Published online Jan 14, 2007. doi: 10.3748/wjg.v13.i2.250
Revised: October 11, 2006
Accepted: November 20, 2006
Published online: January 14, 2007
AIM: To investigate the antiproliferative effect of paeonol (Pae) used alone or in combination with chemotherapeutic agents [cisplatin (CDDP), doxorubicin (DOX) and 5-fluorouracil (5-FU)] on human hepatoma cell line HepG2 and the possible mechanisms.
METHODS: The cytotoxic effect of drugs on HepG2 cells was measured by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetra-zolium bromide (MTT) assay. Morphologic changes were observed by acridine orange (AO) fluorescence staining. Cell cycle and apoptosis rate were detected by flow cytometry (FCM). Drug-drug interactions were analyzed by the coefficient of drug interaction (CDI).
RESULTS: Pae (7.81-250 mg/L) had an inhibitory effect on the proliferation of HepG2 cells in a dose-dependent manner, with the IC50 value of (104.77 ± 7.28) mg/L. AO fluorescence staining and FCM assays showed that Pae induced apoptosis and arrested cell cycle at S phase in HepG2 cells. Further, different extent synergisms were observed when Pae (15.63, 31.25, 62.5 mg/L) was combined with CDDP (0.31-2.5 mg/L), DOX (0.16-1.25 mg/L), or 5-FU (12.5-100 mg/L) at appropriate concentrations. The IC50 value of the three drugs decreased dramatically when combined with Pae (p < 0.01). Of the three different combinations, the sensitivity of cells to drugs was considerably different.
CONCLUSION: Pae had a significant growth-inhibitory effect on the human hepatoma cell line HepG2, which may be related to apoptosis induction and cell cycle arrest. It also can enhance the cytotoxicity of chemotherapeutic agents on HepG2 cells, and the S phase arrest induced by Pae may be one of the mechanisms of these interactions.
- Citation: Xu SP, Sun GP, Shen YX, Wei W, Peng WR, Wang H. Antiproliferation and apoptosis induction of paeonol in HepG2 cells. World J Gastroenterol 2007; 13(2): 250-256
- URL: https://www.wjgnet.com/1007-9327/full/v13/i2/250.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i2.250
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third leading cause of cancer-related death worldwide[1]. Eighty percent of the burden is borne by countries in Asia and Sub-Saharan Africa[2]. Although recent advances in management with a multidisciplinary approach results in improved local and regional disease control, the 5-year survival rate is still less than 10%[3]. Thus it is imperative to develop more effective and low-toxic chemotherapy agents.
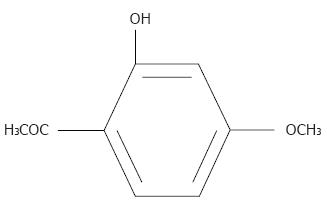
Chinese herbal medicines are now attracting great attention in the world, which also show promising effects in treatment of cancers, including HCC[4]. Paeonol (Pae, 2-hydroxy-4-methoxyacetophenone, Figure 1), is a natural product extracted from the root of Paeonia Suffruticosa Andrew[5]. In our previous study, the antineoplastic activity of Pae has been demonstrated both in various cell lines[6] and in animal models[7,8]. The present study was designed to investigate the antiproliferative effect of Pae used alone or in combination with chemotherapeutic drugs [cisplatin (CDDP), doxorubicin (DOX) and 5-fluorouracil (5-FU)] on human hepatoma cell line HepG2 and the possible mechanisms.
Human hepatocellular carcinoma cell line HepG2 was purchased from Shanghai Institute of Hepatocarcinoma. HepG2 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and incubated at 37°C in a humidified atmosphere with 5% CO2.
Pae Injection was purchased from the First Pharmaceutical Factory of Shanghai, China (Cat. No. 990402, 10 mg/2 mL); CDDP Injection was purchased from Nanjing Pharmaceutical Co. Ltd, China (Cat. No. 20050602, 20 mg/20 mL); DOX was provided by Wanle Pharmaceutical Inc., Shenzhen, China (Cat. No. 0407E1, 10 mg/ampoule); 5-FU Injection was supplied by Shanghai Haipu Pharmaceutical Factory, China (Cat. No. 031109, 0.25 g/10 mL); DMEM was purchased from GIBCO BRL, Life Technologies Inc. (New York, USA); 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetra-zolium bromide (MTT) and acridine orange (AO) were from Sigma Co., USA. DNA-Prep-Reagents Kit was provided by Beckman Coulter Co. USA(Cat. No. 760279K).
