Published online Jan 14, 2007. doi: 10.3748/wjg.v13.i2.244
Revised: October 15, 2006
Accepted: November 24, 2006
Published online: January 14, 2007
AIM: To use the tyrosinase minigene as a visual marker to perform microinjection training and improve the techniques related with transgene to greatly elevate the efficiency of gene transfer.
METHODS: A mouse tyrosinase minigene, i.e., TyBS, in which the 2.25-kb authentic genomic 5’ non-coding flanking sequence of mouse tyrosinase was fused to a mouse tyrosinase cDNA, was introduced into the fertilized eggs of outbred Kunming albino mice.
RESULTS: Of the 11 animals that developed from the injected eggs, two mice (P1 and #8) exhibited pigmented hair (P1) and eyes (P1 and #8), as confirmed by PCR analysis for the tyrosinase minigene integrated into the genome. When founder P1 was bred to Kunming male mouse, six progeny out of 11 offspring inherited the transgene and the pigmented-eye phenotype.
CONCLUSION: Taken together, these results suggest that this minigene encodes the active tyrosinase protein and that its 5’ flanking region contains the sequences regulating the expression of mouse tyrosinase gene as expected. We have rescued the albino phenotype by introduction and expression of a functional tyrosinase minigene in the Kunming albino mouse and the transgene can be passed to subsequent generation. These findings also indicate that TyBS can be a useful visual marker gene in the co-transgenic experiments.
- Citation: Xiao D, Yue Y, Deng XY, Huang B, Guo ZM, Ma Y, Lin YL, Hong X, Tang H, Xu K, Chen XG. Rescue of the albino phenotype by introducing a functional tyrosinase minigene into Kunming albino mice. World J Gastroenterol 2007; 13(2): 244-249
- URL: https://www.wjgnet.com/1007-9327/full/v13/i2/244.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i2.244
Visible pigmentation in the mammals results from the synthesis and distribution of melanin in skin, hair bulbs and eyes[1-3]. Tyrosinase is the first and rate-limiting enzyme in the pathway for melanin production in melanocytes of the skin and eyes[1-3]. Mutation of the tyrosinase gene is a common cause of a similar phenotype in all vertebrates, known as albinism, due to a lack of melanin pigment[1,3]. In mouse, the albino phenotype is characterized by a total absence of pigmentation due to a mutation in the tyrosinase gene; several point mutations within the tyrosinase gene have been found, which can inactivate its function to result in oculocutaneous albinism (OCA)[1,3]. In mouse, the classical albino (c) mutation corresponds to a single-point mutation in the first exon of the tyrosinase gene, which brings about an amino acid mutation Cys103Ser, leading to the accumulation of a non-functional protein[4,5]. When mice are homozygous (c/c) for mutations that inactivate the tyrosinase gene, mice are albino regardless of the genotype at the other loci[1,3]. The entire common albino inbred strains of laboratory mice, such as FVB/N, BALB/c, etc, belonging to OCA, have the same point mutation in the tyrosinase gene, indicating that these strains are derived from a common ancestor[5]. The albino phenotype has been successfully corrected through the tyrosinase transgene, which can express the active tyrosinase in transgenic mice[5-18], rabbits[19], fish[20-22] and other vertebrates expressing tyrosinase functional transgenes[23].
The Human and Model Organism Genome Projects have revealed the sequence information of many genes. A significant challenge for scientists over the next few decades is to annotate the human and model organism genomes with functional information. Genetically engineered mice will play a vital role in the study of the functional genome.
The production of transgenic mice, involving an intensive sequence of procedures in genetics, molecular biology, embryology and animal science, is usually time-consuming and labor-consuming. One problem with learning to do microinjections is that it can be a long wait between the time the microinjections are done and the time that the results are known, particularly if one waits until the microinjected embryos have developed into weaning age mice before screening. How to easily and rapidly assay for a successful pronuclear? There are a number of constructs that are particularly useful when learning to do microinjections. Among them, tyrosinase can be used to allow the visual identification of transgenic mice at birth in the first and all subsequent generations. Microinjection of a tyrosinase minigene into embryos from an albino mouse strain can result in gene cure of the albino defect and the pigment synthesis[5,6,18]. Pigmented mice with dark eyes can be easily identified by simply visible inspection at birth. In fact, the pigment epithelial cells of the retina begin to synthesize melanin by P10.5 of embryonic development[8,26] so that transgenic mice can be typically identified by visual inspection of the fetuses 2 wk after microinjection. The microinjection can be done using albino inbred strains (such as FVB/N and BALB/c) and inexpensive outbred albino strains (such as ICR and Kunming mice). Another advantage of the tyrosinase minigene is the fact that it is not detrimental to the health of the transgenic animals.
Therefore, we decided to use the tyrosinase minigene as a visual marker to perform microinjection training and improve the techniques related with transgene to greatly elevate the efficiency of gene transfer in our center.
The tyrosinase minigene TyBS[5] used for microinjection was generously provided by Dr. P.A. Overbeek (Howard Hughes Medical Institute, Department of Cell Biology, Baylor College of Medicine, Houston, TX, USA) and Dr. F Beermann (Swiss Institute for Experimental Cancer Research, Switzerland).
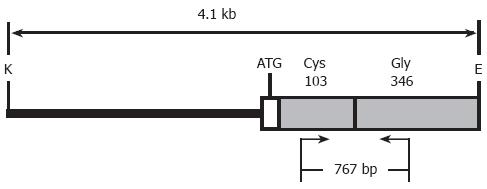
Transgenic mice were generated by microinjection of single cell embryos using standard techniques[27]. The Kunming mouse strain, supplied by Center of Experimental Animals, Sun Yat-Sen University, was used as the source of embryos for the micromanipulation and for the subsequent breeding trials. For microinjection, the 4.1-kb fragment of tyrosinase minigene (Figure 1) was released free from the vector backbone of pTyBS[5] via digestion with EcoR I and Kpn I, thereafter isolated and purified using the QIA quick gel extraction kit (Qiagen, Hilden, Germany), diluted to a final concentration of 2 µg/mL DNA in injection buffer (10 mmol/L Tris/0.1 mmol/L EDTA, pH 7.4), and then microinjected into the pronuclear of one cell-stage fertilized embryos [Kunming mouse (♀) × Kunming mouse (♂)]. About 20-25 DNA-injected fertilized eggs that survived microinjection were implanted into the oviducts of one recipient pseudopregnant Kunming mouse 2-3 h after injection or the next day as previously described[27]. Potential transgenic founders were weaned at 3 wk of age. The offsprings were firstly screened for the presence of the transgene via pigmentation phenotypes derived from the existence of the functional tyrosinase minigene, followed by PCR analysis performed on the tail genomic DNA prepared with standard protocols[28]. All animal care and experimentation were performed according to the Study and Ethical Guidelines for Animal Care, handling and termination established by the Subcommittee of Sun Yat-Sen University on laboratory animal care. The presented work was approved by the ethical committee of Sun Yat-sen University and is covered by Chinese animal husbandary legislation.
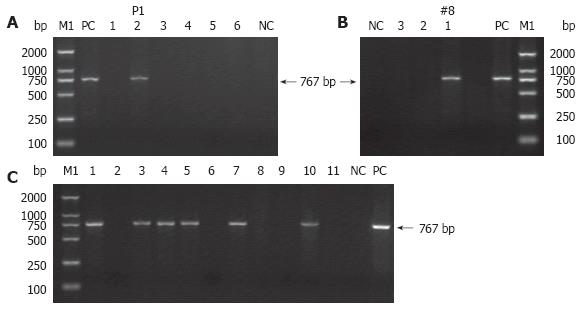
PCR was performed on tail genomic DNA to further identify which mice have tyrosinase minigene integrated into their genome. The sequences of the forward primer (FP) within exon 1 and reverse primer (RP) within exon 4 used to amplify a 767-bp fragment of the tyrosinase minigene were: 5’-GGTTTCAACTGCGGAAACTG-3’ (forward) and 5’-TGTGAGTGGACTGGCAAATC-3’ (reverse) (Figure 1). PCR conditions were as follows: pre-denaturation at 94°C for 7 min, followed by 30 amplification cycles of denaturation at 94°C for 1 min, primer annealing at 58°C for 1 min, and extension at 72°C for 1 min 30 s, and finally an additional extension at 72°C for 10 min. TyBS construct DNA was used as the positive control for each PCR reaction, and genomic DNA from normal Kunming mice was employed as a negative control for each PCR test. DNA samples were considered positive for a particular transgene if a band of the predicted size in the test samples was present with no amplification occurring in the control sample. Endogenous genomic tyrosinase sequence was not amplified under this PCR conditions chosen here.
At 6-8 wk of age, founders shown to be transgenic for the tyrosinase minigene were mated with normal Kunming mice to generate F1. Pigmented F1 animals derived from founder, as well as albino non-transgenic littermates were further analyzed for the inheritance of the tyrosinase transgene by PCR using tyrosinase-FP/RP primers. PCR protocols for TyBS were noted above.
Within the coding sequences of the tyrosinase gene, a G to C transversion at nucleotide 308, leading to a cysteine (Cys) to serine (Ser) mutation at amino acid 103, is sufficient to abrogate pigment production in mice[5]. This same base pair change is fully conserved in the classical albino strains of laboratory mice, such as FVB/N and BALB/c[5]. Albino Kunming mice are an outbred mouse strain that is homozygous mutant at the albino (c) locus. An albino mutation carried in the Kunming mouse strain should be also the result of a base substitution from G to C in exon I. It is, therefore, reasonable to expect that the albino phenotype can be rescued by introducing a functional tyrosinase minigene, such as TyBS, into albino embryos.
The tyrosinase minigene TyBS construct[5] used for microinjection is illustrated in Figure 1. As the expression of the tyrosinase minigene is easily detected by the pigmented phenotype, this gene can be used as a visual marker for the production of transgenic animals.
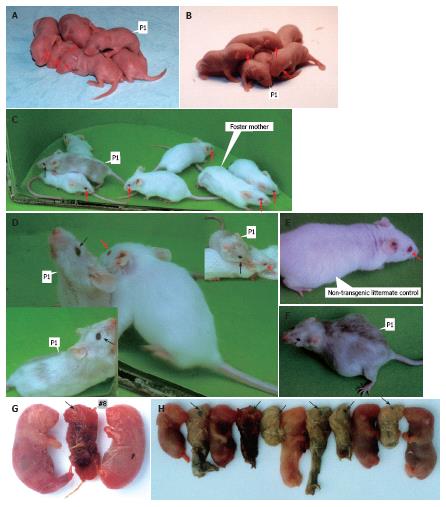
Of the 45 embryos transferred to the recipient females, 11 embryos developed to term. Two individuals of 11 siblings were transgenic, as demonstrated by pigmentation phenotype in the eyes (Figure 2A-D, F and G) and coat (Figure 2C, D and F), and PCR analyses (Figures 3A and B).
Furthermore, two TyBS transgenic mice, i.e. P1 (Figure 2A and B) and #8 (Figure 2G) which died 48 h after birth, had dark eyes at birth, and were immediately identifiable as transgenic mice. Although the extent of the coat pigmentation was non-standard like the wild-type phenotype, founder P1 exhibited the partially pigmented phenotype (Figure 2C, D and F). Over time, the coat of P1 with nearly black eyes (Figure 2A-D, F) became more heavily pigmented (light grey to dark grey) (Figure 2C, D and F), while the eye and fur phenotypes of non-transgenic littermate controls remained pink and albino throughout life (Figure 2C-E), respectively.
To determine whether the TyBS transgene was transmitted to the next generation, at 6 wk of age female P1 was back-crossed to the parental mouse strain to give F1 generation. The progeny of P1 was analyzed for the inheritance of the transgene by eye phenotype, coat pigmentation and PCR.
From the cross between P1 and normal Kunming mouse, 11 offspring were obtained. Although all of littermates from P1 died immediately at birth, it was found that six out of the 11 siblings exhibited the pigmented eyes at birth (Figure 2H), as verified by PCR (Figure 3C).
Non-mosaic transgenic mice with one site of integration should transmit the transgenic DNA in a Mendelian fashion to about 50% of their offspring, whereas mosaic mice generally show a frequency of transmission of 25% or less. Note that founder mice that have more than one site of integration can produce litters where 75% or more of the offspring are transgenic, although the percent transmission for any one site of integration is expected to be average 50% or less[29,30]. It was concluded that founder P1, successfully transmitting the transgene in a Mendelian fashion to about 55% (6/11) of its progeny, is non-mosaic transgenic mouse.
Taken together, these data demonstrate that founder P1 can transmit the transgene to subsequent generation and its progeny show an inherited characteristic phenotype of pigmented eyes.
Pigmentary genes are the first mammalian genes to be studied, mostly because of the obvious phenotypes associated with their mutations[23]. In this study, founder P1, harboring the tyrosinase minigene TyBS, exhibited light pigmentation, but non-standard wild-type coat color in the skin, although over time, P1 coat became more heavily pigmented. Similarly, the transgenic mice carrying TyBS construct showed considerable variation in the intensity of pigmentation, the coat colors were found to range from grayish to brownish, and none of the mice were black[5].
Actually, all these standard tyrosinase constructs, including TyBS, driven by the limited amount of 5’ tyrosinase upstream regulatory sequences (ranging from 270- to 5500-bp promoter sequences) displayed a high degree of variability in coat pigmentation between independent lines[14,30-34], and the coat pigmentation did not reach the normal levels observed in the wild-type phenotype[6,18,30-32,35,36]. For example, in an evaluation of 39 transgenic founder animals and 44 transgenic lines, 5 phenotypic patterns of pigmentation were consistently observed, including albino, dark, light, mottled and Himalayan[32]. In fact, the tyrosinase minigene which is sufficient to produce normal levels of both eumelanin and phaeomelanin can give normal black or brown pigmentation on the appropriate non-agouti genetic backgrounds[5,32]. These abnormally expressional patterns might have been explained by position effects. In summary, these findings demonstrate that other regulatory regions within the tyrosinase gene are required to sustain the faithful expression of tyrosinase transgene, independent of integration site.
By flanking a tyrosinase minigene with tandem copies of the chicken β-globin 5' HS4 insulator, there is a significant reduction in variability among transgenic lines, with the resulting mice exhibiting the similar levels of coat pigmentation, which, in turn, improves the yield of phenotypically expected transgenic founders resulting from each microinjection session, and consequently reduces animal requirements for transgenic production[37].
Screening transgenic animals is usually time-consuming and labor-consuming. It would be very helpful if the transgenic animals could be identified by the visible inspection at birth. The functional tyrosinase gene introduced into an albino mouse strain leads to pigmentation in eyes and skin with high penetrance, and pigmented mice with dark eyes can be immediately identified by simply visible inspection at birth[23], as further confirmed by this study.
When two or several transgenic constructs are co-injected into single-cell fertilized embryos, the co-injected constructs typically co-integrate into the genome, where the transgene can independently express[38]. Theoretically, co-injection of tyrosinase transgenic construct with any other construct(s) should result in a certain percentage of transgenic mice carrying both transgenes at a single chromosomal site[23]. Additionally, co-injection experiments with the agouti transgenes and other transgenes demonstrated co-integration of the two constructs at the same chromosomal site in approximately 95% of F1 progeny, allowing transgene inheritance to be visibly detected[39]. The direct and visual detection of pigmentation in tyrosinase transgenic animals generated in the albino genetic backgrounds was repeatedly proposed by independent teams as a visual marker in co-injection strategies for the rapid detection of the successful transgenesis[12,30-33,35] and by our practices (data not shown). The utility of tyrosinase minigene co-injection with other construct(s) of interest is a useful adjunct to allow rapidly visual identification of transgenic mice at birth.
Moreover, another advantage of co-injection strategy is the fact that homozygous mice in most families can be identified by simply visual inspection, since the homozygous mice have darker coat colors, reflecting the increased gene dosage[32].
The co-injection strategy improves the yield of phenotypically desirable transgenic founder mice resulting from each microinjection session, and consequently reduces animal requirements for the transgenic production and routine genetic validation of transgenic lines.
In summary, we have successfully rescued the albino phenotype by introducing a functional tyrosinase gene into Kunming albino mouse. It should be pointed out here that TyBS and other tyrosinase transgenic constructs can be fused with any of the other genes and microinjected into fertilized eggs from albino murine strains in order to produce melanin pigments as an excellently visible marker for the generation and breeding of transgenic mice.
We thank Dr. CY Fan (Department of Pathology and Otolaryngology, University of Arkansas for Medical Sciences, USA) for his unstinting advices and technical guidance in making transgenic mice, for his supportive and friendly attitude toward our projects. We thank Dr. F Beermann (Swiss Institute for Experimental Cancer Research, Switzerland) for kindly gifting the plasmids of ptrTyr5 and pTyBS, Dr. PA Overbeek (Howard Hughes Medical Institute, Department of Cell Biology, Baylor College of Medicine, Houston, TX, USA) for providing the vectors (e.g., pTyBS and pTy811C), and Professor G Schutz [Deutsches Krebsforschungszentrum (DKFZ), Heidelberg, Germany] for providing the plasmid ptrTyr5. We are also indebted to the expert technical assistance of JY Han, HH Zhang, GG Qiu, WG Huang, FY Chen, FR Ni, JY Xie and JH Wang, Center of Experimental Animals, Sun Yat-Sen University.
S- Editor Liu Y L- Editor Kumar M E- Editor Liu WF
| 1. | del Marmol V, Beermann F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 1996;381:165-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 323] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 2. | Nadeau JH. Modifier genes in mice and humans. Nat Rev Genet. 2001;2:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 393] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 3. | Oetting WS. The tyrosinase gene and oculocutaneous albinism type 1 (OCA1): A model for understanding the molecular biology of melanin formation. Pigment Cell Res. 2000;13:320-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 150] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Jackson IJ. Molecular and developmental genetics of mouse coat color. Annu Rev Genet. 1994;28:189-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 183] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Yokoyama T, Silversides DW, Waymire KG, Kwon BS, Takeuchi T, Overbeek PA. Conserved cysteine to serine mutation in tyrosinase is responsible for the classical albino mutation in laboratory mice. Nucleic Acids Res. 1990;18:7293-7298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 161] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Beermann F, Ruppert S, Hummler E, Bosch FX, Müller G, Rüther U, Schütz G. Rescue of the albino phenotype by introduction of a functional tyrosinase gene into mice. EMBO J. 1990;9:2819-2826. [PubMed] |
| 7. | Beermann F, Schmid E, Ganss R, Schütz G, Ruppert S. Molecular characterization of the mouse tyrosinase gene: pigment cell-specific expression in transgenic mice. Pigment Cell Res. 1992;5:295-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Beermann F, Schmid E, Schütz G. Expression of the mouse tyrosinase gene during embryonic development: recapitulation of the temporal regulation in transgenic mice. Proc Natl Acad Sci USA. 1992;89:2809-2813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Ganss R, Montoliu L, Monaghan AP, Schütz G. A cell-specific enhancer far upstream of the mouse tyrosinase gene confers high level and copy number-related expression in transgenic mice. EMBO J. 1994;13:3083-3093. [PubMed] |
| 10. | Jeffery G, Brem G, Montoliu L. Correction of retinal abnormalities found in albinism by introduction of a functional tyrosinase gene in transgenic mice and rabbits. Brain Res Dev Brain Res. 1997;99:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Jeffery G, Schütz G, Montoliu L. Correction of abnormal retinal pathways found with albinism by introduction of a functional tyrosinase gene in transgenic mice. Dev Biol. 1994;166:460-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Kang JK, Kim JH, Lee SH, Kim DH, Kim HS, Lee JE, Seo JS. Development of spontaneous hyperplastic skin lesions and chemically induced skin papillomas in transgenic mice expressing human papillomavirus type 16 E6/E7 genes. Cancer Lett. 2000;160:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Klüppel M, Beermann F, Ruppert S, Schmid E, Hummler E, Schütz G. The mouse tyrosinase promoter is sufficient for expression in melanocytes and in the pigmented epithelium of the retina. Proc Natl Acad Sci USA. 1991;88:3777-3781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Porter SD, Hu J, Gilks CB. Distal upstream tyrosinase S/MAR-containing sequence has regulatory properties specific to subsets of melanocytes. Dev Genet. 1999;25:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Schedl A, Beermann F, Thies E, Montoliu L, Kelsey G, Schütz G. Transgenic mice generated by pronuclear injection of a yeast artificial chromosome. Nucleic Acids Res. 1992;20:3073-3077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Takeuchi T, Tanaka S, Tanaka M. Expression of tyrosinase gene in transgenic mice: programmed versus non-programmed expression. J Invest Dermatol. 1993;100:141S-145S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Tanaka S, Takeuchi T. Expression of tyrosinase gene in transgenic albino mice: the heritable patterned coat colors. Pigment Cell Res. 1992;5:300-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Tanaka S, Yamamoto H, Takeuchi S, Takeuchi T. Melanization in albino mice transformed by introducing cloned mouse tyrosinase gene. Development. 1990;108:223-227. [PubMed] |
| 19. | Aigner B, Brem G. Tyrosinase as a marker gene and model for screening transgenes in mice and rabbits. Theriogenology. 1993;39:177. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Hyodo-Taguchi Y, Winkler C, Kurihara Y, Schartl A, Schartl M. Phenotypic rescue of the albino mutation in the medakafish (Oryzias latipes) by a mouse tyrosinase transgene. Mech Dev. 1997;68:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Fu L, Mambrini M, Perrot E, Chourrout D. Stable and full rescue of the pigmentation in a medaka albino mutant by transfer of a 17 kb genomic clone containing the medaka tyrosinase gene. Gene. 2000;241:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Tseng FS, Liao IC, Tsai HJ. Transient expression of mouse tyrosinase gene in albino walking catfish Clarias fuscus by subcutaneous microinjection. Fish Sci. 1995;61:163. |
| 23. | Giraldo P, Montoliu L. Artificial chromosome transgenesis in pigmentary research. Pigment Cell Res. 2002;15:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Bockamp E, Maringer M, Spangenberg C, Fees S, Fraser S, Eshkind L, Oesch F, Zabel B. Of mice and models: improved animal models for biomedical research. Physiol Genomics. 2002;11:115-132. [PubMed] |
| 25. | van der Weyden L, Adams DJ, Bradley A. Tools for targeted manipulation of the mouse genome. Physiol Genomics. 2002;11:133-164. [PubMed] |
| 26. | Le Douarin N. The Neural Crest. Cambridge: Cambridge University Press 1997; 1-600. |
| 27. | Nagy A, Gertsensten M, Vintersten K, Behringer R. Manipulating the Mouse Embryo: A Laboratory Manual. 3rd ed. New York: Cold Spring Harbor Press 2003; 1-600. |
| 28. | Sambrook JE, Fritsch F, Maniatis T. Molecular Cloning: A Laboratory Manual. 3rd ed. New York: Cold Spring Harbor Laboratory Press 2001; 1-800. |
| 29. | Tymms MJ, Kola I. Gene knockout protocols. Totowa: Humana Press Inc 2001; 1-370. [DOI] [Full Text] |
| 30. | Overbeek PA. Factors affecting transgenic animal production. Transgenic Animal Technology: A Laboratory Handbook. San Diego: Academic Press Inc 1994; 69-114. [DOI] [Full Text] |
| 31. | Aigner B, Brem G. Tyrosinase as a marker gene for studying transmission and expression of transgenes in mice. Transgenics. 1994;1:417-429. |
| 32. | Methot D, Reudelhuber TL, Silversides DW. Evaluation of tyrosinase minigene co-injection as a marker for genetic manipulations in transgenic mice. Nucleic Acids Res. 1995;23:4551-4556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Overbeek PA, Aguilar-Cordova E, Hanten G, Schaffner DL, Patel P, Lebovitz RM, Lieberman MW. Coinjection strategy for visual identification of transgenic mice. Transgenic Res. 1991;1:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Porter SD, Meyer CJ. A distal tyrosinase upstream element stimulates gene expression in neural-crest-derived melanocytes of transgenic mice: position-independent and mosaic expression. Development. 1994;120:2103-2111. [PubMed] |
| 35. | Beermann F, Ruppert S, Hummler E, Schütz G. Tyrosinase as a marker for transgenic mice. Nucleic Acids Res. 1991;19:958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Montoliu L, Schedl A, Kelsey G, Lichter P, Larin Z, Lehrach H, Schütz G. Generation of transgenic mice with yeast artificial chromosomes. Cold Spring Harb Symp Quant Biol. 1993;58:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Potts W, Tucker D, Wood H, Martin C. Chicken beta-globin 5'HS4 insulators function to reduce variability in transgenic founder mice. Biochem Biophys Res Commun. 2000;273:1015-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Behringer RR, Ryan TM, Palmiter RD, Brinster RL, Townes TM. Human gamma- to beta-globin gene switching in transgenic mice. Genes Dev. 1990;4:380-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 243] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 39. | Kucera GT, Bortner DM, Rosenberg MP. Overexpression of an Agouti cDNA in the skin of transgenic mice recapitulates dominant coat color phenotypes of spontaneous mutants. Dev Biol. 1996;173:162-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |