Published online Jan 14, 2007. doi: 10.3748/wjg.v13.i2.270
Revised: November 13, 2006
Accepted: December 12, 2006
Published online: January 14, 2007
AIM: To investigate the proximal gastric motor response to duodenal nutrients in critically ill patients with long-standing type 2 diabetes mellitus.
METHODS: Proximal gastric motility was assessed (using a barostat) in 10 critically ill patients with type 2 diabetes mellitus (59 ± 3 years) during two 60-min duodenal infusions of Ensure® (1 and 2 kcal/min), in random order, separated by 2 h fasting. Data were compared with 15 non-diabetic critically ill patients (48 ± 5 years) and 10 healthy volunteers (28 ± 3 years).
RESULTS: Baseline proximal gastric volumes were similar between the three groups. In diabetic patients, proximal gastric relaxation during 1 kcal/min nutrient infusion was similar to non-diabetic patients and healthy controls. In contrast, relaxation during 2 kcal/min infusion was initially reduced in diabetic patients (p < 0.05) but increased to a level similar to healthy humans, unlike non-diabetic patients where relaxation was impaired throughout the infusion. Duodenal nutrient stimulation reduced the fundic wave frequency in a dose-dependent fashion in both the critically ill diabetic patients and healthy subjects, but not in critically ill patients without diabetes. Fundic wave frequency in diabetic patients and healthy subjects was greater than in non-diabetic patients.
CONCLUSION: In patients with diabetes mellitus, proximal gastric motility is less disturbed than non-diabetic patients during critical illness, suggesting that these patients may not be at greater risk of delayed gastric emptying.
- Citation: Nguyen NQ, Fraser RJ, Bryant LK, Chapman M, Holloway RH. Proximal gastric motility in critically ill patients with type 2 diabetes mellitus. World J Gastroenterol 2007; 13(2): 270-275
- URL: https://www.wjgnet.com/1007-9327/full/v13/i2/270.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i2.270
In the community, approximately 50% of patients with type 1 or 2 diabetes mellitus (DM) have gastroparesis[1]. Although gastric emptying of either a solid or semi-solid meal is consistently slow in these patients, gastric emptying of liquid meals is variable[1-4]. The aetiology of slow gastric emptying and the variable rate of liquid emptying are unclear, but may be related to hyperglycemia or autonomic neuropathy[1,5-7], factors that result in motor dysfunction of both the proximal and distal stomach[1,7,8].
Delayed gastric emptying is also common in critically ill patients[9-11] and is associated with disturbed motility of both the proximal and distal stomach[10,12,13]. In health, the proximal stomach is a major determinant of liquid gastric emptying and is regulated by feedback from the small intestine. In health, the fundus relaxes in response to the presence of nutrient in the duodenum[14]. Critically ill patients without DM have been reported to have impaired proximal gastric relaxation, reduced fundic wave activity and a failed recovery of proximal gastric volume to pre-stimulation level[12]. Currently, there are no data on the impact of DM on gastric motor function or emptying during critical illness, despite the fact that one-third of patients admitted to critical care units have DM[15]. Given that both DM and critical illness are risk factors for disturbed gastric motility, we hypothesized that critically ill patients with DM would have abnormal proximal gastric motor activity during fasting and in response to duodenal nutrient infusion, compared to non-diabetic critically ill patients and healthy humans.
Studies were performed in 25 sedated and mechanically ventilated critically ill patients, who were admitted to a level-3 mixed intensive care unit between January and September 2004. All patients required enteral nutrition. Ten patients had documented type 2 DM with a mean duration of 7.9 ± 1.8 years. Seven of the diabetic patients had required insulin therapy prior to ICU admission. Formal testing for the presence of autonomic neuropathy was not performed. Fifteen critically ill patients without diabetes mellitus served as patient controls. Exclusion criteria for all patients were (1) recent major abdominal surgery, (2) any contra-indication to passage of an enteral tube, (3) administration of opioid analgesia, benzodiazepines or prokinetic therapy within the previous 24 h, and (4) previous gastric, oesophageal or intestinal surgery. All patients received an insulin infusion for blood glucose control according to a standardized protocol that started on admission and aimed to maintain blood glucose concentrations between 5.0 and 7.9 mmol/L. Data from both patient groups were compared to 10 healthy volunteers, who had no history of systemic or gastrointestinal disease and were not taking any medication. Healthy volunteers were instructed to refrain from smoking for 24 h prior to the study.
Written informed consent was obtained from healthy subjects and the next of kin of patients prior to enrolment into the study. The protocol was approved by the Human Research Ethics Committee of the Royal Adelaide Hospital.
Proximal gastric motility was measured using an electronic gastric barostat[16] (Distender Series II; G&J Electronics, Ontario, Canada). A thin flaccid-walled bag with a maximum capacity of 1000 mL was attached to the distal end of the assembly, which was connected to the system via pressure and volume ports. Changes in proximal gastric volume were measured indirectly by changes in the volume of the polyethylene bag.
Data were stored onto a Powermac 7100 computer (Apple Computer, Cupertino, CA), using custom-written data-acquisition software (Labview: National Instruments, Austin, TX). This software was also used to program the barostat to perform distensions in stepwise increments. Recorded data were imported into a display and analysis program (Acqknowledge, Biopac System, Goleta, CA) for manual analysis.
Marked hyperglycaemia may alter small intestinal feedback and adversely affect gastric motility[5-7]. Blood glucose concentrations were measured using a portable glucometer (Precision Plus, Abbott Laboratories, Bedford, USA) immediately before and every 20 min during nutrient infusion.
All subjects were studied after at least 6 h fasting and in a 30 degree recumbent position. To standardise the sedative regimen in patients, propofol alone was used, and opioids, benzodiazepines or prokinetic agents were not administered for 24 h prior to and during the study. In patients, placement of both the barostat catheter and post-pyloric feeding tube were performed at the bedside with endoscopic assistance, without additional sedation to that required for ventilation. A 12 French × 114 cm naso-duodenal feeding tube (Flexiflo, Abbott, Ireland) was inserted into the duodenum over a guidewire (THSF-35-260, Cook, Australia). The barostat catheter was then guided through the mouth into the stomach by the endoscope. The barostat bag was inflated with 400 mL of air and gently retracted into the fundus under direct vision. Gastric contents (air and fluid) were aspirated completely prior to withdrawal of the endoscope. Correct placement of the feeding tube was confirmed by measurement of the duodenal trans-mucosal potential difference (TMPD)[16] and subsequently by radiography[13].
In healthy subjects, the barostat assembly and feeding tube were swallowed and allowed to pass into the correct position spontaneously, without the assistance of endoscopy. Duodenal nutrient infusion was achieved by inserting a silicon-rubber catheter (Dentsleeve, Adelaide, Australia) with a central feeding lumen and lead-weighted tip into the stomach. The tube passed spontaneously into the duodenum using phase 2 and 3 of the migrating motor complex. Movement of the catheter into the correct position was monitored continuously by measurement of the antro-duodenal TMPD gradient[16]. Radiological confirmation was not performed in healthy subjects. The barostat catheter was then inserted to a depth of 55 cm, the bag inflated with 400 mL of air and the assembly pulled back until resistance was felt[17].
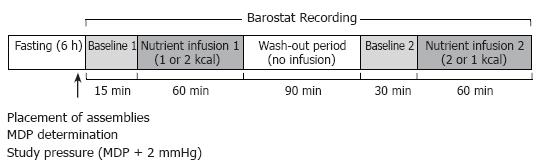
Following confirmation that both assemblies were positioned correctly, air in the barostat bag was aspirated manually and the catheter was connected to the barostat pump. The minimum distending pressure (MDP), defined as the first pressure level that provided an intragastric bag volume of more than 30 mL, was determined[17]. The intra-bag pressure for the study was set at MDP + 2 mmHg[17]. All studies began with a 15-min baseline recording, during which normal saline (0.9% NaCl) was infused into the duodenum at a rate of 240 mL/h (baseline 1). Each subject then received two 60 minute duodenal infusions of Ensure® (Abbott Laboratories, Ohio, USA; nutrient content: 13% protein, 64% carbohydrate, 21% fat; energy density: 1 kcal/mL) at 1 and 2 kcal/min, in a randomised order. Ensure® was diluted with normal saline to 1:4 for the 1 kcal/min infusion and to 1:2 for 2 kcal/min infusion, and infused at a rate of 240 mL/h. The nutrient infusions were separated by a 2 h ‘washout period’, consisting of 90 min of no infusion, followed by 30 min of intraduodenal saline infusion (baseline 2). Blood samples for the measurement of blood glucose concentration were collected at baseline and every 20 min during nutrient infusion. Barostat recordings were performed continuously over 4 h. The study protocol is outlined in Figure 1.
Intra-bag volumes were determined at 2-min intervals and the mean baseline volume was measured over 10 min before each infusion. Changes in intra-bag volume during nutrient infusion were calculated as the difference between the actual volume and the mean baseline volume. The serial changes in bag volume during the infusions were plotted and compared. Proximal gastric relaxation was indirectly inferred by an increase in bag volume[17]. The time course for the proximal stomach to return to baseline volume after nutrient stimulation was assessed by analysis of the 2 h “no-infusion” period; and was defined as the time taken for the relaxed fundus to return to pre-stimulation level for > 5 min. Assessment of fundic slow volume waves (FW) was also performed. These were defined as changes in proximal gastric volume of greater than 30 mL that reverted in less than 2 min to a volume within 50% of the previous level[17]. The number and amplitude of FWs (per 10 min) was determined during fasting and in response to duodenal nutrient infusion.
Data are expressed as mean ± SE. Differences in demographic characteristics, baseline volumes, MDP, FW frequency, peak volume response and the time required for the proximal stomach to return to baseline volume, were compared between the three groups using Student's unpaired t-test. ANOVA was used to compare the proximal gastric volume, fundic wave and blood glucose responses to nutrient infusion between the groups, with time and treatment as the factors. A P value < 0.05 was considered statistically significant.
Oral intubation of the assembly was tolerated well by all subjects and no complications occurred in any group. Demographic characteristics of the study groups are summarized in Table 1. The MDP was higher in both patient groups compared to healthy subjects (P < 0.05), but was similar between diabetic and non-diabetic patients (Table 2). Baseline proximal gastric volumes were similar between the three groups.
| Diabetic ICUpatients(n = 10) | Non-diabeticICU patients(n = 15) | Healthysubjects(n = 10) | |
| Age (yr) | 59 ± 3a | 48 ± 5a | 28 ± 3 |
| Gender (M:F) | 5:5 | 12:3 | 7:3 |
| BMI (kg/m2) | 35 ± 3c | 27 ± 1 | 25 ± 1 |
| APACHE II score | |||
| On admission | 28.6 ± 1.5 | 23.2 ± 0.8 | N/A |
| On study day | 24.7 ± 1.5 | 21.1 ± 1.3 | |
| Diagnoses | Sepsis (3) | Sepsis (3) | N/A |
| Pneumonia (3) | Pancreatitis (2) | ||
| Severe asthma (1) | Head trauma (2) | ||
| MVA (1) | MVA (3) | ||
| Angioedema (1) | Cardiac failure (2) | ||
| Sub-arachnoid | Burn (1) | ||
| haemorrhage (1) | Lung abscess (1) Meningitis (1) | ||
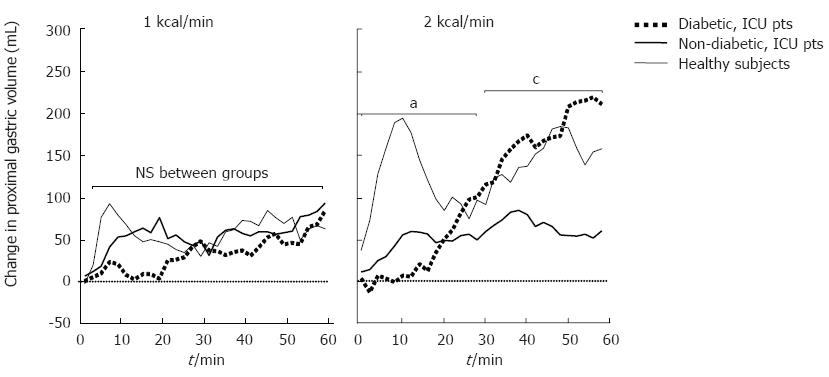
In response to duodenal nutrients, healthy volunteers demonstrated a “biphasic” proximal gastric volume response. Following an initial rapid relaxation, the fundus partially contracted and then exhibited a sustained relaxation throughout the remainder of the infusion (Figure 2). In non-diabetic critically ill patients, there was an overall impairment of both the initial and later phase of the response. In diabetic patients, however, there was an absence of the initial response in the first 20 min during both 1 and 2 kcal/min infusions. Thereafter, the proximal gastric volume increased to the level observed in healthy volunteers.
During the 1 kcal/min infusion there was no difference in the proximal gastric volume response between diabetic critically ill patients and the other two groups. However, during the first 20 min of the 2 kcal/min infusion, the proximal gastric volume was significantly smaller in diabetic critically ill patients than in non-diabetic critically ill patients and healthy subjects. Thereafter, the proximal gastric volume of diabetic patients was greater than that of non-diabetic patients and similar to healthy subjects (Figure 2).
The proximal gastric volume returned to baseline level within 60 min following cessation of nutrient stimulation in all diabetic patients and healthy subjects, but in only 2 of the 15 non-diabetic patients. In diabetic patients, the time taken for the proximal gastric volume to return to baseline level was significantly shorter than in non-diabetic patients and longer than healthy subjects (Table 2).
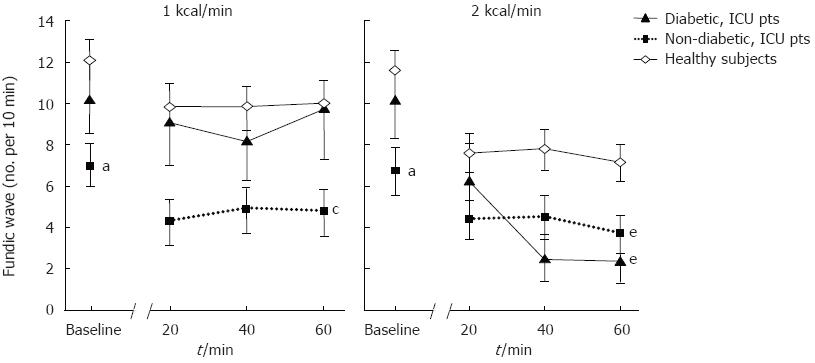
At baseline, the mean frequency of FWs in diabetic patients (10.2 ± 1.7 waves/10 min) was similar to that of healthy subjects (11.8 ± 0.9 waves/10 min; P = 0.38), but was higher than non-diabetic patients (7.0 ± 0.8 waves/10 min; P < 0.05; Figure 3).
The impact of duodenal nutrient stimulation on the frequency of FWs in diabetic patients was similar to that of healthy subjects but differed from non-diabetic patients (Figure 3). Nutrient stimulation with 1 kcal/min infusion did not reduce the mean frequency of FWs in either diabetic patients (9.0 ± 2.0 waves/10 min) or healthy subjects (9.9 ± 1.0 waves/10 min), in contrast to non-diabetic patients (4.4 ± 0.9 waves/10 min; P < 0.05). However, in all 3 groups the 2 kcal/min nutrient infusion significantly reduced the mean frequency of FWs compared to baseline (diabetic: 3.9 ± 1.1 waves/10 min, P < 0.05; healthy: 7.6 ± 0.8 waves/10 min, P < 0.001; non-diabetic: 4.2 ± 0.9 waves/10 min, P < 0.05). The magnitude of reduction in FW frequency during 2 kcal/min was greatest in diabetic patients (-6.6 ± 1.7 waves/10 min), compared to healthy subjects (-4.0 ± 0.7 waves/10 min, P < 0.05) and non-diabetic patients (-1.9 ± 0.6, P < 0.001; Figure 3).
Overall, the frequency of FWs in diabetic patients during 1 kcal/min infusion was similar to that of healthy subjects, but was higher than non-diabetic patients. In contrast, due to the greater magnitude of reduction in FW frequency by a higher nutrient load, fundic wave activity during 2 kcal/min infusion in diabetic patients was similar to that of non-diabetic patients, but less than that of healthy subjects (Figure 3).
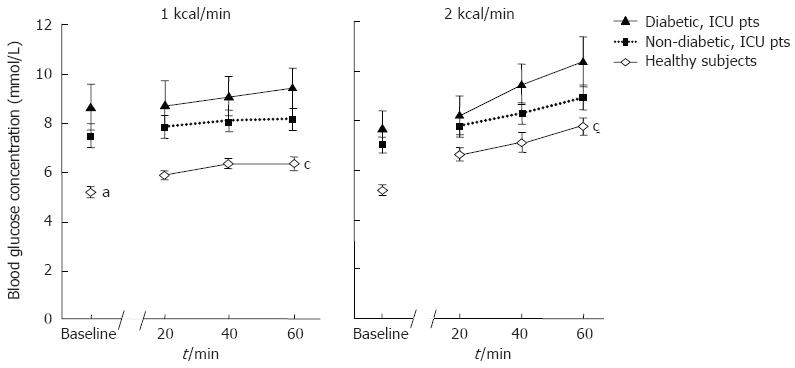
Overall, both fasting and nutrient-stimulated blood glucose concentrations were higher in critically ill patients than in healthy subjects (Figure 4). There were no significant differences in blood glucose concentrations between diabetic and non-diabetic patients, reflecting the use of an insulin infusion protocol in all patients.
This is the first study to examine proximal gastric motor activity in critically ill patients with type 2 diabetes mellitus. The results show that the response to intestinal nutrient feedback is characterized by an initial absence of proximal gastric relaxation, after which the volume increased to a level similar to that seen in healthy volunteers. Furthermore, the delayed volume response was associated with a nutrient load-dependent reduction in fundic wave activity and a slightly impaired recovery of the proximal gastric volume to baseline level. This is in contrast to non-diabetic critically ill patients, who demonstrated a sustained impairment of proximal gastric relaxation, a reduction in fundic wave activity even during 1 kcal/min infusion and a failure of the fundus to recover to baseline volume. These findings support a recent retrospective study, which suggested that type 2 diabetes mellitus may not be a risk factor for delayed gastric emptying in critical illness[18].
A notable feature of the proximal gastric response to nutrient stimulation in diabetic patients was the complete absence of proximal gastric relaxation during the first 20 min of nutrient infusion, however the reason for this response remains unclear. In health, gastro-enteric feedback is regulated by neuro-hormonal pathways[14,16-19], and proximal gastric tone is modulated by a balance between the excitatory cholinergic nerves and the inhibitory nitrergic neural inputs from the vagus[20]. As autonomic dysfunction is common in patients with diabetic autonomic neuropathy[8] and during critical illness[21] the greater degree of impaired relaxation in our diabetic patients may relate to an adverse ‘additive-effect’ of both factors on the autonomic nervous system[20-22]. Formal testing for autonomic neuropathy was not performed in the current study as this was not feasible in the acute critical care setting. A prevalence of autonomic neuropathy of more than 50% would be expected, based on a previous study in diabetic patients with a similar mean duration of disease[23]. Furthermore, disturbances in the metabolism of nitric oxide, a key transmitter in the regulation of gastrointestinal motor function, may also be important[22]. Impaired proximal gastric relaxation is associated with altered levels of nitric oxide[1,8], and has been reported in both patients with diabetes mellitus[1] and critical illness[24]. Whilst increased cytokine production[25] and drug usage[26] can also contribute to impaired relaxation, the impact of these factors are likely to be similar between diabetic and non-DM critically ill patients and probably do not account for the differences in gastric motility seen between the groups.
Following the initial impairment of relaxation, the proximal gastric volume in diabetic critically ill patients increased to a level comparable to that of healthy subjects. This finding has not been previously reported in either patients with critical illness or diabetes mellitus alone. In the later, proximal gastric motor responses to gastric but not duodenal nutrients have been evaluated and further studies to assess this may provide useful information. The mechanisms underlying this normalization of proximal gastric motility are unknown. Despite the use of a standardized insulin protocol in all patients during the study, a small degree of hyperglycaemia occurred in diabetic patients during the latter half of the 2 kcal/min infusion (Figure 4). Although hyperglycaemia has been shown to induce proximal gastric relaxation[7], the absolute increase in blood glucose level was small and therefore unlikely to have contributed significantly to the subsequent response. The diabetic patients had a higher BMI than the healthy volunteers, however no differences in either proximal gastric volume or compliance have been reported between obese and lean subjects[27,28]. Thus, a higher BMI in diabetic patients seems unlikely to have contributed to the differences in proximal gastric motility. Opiate drugs such as morphine were excluded 24 h prior to the study, hence are unlikely to explain our findings[29].
To standardize the nutrient stimulation and enable a reliable assessment of the entero-gastric feedback response, feeds were delivered directly into the duodenum at a rate consistent with normal gastric emptying. Intra-gastric delivery of nutrients was not used in the current study because gastric emptying is frequently impaired in the critically ill. Furthermore, both gastric motility and emptying of a liquid meal may be altered by the presence of a barostat balloon[30]. In addition, intra-duodenal nutrient stimulation with 1 and 2 kcal/min nutrient loads enabled examination of the dose-dependency of the proximal gastric motor response[16,17,19].
Previous studies in critically ill patients have suggested enhanced entero-gastric feedback in response to duodenal nutrient stimulation[12]. In contrast to the non-diabetic patients, diabetic critically ill patients had a dose-dependent reduction in fundic wave activity, similar to the healthy subjects. The different responses in fundic wave activity during the 1 and 2 kcal/min nutrient loads between the diabetic and non-diabetic patients suggest that entero-gastric feedback is not increased in diabetic patients. Furthermore, apart from an initial delay in relaxation, the overall proximal gastric motor responses to nutrients in diabetic critically ill patients were similar to those of healthy subjects. As the proximal stomach is a major determinant of liquid gastric emptying[16,17,19], these findings may explain the relatively normal gastric emptying observed in this group of patients[14].
Whether differences in the ‘accommodative’ response to nutrients between the two patient groups affect their ability to tolerate bolus or continuous gastric feeds remains to be determined and requires further study. It is conceivable that slow continuous feeds may be better tolerated in non-diabetic critically ill patients as proximal gastric relaxation is small and slow during nutrient stimulation[9,11]. In contrast, the relatively normal ‘biphasic’ proximal gastric response in the diabetic critically ill patients may allow better tolerance to bolus gastric feeds.
In conclusion, proximal gastric motor responses to duodenal nutrient are relatively normal in type 2 diabetic patients during critical illness. These motor findings support data which suggests these patients may have normal gastric emptying[18] and may be less prone to developing naso-gastric feed intolerance than non-diabetic, critically ill patients.
S- Editor Liu Y L- Editor Iqbal A E- Editor Liu WF
| 1. | Horowitz M, Wishart JM, Jones KL, Hebbard GS. Gastric emptying in diabetes: an overview. Diabet Med. 1996;13:S16-S22. [PubMed] |
| 2. | Weytjens C, Keymeulen B, Van Haleweyn C, Somers G, Bossuyt A. Rapid gastric emptying of a liquid meal in long-term Type 2 diabetes mellitus. Diabet Med. 1998;15:1022-1027. [PubMed] [DOI] [Full Text] |
| 3. | Bertin E, Schneider N, Abdelli N, Wampach H, Cadiot G, Loboguerrero A, Leutenegger M, Liehn JC, Thiefin G. Gastric emptying is accelerated in obese type 2 diabetic patients without autonomic neuropathy. Diabetes Metab. 2001;27:357-364. [PubMed] |
| 4. | Phillips WT, Schwartz JG, McMahan CA. Rapid gastric emptying in patients with early non-insulin-dependent diabetes mellitus. N Engl J Med. 1991;324:130-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Rayner CK, Samsom M, Jones KL, Horowitz M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care. 2001;24:371-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 307] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 6. | Fraser RJ, Horowitz M, Maddox AF, Harding PE, Chatterton BE, Dent J. Hyperglycaemia slows gastric emptying in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1990;33:675-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 341] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 7. | Hebbard GS, Sun WM, Dent J, Horowitz M. Hyperglycaemia affects proximal gastric motor and sensory function in normal subjects. Eur J Gastroenterol Hepatol. 1996;8:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Samsom M, Roelofs JM, Akkermans LM, van Berge Henegouwen GP, Smout AJ. Proximal gastric motor activity in response to a liquid meal in type I diabetes mellitus with autonomic neuropathy. Dig Dis Sci. 1998;43:491-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Heyland DK, Tougas G, King D, Cook DJ. Impaired gastric emptying in mechanically ventilated, critically ill patients. Intensive Care Med. 1996;22:1339-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 163] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Dive A, Moulart M, Jonard P, Jamart J, Mahieu P. Gastroduodenal motility in mechanically ventilated critically ill patients: a manometric study. Crit Care Med. 1994;22:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 131] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Mutlu GM, Mutlu EA, Factor P. GI complications in patients receiving mechanical ventilation. Chest. 2001;119:1222-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 228] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Nguyen NQ, Fraser RJ, Chapman M, Bryant LK, Holloway RH, Vozzo R, Feinle-Bisset C. Proximal gastric response to small intestinal nutrients is abnormal in mechanically ventilated critically ill patients. World J Gastroenterol. 2006;12:4383-4388. [PubMed] |
| 13. | Chapman M, Fraser R, Vozzo R, Bryant L, Tam W, Nguyen N, Zacharakis B, Butler R, Davidson G, Horowitz M. Antro-pyloro-duodenal motor responses to gastric and duodenal nutrient in critically ill patients. Gut. 2005;54:1384-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Lin HC, Doty JE, Reedy TJ, Meyer JH. Inhibition of gastric emptying by glucose depends on length of intestine exposed to nutrient. Am J Physiol. 1989;256:G404-G411. [PubMed] |
| 15. | Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87:978-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1203] [Cited by in RCA: 1268] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 16. | Heddle R, Collins PJ, Dent J, Horowitz M, Read NW, Chatterton B, Houghton LA. Motor mechanisms associated with slowing of the gastric emptying of a solid meal by an intraduodenal lipid infusion. J Gastroenterol Hepatol. 1989;4:437-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 135] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Azpiroz F, Malagelada JR. Intestinal control of gastric tone. Am J Physiol. 1985;249:G501-G509. [PubMed] |
| 18. | Nguyen NQ, Chapman M, Fraser RJ, Ritz M, Bryant LK, Butler R, Davidson G, Zacharakis B, Holloway RH. Long-standing type II diabetes mellitus is not a risk factor for slow gastric emptying in critically ill patients. Intensive Care Med. 2006;32:1365-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Kelly KA. Gastric emptying of liquids and solids: roles of proximal and distal stomach. Am J Physiol. 1980;239:G71-G76. [PubMed] |
| 20. | Paterson CA, Anvari M, Tougas G, Huizinga JD. Nitrergic and cholinergic vagal pathways involved in the regulation of canine proximal gastric tone: an in vivo study. Neurogastroenterol Motil. 2000;12:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Schmidt HB, Werdan K, Müller-Werdan U. Autonomic dysfunction in the ICU patient. Curr Opin Crit Care. 2001;7:314-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Kellow JE, Delvaux M, Azpiroz F, Camilleri M, Quigley EM, Thompson DG. Principles of applied neurogastroenterology: physiology/motility-sensation. Gut. 1999;45 Suppl 2:II17-II24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Valensi P, Pariès J, Attali JR. Cardiac autonomic neuropathy in diabetic patients: influence of diabetes duration, obesity, and microangiopathic complications--the French multicenter study. Metabolism. 2003;52:815-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 156] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 24. | Argaman Z, Young VR, Noviski N, Castillo-Rosas L, Lu XM, Zurakowski D, Cooper M, Davison C, Tharakan JF, Ajami A. Arginine and nitric oxide metabolism in critically ill septic pediatric patients. Crit Care Med. 2003;31:591-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Emch GS, Hermann GE, Rogers RC. TNF-alpha activates solitary nucleus neurons responsive to gastric distension. Am J Physiol Gastrointest Liver Physiol. 2000;279:G582-G586. [PubMed] |
| 26. | Lee TL, Ang SB, Dambisya YM, Adaikan GP, Lau LC. The effect of propofol on human gastric and colonic muscle contractions. Anesth Analg. 1999;89:1246-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Park MI, Camilleri M. Gastric motor and sensory functions in obesity. Obes Res. 2005;13:491-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Kim DY, Camilleri M, Murray JA, Stephens DA, Levine JA, Burton DD. Is there a role for gastric accommodation and satiety in asymptomatic obese people? Obes Res. 2001;9:655-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Lefebvre RA, Willems JL, Bogaert MG. Gastric relaxation and vomiting by apomorphine, morphine and fentanyl in the conscious dog. Eur J Pharmacol. 1981;69:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Ropert A, des Varannes SB, Bizais Y, Rozé C, Galmiche JP. Simultaneous assessment of liquid emptying and proximal gastric tone in humans. Gastroenterology. 1993;105:667-674. [PubMed] |