HepG2 cells were seeded in 96-well plates at a density of 1-5 × 103 cells/well in 100 μL DMEM containing 10% FBS overnight. Nonadherent cells were removed by gentle washing. Then cells were treated with various concentrations of the drugs. After 44 h of drug exposure, 20 μL MTT solution (5 g/L) was added to each well for another 4 h at 37°C. The formazine was solved in 150 μL/well dimethyl sulfoxide (DMSO) and the absorbance was detected at 490 nm using ELx800 Strip reader (Bio-Tek, USA). The percentage of cytotoxicity was calculated as follows: Cytotoxicity (%) = (1-A490 of experimental well)/ A490 of control well. The median inhibitory concentration (IC50) (defined as the drug concentration at which cell growth was inhibited by 50%) was assessed from the dose-response curves.
The coefficient of drug interaction (CDI) was used to analyze the synergistically inhibitory effect of drug combinations[9]. CDI is calculated as follows: CDI = AB/ (A × B). According to the absorbance of each group, AB is the ratio of the combination groups to control group; A or B is the ratio of the single agent groups to control group. Thus CDI value less than, equal to or greater than 1 indicates that the drugs are synergistic, additive or antagonistic, respectively. CDI less than 0.7 indicates that the drugs are significantly synergistic.
Cells were cultured in 6-well plates containing cover slips overnight. After incubation with Pae for 24 h, the cover slips were washed twice with PBS, fixed with 95% ethanol for 15 min, acidified with 1% acetic acid for 30 s, dyed with 0.1 g/L AO for 10 min, differentiated with 0.1 mol/L CaCl2 for 2 min, and then washed with PBS 3 times. The cover slips were sealed and observed under a fluorescence microscope (OLYMPUS, Japan).
Cells were cultured in 6-well plates and allowed to grow to 75%-80% confluency. Nonadherent cells were removed by gentle washing, and the media were removed and replaced with fresh medium containing Pae at the desired concentrations. After exposure to drugs for 24 h, cells were collected and centrifuged at 1500 r/min in a 15 mL tube for 10 min. The cells were washed twice with PBS and resuspended in 50 μL fixing buffer at a room temperature for 20 s, then 500 μL propidium iodide (PI) staining buffer was added in the dark at room temperature for 30 min (according to the procedure program of the DNA-Prep Coulter reagents kit). A minimum of 1 × 105 cells for each group was analyzed using an EPICS XL-MCL model Coulter counter. Cell cycle distribution was analyzed using Mcycle software.
Biostatistical analyses were done using the SPSS 11.5 software package. All experiments were repeated at least three times. Results of multiple experiments are given as the mean ± SE. Non-parametric Kruskal-Wallis test was used to detect differences among the different experimental groups. Mann-Whitney U test was subsequently used for statistical evaluation in two-group comparisons. Pearson correlation coefficients were used for continuous independent and dependent variables. A level of P < 0.05 was accepted as statistically significant.
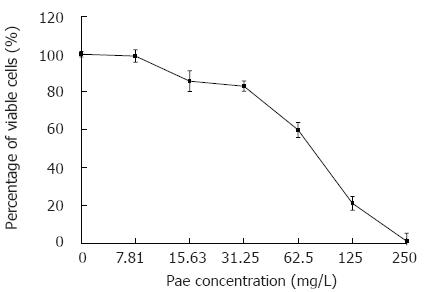
We first examined the effect of Pae on the proliferation of HepG2 cells. As shown in Figure 2, a dramatic dose-dependent reduction of cell viability was seen in cells incubated with Pae at concentrations of 7.81-250 mg/L for 48 h. The r value of dose-effect curves was 0.959 (P < 0.01) and the IC50 value of Pae was (104.77 ± 7.28) mg/L (P < 0.01).
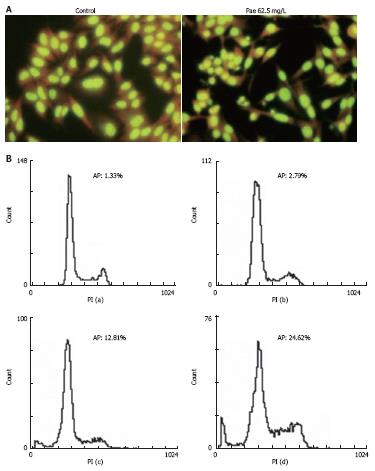
Morphological evidence of apoptosis was demonstrated by AO fluorescence staining. AO could be seen in all cells and the nuclei appeared green and chromatin was stained yellow (Figure 3A). Cells treated with Pae showed typically apoptotic changes, such as chromatin condensation, membrane blebing, deformed and fragmented nuclei.
FCM assay was performed to analyze apoptosis in HepG2 cells treated with various concentrations of Pae for 24 h. It was found that the sub-G1 peak appeared before G1 phase, which represents apoptotic cell population (Figure 3B), in a dose- and time-dependent manner (Figure 4).
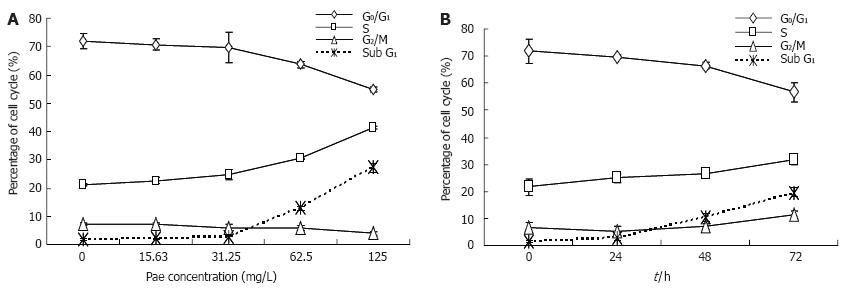
Mcycle software was used to analyze the kinetic changes of cell cycle distribution. In untreated HepG2 controls, cells were present in G0/G1 (71.79% ± 2.76%), S (20.31% ± 0.58%), and G2/M (7.16% ± 0.57%) phases. For HepG2 cells exposed to various concentrations of Pae, the S-phase fraction increased while G0/G1 fraction decreased in a dose-dependent manner (Figure 4A). And the percentages of cells in S phase increased to 24.98% ± 1.63%, 26.54% ± 1.53%, 31.72% ± 4.85% after 24, 48, and 72 h, respectively, when compared with untreated control cells, which was accompanied by a concomitant decrease of cells in the G0/G1 phase of the cell cycle (Figure 4B). It indicated that Pae might arrest the cell cycle at the S phase, and this blockage of cell cycle may prevent cells from entering M phase.
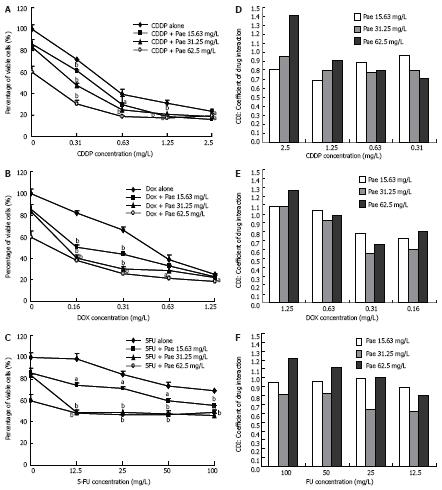
Growth-inhibition assays were performed to investigate whether Pae can enhance the antiproliferative effects of chemotherapeutic agents on HepG2 cells. Three doses of Pae (15.63, 31.25 and 62.5 mg/L) were combined with different concentrations of CDDP, DOX, and 5-FU, respectively. For each experiment, a dose-response curve of each single chemotherapeutic agent and its combination with Pae was drawn, which showed that Pae increased the cytotoxicity of CDDP, DOX, and 5-FU on HepG2 cells. The IC50 value of the three drugs decreased dramatically at different extents when combined with Pae. For example, in the presence of 15.63, 31.25 and 62.5 mg/L Pae, the IC50 of CDDP reduced from 0.591 ± 0.053 mg/L to 0.366 ± 0.011, 0.161 ± 0.018, 0.007 ± 0.002 mg/L, respectively. That of DOX reduced from 0.489 ± 0.124 mg/L to 0.175 ± 0.043, 0.037 ± 0.012, 0.032 ± 0.005 mg/L. And that of 5-FU reduced from 310.783 ± 13.094 mg/L to 161.759 ± 9.507, 8.646 ± 2.331, 5.021 ± 0.962 mg/L, respectively (P < 0.01, Figure 5A-C).
We analyzed the nature of the interaction between Pae and the three drugs using CDI, which quantitatively measures the interaction of two drugs. As shown in Figure 5D, Pae and CDDP yielded synergistic interactions across a wide concentration range. The synergistic effect was most prominent when 15.63 mg/L Pae was combined with 1.25 mg/L CDDP (CDI < 0.7). While a significant synergistic effect was only obtained when Pae concentration reached 31.25 and 62.5 mg/L in combination with 0.16 mg/L and 0.31 mg/L DOX, respectively. When DOX reached 1.25 mg/L, the interaction was antagonistic (Figure 5E). Pae had a relatively weak activity to enhance the antiproliferative effect of 5-FU in HepG2 cells. If the concentrations of drugs were too high or too low, the synergistic cytotoxic effects could not be achieved. The combinations of Pae at 31.25 mg/L and 5-FU at 12.5 and 25 mg/L exhibited significantly synergistic activity against HepG2 cells, while an antagonistic effect was observed at 62.5 mg/L of Pae in combination with 25-100 mg/L of 5-FU (Figure 5F).
Currently, a variety of cytotoxic and antiproliferative agents have been tested in HCC treatment, which are used alone, or in combination with other drugs or other treatment modalities[10]. Agents with partial response rates near or above 10% include DOX, CDDP and 5-FU[11-13]. However, high doses of these drugs lead to severe toxicities, which have a negative effect on patients’ survival. The use of less toxic doses in combination with other anti-proliferative agents would be desirable[14-17].
Pae is isolated from the herb Pycnostelma paniculatum (Bunge) K.S., and the root of the plant Paeonia Suffruticosa Andrew[5]. It is a white needle crystal with a relatively low-melting point of 51°C-52°C. The molecular weight of Pae is 166.18 ku and the molecular formula is C9H10O3[18] . Pae possesses extensive pharmacological activities such as sedation, hypnosis, antipyresis, analgesic, antioxidation, antiinflammation, and immunoregulation[19]. Additionally, Pae had minimal systemic toxicity (LD50 3430 mg/kg) when it was orally administrated to mice[20]. In our previous study, the antineoplastic activity of Pae has been demonstrated both in cell lines, such as human erythromyeloid cell line K562, breast cancer gene cell line T6-17, human hepatoma cell line Bel-7404, and cervical cancer cell line Hela[6], and in animal models bearing HepA hepatocarcinoma[7,8]. Ji et al[21] demonstrated that Pae at a low concentration had synergetic effects with 5-FU, MMC and CDDP on inhibiting the proliferation of human colorectal cancer cell line HT-29.
In the present study, Pae exhibited growth inhibition to HepG2 cells in a dose-dependent manner, with the IC50 value of 104.77 (± 7.28 mg/L) mg/L. Although the exact mechanism of the cytotoxicity of Pae against HepG2 cells is not entirely clear, many potential mechanisms have been proposed for the growth inhibition of Pae in cultured cells and animal models. These mechanisms include induction of apoptosis[22-23] and immunoregulation such as promoted lymphocyte proliferation, IL-2 production by splenocytes, and TNF-α production by PMφ from the model mice[7,8]. Apoptosis is a mechanism by which cells undergo death to control cell proliferation or in response to DNA damage. A tumor occurs when the balance of cell proliferation and cell death is broken[24]. Induction of apoptosis is an effective strategy for cancer therapy[25]. In the present study, the cells treated with Pae showed typical characteristics of apoptosis. Similarly, apoptotic peak appeared before G1 phase after treatment with Pae, which resulted from the internucleosomal degradation of DNA, in a dose- and time-dependent manner. Moreover, the HepG2 cells exposed to Pae for 24 h showed depletion of the G0/G1 fraction and accumulation in S-phase. Accumulation in S-phase has also been reported by Liu et al[22], in which Pae could induce cell cycle disturbance and S phase of the HT-29 cells was increased, while G0/G1 and G2/M phase of the cells were decreased. The S phase arrest and apoptosis induction of Pae on HepG2 cells might be its main mechanism.
Meanwhile, HepG2 cells were treated with the combinations of Pae and different chemotherapeutic agents. The results indicated that the growth inhibitory effect of CDDP, DOX, or 5-FU, respectively, was enhanced significantly by Pae at appropriate concentra-tions. Among the three agents examined, CDDP showed the most wide synergistic effect with Pae. The synergistic effect was most prominent (CDI < 0.7 =) when 15.63 mg/L Pae was combined with 1.25 mg/L CDDP. This indicated that the combination of Pae and CDDP at certain concentrations might help reduce nausea, vomiting and serious kidney toxicity of CDDP. Similar results that Pae in combination with anticancer drugs had synergistic effects at lower concentrations and had antagonistic effects at higher concentrations were observed in DOX and 5-FU, but with different sensitivities. The S phase arrest of Pae may be one of the mechanisms related with these interactions. 5-FU belongs to cell cycle specific agents, which acts specifically on cells in S phase[26]. The cytotoxic effects of CDDP and DOX are generally considered to be non-cell-cycle specific[27-28]. Nevertheless, DOX has the most killing effect on S phase cells[28]. CDDP is most specific to G1 phase cells, while it also has strong effects on cells in S phase[29]. Further studies are needed to investigate the mechanisms of these synergisms, which favor the reasonable application of Pae to HCC treatment.
We thank Dr. Zhimin Zhai and Qing Li for FACS analysis, the Central Laboratory of the Provincial Hospital of Anhui.
Hepatocellular carcinoma (HCC) is a major contributor to cancer incidence and mortality in the world. No effective treatment is available by now. Therefore, there is a critical need to develop more strategies for chemotherapy of hepatoma.
Currently, a variety of cytotoxic and antiproliferative agents have been tested in HCC treatment, which are used alone, or in combination with other drugs or with different modalities of treatment. Chinese herbal medicines are now attracting great attention in the world, which also show promising effects in HCC therapy. Paeonol, a natural product extracted from the root of Paeonia Suffruticosa Andrew, has shown antineoplastic activities both in cell lines and in animal models.
This is the first report on the antiproliferation, induction of apoptosis and cell cycle arrest by Pae in HepG2 cells.
Pae may be expected to be effective and useful as a new agent in hepatoma chemotherapy.
The authors examine the cytotoxic effect of Pae only in HepG2 cells. It remains unclear whether the effect of Pae in HepG2 cells can be generalized to other hepatoma cells. The authors should examine the effect of Pae using a panel of hepatoma cell lines; The data in Figure 4 suggested that Pae induces S-phase arrest in HepG2 cells. However, the molecular basis for S-phase arrest is not clearly shown. The authors should examine whether Pae has an effect in cells arrested at G1/S phase using pre-treatment of cells such as hydroxyurea. It would be important to examine the expression of p21, p27 CDK1 and cyclinA after treatment with Pae.
S- Editor Liu Y L- Editor Zhu LH E- Editor Ma WH
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2651] [Cited by in RCA: 2598] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 2. | McGlynn KA, London WT. Epidemiology and natural history of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2005;19:3-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 251] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 3. | Johnson PJ. Hepatocellular carcinoma: is current therapy really altering outcome? Gut. 2002;51:459-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Shu X, McCulloch M, Xiao H, Broffman M, Gao J. Chinese herbal medicine and chemotherapy in the treatment of hepatocellular carcinoma: a meta-analysis of randomized controlled trials. Integr Cancer Ther. 2005;4:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Riley CM, Ren TC. Simple method for the determination of paeonol in human and rabbit plasma by high-performance liquid chromatography using solid-phase extraction and ultraviolet detection. J Chromatogr. 1989;489:432-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Sun GP, Wang H, Shen YX, Xu SY. Inhibitory effects of paeonol on the proliferation on four tumor cell lines. Anhui Yiyao. 2004;8:85-87. |
| 7. | Sun GP, Shen YX, Zhang LL, Zhou AW, Wei W, Xu SY. Study on immunomodulation and antitumor activity of paeonol in HepA tumor mice. Zhongguo Yaolixie Tongbao. 2003;19:160-162. |
| 8. | Sun GP, Shen YX, Zhang LL, Wang H, Wei W, Xu SY. Anti-tumor effect of paeonol in vitro and in vivo. Anhui Keji Xueyuan Xuebao. 2002;37:183-185. |
| 9. | Cao SS, Zhen YS. Potentiation of antimetabolite antitumor activity in vivo by dipyridamole and amphotericin B. Cancer Chemother Pharmacol. 1989;24:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Zhu AX. Systemic therapy of advanced hepatocellular carcinoma: how hopeful should we be? Oncologist. 2006;11:790-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 200] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 11. | Okada S, Okazaki N, Nose H, Shimada Y, Yoshimori M, Aoki K. A phase 2 study of cisplatin in patients with hepatocellular carcinoma. Oncology. 1993;50:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Lai CL, Wu PC, Chan GC, Lok AS, Lin HJ. Doxorubicin versus no antitumor therapy in inoperable hepatocellular carcinoma. A prospective randomized trial. Cancer. 1988;62:479-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Lin DY, Lin SM, Liaw YF. Non-surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 1997;12:S319-S328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Leung TW, Patt YZ, Lau WY, Ho SK, Yu SC, Chan AT, Mok TS, Yeo W, Liew CT, Leung NW. Complete pathological remission is possible with systemic combination chemotherapy for inoperable hepatocellular carcinoma. Clin Cancer Res. 1999;5:1676-1681. [PubMed] |
| 15. | Yeo W, Mok TS, Zee B, Leung TW, Lai PB, Lau WY, Koh J, Mo FK, Yu SC, Chan AT. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 455] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 16. | Lee J, Park JO, Kim WS, Park SH, Park KW, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC. Phase II study of doxorubicin and cisplatin in patients with metastatic hepatocellular carcinoma. Cancer Chemother Pharmacol. 2004;54:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Yin XY, Lü MD, Liang LJ, Lai JM, Li DM, Kuang M. Systemic chemo-immunotherapy for advanced-stage hepatocellular carcinoma. World J Gastroenterol. 2005;11:2526-2529. [PubMed] |
| 18. | Mimura K, Baba S. Determination of paeonol metabolites in man by the use of stable isotopes. Chem Pharm Bull (Tokyo). 1981;29:2043-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Sun YC, Shen YX, Sun GP. Advances in the studies of major pharmacological activity of paeonol. Zhongchengyao Zazhi. 2004;26:579-582. |
| 20. | Jiang SP, Chen YX. Advances in the research and its clinical application of Cynanchum paniculatum (Bge.) Kitag. Zhongguo Zhongyao Zazhi. 1994;19:311-314. [PubMed] |
| 21. | Ji CY, Tan SY, Liu CQ. Inhibitory effect of paeonol on the proliferation of human colorectal cancer cell line HT-29 and its synergistic effect with chemotherapy agents. Zhongguo Zhongyao Zazhi. 2005;17:122-124. |
| 22. | Liu CQ, Tan SY, Ji CY, Luo HS, Yu JP. The effects of paeonol on inhibiting the proliferation of human colorectal cancer cell line HT-29 and its molecule mechanism. Zhongguo Yaolixie Tongbao. 2005;21:1251-1254. |
| 23. | Sun GP, Wang H, Shen YX, Zhai ZM, Wei W, Xu SY. Study on effects of paeonol in inhibiting growth of K562 and inducing its apoptosis. Zhongguo Yaolixie Tongbao. 2004;20:550-552. |
| 24. | Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2445] [Article Influence: 101.9] [Reference Citation Analysis (0)] |
| 25. | Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013-2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 26. | Petru E, Sevin BU, Haas J, Ramos R, Perras J. A correlation of cell cycle perturbations with chemosensitivity in human ovarian cancer cells exposed to cytotoxic drugs in vitro. Gynecol Oncol. 1995;58:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Bergerat JP, Barlogie B, Göhde W, Johnston DA, Drewinko B. In vitro cytokinetic response of human colon cancer cells to cis-dichlorodiammineplatinum(II). Cancer Res. 1979;39:4356-4363. [PubMed] |
| 28. | Takahashi K, Ebihara K, Honda Y, Nishikawa K, Kita M, Oomura M, Shibasaki C. Antitumor activity of cis-dichlorodiammineplatinum(II) and its effect on cell cycle progression. Gan To Kagaku Ryoho. 1982;9:624-631. [PubMed] |
| 29. | Potter AJ, Rabinovitch PS. The cell cycle phases of DNA damage and repair initiated by topoisomerase II-targeting chemotherapeutic drugs. Mutat Res. 2005;572:27-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |