound-guided fine-needle aspiration. Diagn Cytopathol. 2004;31:313-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Binmoeller KF, Thul R, Rathod V, Henke P, Brand B, Jabusch HC, Soehendra N. Endoscopic ultrasound-guided, 18-gauge, fine needle aspiration biopsy of the pancreas using a 2.8 mm channel convex array echoendoscope. Gastrointest Endosc. 1998;47:121-127. [PubMed] |
| 8. | Harada N, Kouzu T, Arima M, Isono K. Endoscopic ultrasound-guided histologic needle biopsy: preliminary results using a newly developed endoscopic ultrasound transducer. Gastrointest Endosc. 1996;44:327-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Levy MJ, Jondal ML, Clain J, Wiersema MJ. Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc. 2003;57:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Varadarajulu S, Fraig M, Schmulewitz N, Roberts S, Wildi S, Hawes RH, Hoffman BJ, Wallace MB. Comparison of EUS-guided 19-gauge Trucut needle biopsy with EUS-guided fine-needle aspiration. Endoscopy. 2004;36:397-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 167] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Larghi A, Verna EC, Stavropoulos SN, Rotterdam H, Lightdale CJ, Stevens PD. EUS-guided trucut needle biopsies in patients with solid pancreatic masses: a prospective study. Gastrointest Endosc. 2004;59:185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Robins DB, Katz RL, Evans DB, Atkinson EN, Green L. Fine needle aspiration of the pancreas. In quest of accuracy. Acta Cytol. 1995;39:1-10. [PubMed] |
| 13. | Voss M, Hammel P, Molas G, Palazzo L, Dancour A, O'Toole D, Terris B, Degott C, Bernades P, Ruszniewski P. Value of endoscopic ultrasound guided fine needle aspiration biopsy in the diagnosis of solid pancreatic masses. Gut. 2000;46:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 248] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 14. | Wiersema MJ, Kochman ML, Cramer HM, Tao LC, Wiersema LM. Endosonography-guided real-time fine-needle aspiration biopsy. Gastrointest Endosc. 1994;40:700-707. [PubMed] |
| 15. | Chang KJ, Katz KD, Durbin TE, Erickson RA, Butler JA, Lin F, Wuerker RB. Endoscopic ultrasound-guided fine-needle aspiration. Gastrointest Endosc. 1994;40:694-699. [PubMed] |
| 16. | Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 299] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 17. | Binmoeller KF, Rathod VD. Difficult pancreatic mass FNA: tips for success. Gastrointest Endosc. 2002;56:S86-S91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Bhutani MS, Hawes RH, Baron PL, Sanders-Cliette A, van Velse A, Osborne JF, Hoffman BJ. Endoscopic ultrasound guided fine needle aspiration of malignant pancreatic lesions. Endoscopy. 1997;29:854-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 170] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Harewood GC, Wiersema MJ. Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol. 2002;97:1386-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 291] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 20. | Giovannini M, Seitz JF, Monges G, Perrier H, Rabbia I. Fine-needle aspiration cytology guided by endoscopic ultrasonography: results in 141 patients. Endoscopy. 1995;27:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 264] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Gress FG, Hawes RH, Savides TJ, Ikenberry SO, Lehman GA. Endoscopic ultrasound-guided fine-needle aspiration biopsy using linear array and radial scanning endosonography. Gastrointest Endosc. 1997;45:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 262] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 22. | Raut CP, Grau AM, Staerkel GA, Kaw M, Tamm EP, Wolff RA, Vauthey JN, Lee JE, Pisters PW, Evans DB. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration in patients with presumed pancreatic cancer. J Gastrointest Surg. 2003;7:118-126; discussion 127-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 192] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 23. | Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 874] [Cited by in RCA: 736] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 24. | Chhieng DC, Jhala D, Jhala N, Eltoum I, Chen VK, Vickers S, Heslin MJ, Wilcox CM, Eloubeidi MA. Endoscopic ultrasound-guided fine-needle aspiration biopsy: a study of 103 cases. Cancer. 2002;96:232-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Williams DB, Sahai AV, Aabakken L, Penman ID, van Velse A, Webb J, Wilson M, Hoffman BJ, Hawes RH. Endoscopic ultrasound guided fine needle aspiration biopsy: a large single centre experience. Gut. 1999;44:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 436] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 26. | Erickson RA. EUS-guided FNA. Gastrointest Endosc. 2004;60:267-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Klapman JB, Logrono R, Dye CE, Waxman I. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol. 2003;98:1289-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 362] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 28. | Jhala NC, Jhala DN, Chhieng DC, Eloubeidi MA, Eltoum IA. Endoscopic ultrasound-guided fine-needle aspiration. A cytopathologist's perspective. Am J Clin Pathol. 2003;120:351-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 139] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 29. | Chang KJ, Nguyen P, Erickson RA, Durbin TE, Katz KD. The clinical utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of pancreatic carcinoma. Gastrointest Endosc. 1997;45:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 402] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 30. | Klöppel G, Hruban RH, Longnecker DS, Adler G, Kern SE, Partanen TJ. Ductal adenocarcinoma of the pancreas. Pathology and Genetics of Tumours of the Digestive System. WHO Classification of Tumours. Lyon: IARC Press 2000; 221-230. |
| 31. | O'Toole D, Palazzo L, Arotçarena R, Dancour A, Aubert A, Hammel P, Amaris J, Ruszniewski P. Assessment of complications of EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53:470-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 234] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 32. | Micames C, Jowell PS, White R, Paulson E, Nelson R, Morse M, Hurwitz H, Pappas T, Tyler D, McGrath K. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003;58:690-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 278] [Article Influence: 12.6] [Reference Citation Analysis (0)] |