Published online May 21, 2007. doi: 10.3748/wjg.v13.i19.2675
Revised: January 11, 2007
Accepted: February 25, 2007
Published online: May 21, 2007
Recent advances in cell and molecular radiobiology clearly showed that tissue response to radiation injury cannot be restricted to a simple cell-killing process, but depends upon continuous and integrated pathogenic processes, involving cell differentiation and crosstalk between the various cellular components of the tissue within the extracellular matrix. Thus, the prior concept of primary cell target in which a single-cell type (whatever it’s epithelial or endothelial cells) dictates the whole tissue response to radiation injury has to be replaced by the occurrence of coordinated multicellular response that may either lead to tissue recovery or to sequel development. In this context, the present review will focus on the maintenance of the radiation-induced wound healing and fibrogenic signals triggered by and through the microenvironment toward the mesenchymal cell compartment, and will highlight how sequential and sustained modifications in cell phenotypes will in cascade modify cell-to-cell interactions and tissue composition.
- Citation: Haydont V, Vozenin-Brotons MC. Maintenance of radiation-induced intestinal fibrosis: Cellular and molecular features. World J Gastroenterol 2007; 13(19): 2675-2683
- URL: https://www.wjgnet.com/1007-9327/full/v13/i19/2675.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i19.2675
Treatment of tumor by radiation therapy faces a crucial dilemma that is delivering sufficient radiation rate for tumor cure, while limiting, as far as possible, normal tissue exposure and injury. Despite the recent sophisticated irradiation modalities development, as 3D-conformal or intensity-modulated radiation therapy, increased radiation ballistic performance in pelvic and abdominal cancer treatment[1], the intestine remains a major dose-limiting organ. Indeed, chronic gastro-intestinal side effects (diarrhea, fecal urgency, proctitis, bleeding, fistula, etc.) affect the daily quality of life of 6% to 78% patients[2]. Moreover, 5% to 10% of patients will develop severe intestinal toxicity mainly characterized by intestinal narrowing and transmural fibrosis leading to obstruction[3]. Excessive deposition of collagens and extracellular matrix components in the submucosa induces the loss of compliance of the mucosa over the muscularis propria required for aboral propulsion. In addition, thickening of the intestinal wall contributes to stricture formation. Globally, this loss of compliance and the stricture formation lead to intestinal obstruction[4]. Although antioxidant-based anti-fibrotic treatments have been proposed to patients, including the combination of pentoxifylline and tocopherol[5,6], their efficacy in delayed radiation-induced intestinal toxicity is disputed[7] and surgical resection remains today the only therapeutic option for patients with delayed radiation enteropathy. These inconsistent clinical reports add confusion to the old, yet unresolved controversy about the reversibility of radiation fibrosis[8]. Thus, one challenge for translational research in radiopathology/radiotherapy is to characterize the specific molecular mechanisms and cellular contributions involved in the maintenance of fibrosis to define efficient curative strategies. We will see in the present review that contrary to the conventional wisdom, severe fibrotic lesions observed in human radiation enteropathy are highly dynamic[9,10], thus opening real perspective for therapeutic interventions. These curative strategies are particularly relevant in oncolology as they won’t interfere with anti-cancer treatments and would be applicable to treat established radiation injury in case of radiation accidents or acts of terrorism[11].
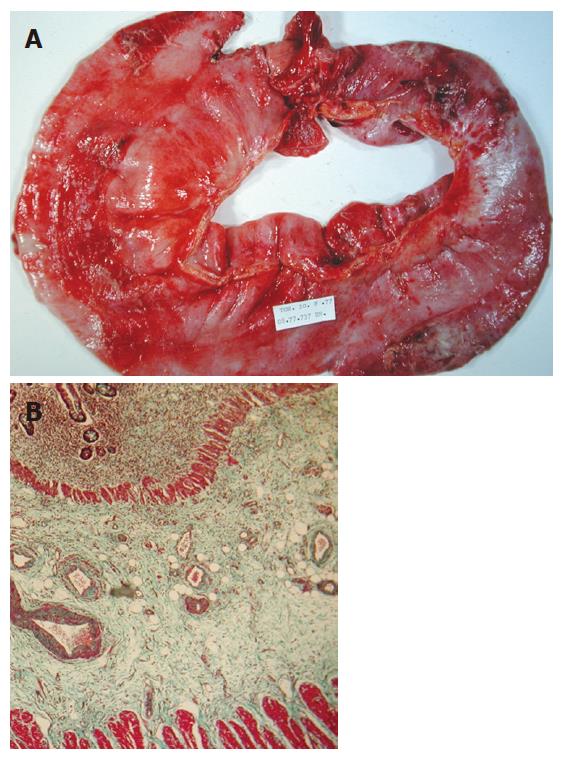
The main pathological feature of delayed radiation toxicity is the transmural fibrosis consisting of severe deposition of extracellular matrix component within the mucosa, submucosa, muscularis propria and subserosa (Figure 1). The number of crypts is reduced and a collagenous infiltration in the lamina propria is observed. Around the microvessels, accumulation of inflammatory cells suggesting an increased vascular permeability likely caused by endothelial cell damages. The muscularis mucosa is thickened with zones of complete disruption with infiltration of muscular-like structures within the submucosa. Submucosal layers always exhibit an altered but heterogeneous morphology. Some zones are composed of dense cords of collagen fibers with few fibroblasts whereas others are edematous or contained fibrosis-related fibroblasts and inflammatory cells located around hyalinized vessels with interlaced fibers. The muscularis propria is thickened and dystrophic with infiltration of connective septa. The Auerbach plexus, located between the circular and longitudinal muscular layers, are mostly hypertrophied. The subserosa also revealed a severe heterogeneous fibrosis containing newly formed microvessels, myofibroblasts, inflammatory cells, and paucicellular zones composed of stromal accumulation.
More than 30 years ago the World Health Organization proposed to define fibrosis “as the presence of excess collagen due to new fiber formation”[12]. The same is true for radiation fibrosis that was classically considered as a chronic and progressive process in which normal tissue is replaced by fixed and irreversible fibrotic tissue. This view has however been challenged and fibrosis has been recently redefined as a dynamic process resembling chronic wound healing[9,10,13].
The pathophysiological mechanisms of acute intestinal lesions after irradiation have been well investigated but the mechanisms underlying delayed radiation-induced intestinal complications and the precise sequence of cellular and molecular events that initiates fibrogenesis are still discussed. Classical radiobiological views presents radiation-induced tissue injury as the direct consequence of DNA damages and cell death induction in target cells, meaning that the severity of tissue damages would be directly related to cell depletion during the acute phase.
In murine models of acute gastrointestinal syndromes, the injury has been mainly attributed to apoptosis and depletion of both microvascular endothelial cells[14] and epithelial stem cells[15]. The primary role of the vascular compartment in triggering radiation-induced normal tissue damages was introduced more than 40 years ago by Rubin and Casarett[16]. Endothelial cell dysfunctions precede other cell-type response as well as fibrin deposition. In addition, peri-vascular edema always precedes collagen accumulation[17]. This vascular hypothesis represents a matter of debate because the endothelial compartment was seen as a single entity. The detractors of the vascular hypothesis argued that if endothelial cells were at the initiation of late damages the relationship between radiation dose and tissue lesion should be similar in all organs[18]. Today, the progress made in cell biology clearly demonstrate that endothelial cell phenotype depends upon the vessel type (artery, veins, micro-vessels) and the tissue[19]. These observations suggest that the biological effects of irradiation on endothelial cells might be tissue specific. Although specific responses of endothelial cells isolated from various tissues to ionizing radiation remains to be studied, their role in normal tissue response to radiation-injury is today undisputable.
In rodents, acute mucosal damage is required for the development of delayed intestinal complications[20,21]. These observations suggest that acute mucosal lesions contributed to late toxicity[22] and lead to the idea that increasing the pool of epithelial cells before irradiation would improve acute damages and inhibit the development of late injury[22]. This hypothesis has been fully validated in experimental models using mucosal trophic growth factors like KGF[23,24] and GLP-2[25]. Yet, the use of trophic factors faced a crucial problem in cancer patient related to their stimulatory action on tumor growth[26,27]. The functional consequences of radiation-induced epithelial depletion are probably far beyond the barrier function. One indirect consequence of the epithelial rupture is the exposure of the intestinal stroma to luminal flora, involved in specific lymphocyte T helper (TH) polarization. The role of TH orientation and the local production of specific cytokines associated with this polarization have been well investigated. On the one hand TH1 orientation, notably characterized by the secretion of interferon γ, is associated with resolution of the wound healing process. On the other hand TH2 orientation, characterized by the secretion of IL-4, IL-13 and TGF-β1, triggers tissue response toward fibrosis probably mediated by the pro-fibrotic growth factor: TGF-β1[28-30]. Exposure of intestinal stroma to bacteria is known to induce a TH1 polarization[29], but in the lung persistent exposure to bacterial antigens reorients TH1 polarization toward a TH2 profile suggesting that chronic epithelial depletion is fibrosis-prone[30]. In addition, seven days after γ-irradiation (10 Gy) a TH2 orientation has been described in rats[31], suggesting that a fibrosis-prone polarization occurs that remains to be fully characterized. The importance of the immune compartment to intestinal response to ionizing radiation.
The extrapolation from observations obtained in rodents to patients probably has to be moderated. Indeed, whether the consequential component occurs in radiotherapy patients is more controversial. Whereas, fractionation protocols significantly minimizes mucosal damages[32], late toxicity does occur[33]. In addition, several clinical reports showed the absence of correlation between the severity of early lesions and the probability of late effect development[34-37]. These data suggest that delayed tissue response to radiation injury depends upon continuous and integrated pathogenic processes, involving cell differentiation and crosstalk between the various cellular components of the tissue, within the extracellular matrix[38]. In this context, the role of the mesenchymal compartment (i.e. in the gut, smooth muscle cells, submucosa fibroblasts, subepithelial myofibroblasts and the extracellular matrix) is probably the most indubitable for the development and the maintenance of fibrosis as these cells are responsible of the pathological extracellular matrix accumulation observed in fibrosis.
Radiation-induced intestinal fibrosis is characterized by the accumulation of extracellular matrix due to a global deregulation of the synthesis/degradation balance[9,10]. Whether this dynamic remodeling process is a cause or a consequence of the phenotypic alteration of the resident mesenchymal cells is not known[33]. However, this pathological differentiation contributes to the intestinal loss of function and obstruction. After injury, tissue regeneration relies on the differentiation capacity of its resident cells. Thus, understanding the mechanisms involved in the differentiation of intestinal mesenchymal cells would provide new insight to design new therapeutic strategies.
After injury, the loss of dermal homeostasis induces mechanical tension in the clot. This contractile stress added to the secretion of cytokines, such as PDGF, triggers fibroblast recruitment and their morphological change[39] into proto-myofibroblasts[40]. Then, the mechanical tension associated with ED-A fibronectin and TGF-β1 deposition induce the differentiation of proto-myofibroblasts into myofibroblasts. The latters are mainly characterized by altered cytoskeleton with prominent stress fibers (composed of α-Sm actin, myosin, tropomyosin, α-actinin and filamin), anchored at the cytoplasmic membrane by molecular complex named the focal adhesion point in-vitro and the fibronexus in-vivo[41]. These focal adhesion points connect the actin cytoskeleton to the extracellular matrix via integrin receptors and control the mechanical exchanges between the myofibroblats and the extracellular matrix. Subsequently, contraction of the granulation tissue occurs and leads to wound healing closure[42,43]. In addition, myofibroblasts are connected directly to each other through gap junctions, composed of several hemichannels containing distinct but functionally related proteins called connexins[44]. Thus, myofibroblasts might form a syncytial structure composed of multicellular contractile units. In summary, the myofibroblastic differentiation is an intermediate differentiation between fibroblast and smooth muscle cells and ensured the contraction of the granulation tissue and the neo-synthesis of the extracellular matrix.
In the intestine, the mesenchymal compartment is composed of 3 cell types: the sub-epithelial myofibroblasts, the submucosal fibroblasts and the smooth muscle cells of muscularis mucosa and muscularis propria. The respective contribution of these 3 cell types to fibrosis is not clearly defined, but the pathological collagen deposition seemed mainly achieved by smooth muscle cells[45], and their differentiation profile seemed comparable at the molecular level[46].
After injury, the differentiation of smooth muscle cells is characterized by a phenotypic switch from a contractile function to a secretory activity[47]. This switch is associated with cytoskeleton modifications defined using the VDA classification proposed by Gabbiani : Vimentin-Desmin-α-sm Actin[48]. In human intestinal radiation-induced fibrosis, an overall increase in the relative number of cells defined as fibroblasts/myofibroblasts were detected in the mucosa (V+/D-/A+) and submucosa (V+/D-/A-/+), differentiated smooth muscle cells are also found in the hyalinized vessel wall (V+/D-/A+) and in the dystrophic muscularis propria (V-/D+/A+)33. The strong plasticity of smooth muscle cells allowed profound alterations in their phenotype in response to changes in local environment[47,49] and in return these differentiated smooth muscle cells controlled tissue response.
For a long time, the conventional wisdom presented mesenchymal cell differentiation in radiation-induced fibrosis as terminal and thus irreversible[50,51]. This hypothesis was based on phenotypical characterizations performed on fibroblasts irradiated in vitro, which exhibit a premature senescent and pro-secretory phenotype (extracellular matrix secretion). Thus, necrosis or apoptosis of these fibrosis-activated mesenchymal cells were the unique solution conceivable to cure fibrosis[13,52].
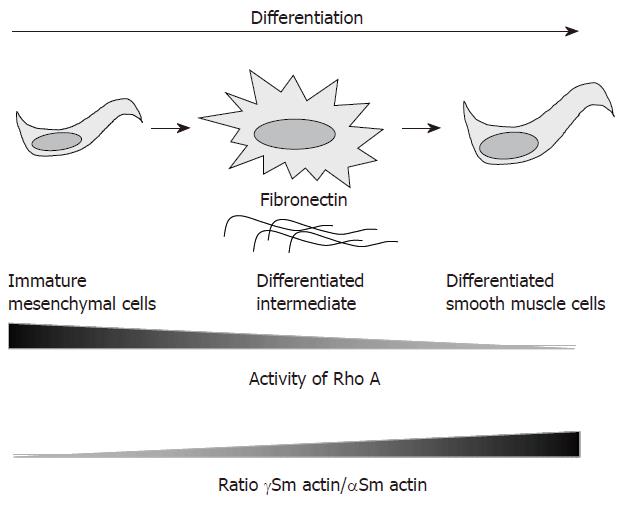
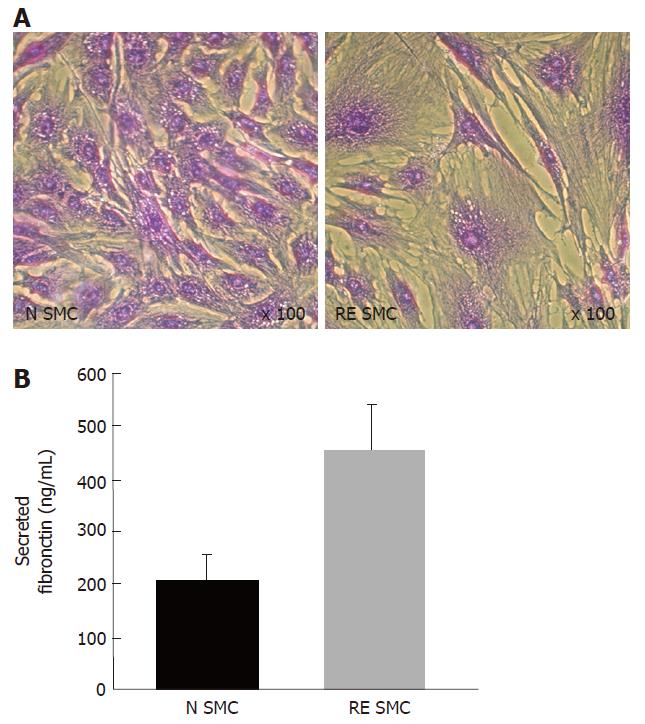
Further investigation on the characterization of the fibrogenic differentiation of intestinal smooth muscle cells isolated from radiation enteropathy suggested another hypothesis, in which fibrosis-derived intestinal smooth muscle cells (RE-SMC) seemed more immature than their normal counterpart (N-SMC). The first evidence was given by cytoskeleton analysis since an alteration of the γ-Sm actin/α-Sm actin ratio was found. Indeed, RE-SMCs exhibit higher expression level of the α-Sm actin than their normal counterparts whereas the level γ-Sm actin remained stable[53]. Because this ratio is an indicator of intestinal smooth muscle cell differentiation[54] i.e. increased ratio indicates a differentiated phenotype whereas decreased ratio reveals immaturity, the profile found in RE-SMC suggests the maintenance of an immature phenotype (Figure 2). This immaturity is further supported by the comparison study by Beqaj et al[55], who demonstrated an inverse correlation between smooth muscle cell differentiation during bronchial myogenesis and Rho activity: i.e. Rho activity decreased when cells became mature. In RE-SMC, a global profiling approach performed by cDNA array revealed a deregulation of the genes coding for Rho pathway as compared to N-SMC[53]. Furthermore the Rho pathway is preferentially activated upon TGF-β1 stimulation in RE-SMC[56]. The last evidence is related to the extracellular matrix composition and in particular to the secretion of fibronectin. Fibronectin is known to control the differentiation of smooth muscle cells in the lung. Indeed, Bequaj et al[55] have been able to isolate a specific cell-type that spreads on fibronectin and exhibits an intermediate differentiation status between mesenchyme precursors and bronchial smooth muscle cells. These intermediate cells are larger and more spread than the differentiated lung smooth muscle cells (Figure 2). Similarly RE-SMC are more spread and larger than their normal counterparts (Figure 3A) and secrete high level of fibronectin (Figure 3B). This immaturity concept has important clinical implications as it suggests that fibrotic tissue has high regenerative potential and imply that fibrosis might be reversible.
If defining the differentiation status of fibrosis-related cells is essential to investigate the regenerative potential of irradiated tissue, another important issue is to characterize the mediators involved in the maintenance of this pathological phenotype. Numerous actors are involved, yet we choose to focus on important mediators involved in the criss-cross relationship between mesenchymal cells and their micro-environment leading to the establishment of sequential and chronic activation loops.
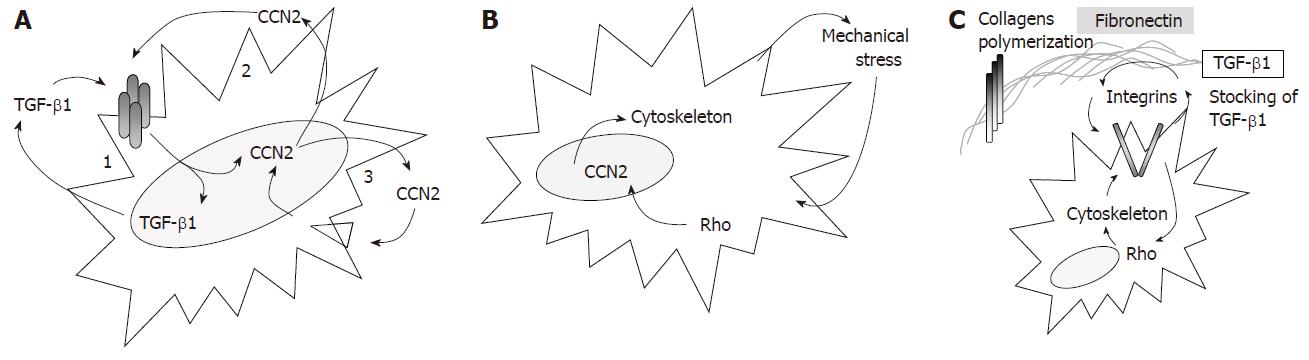
TGF-β1 is a pleïotropic cytokine involved in the regulation of various biological processes including maturation of the immune cells, proliferation, differentiation, apoptosis as well as normal and patholo-gical wound healing response[57]. In the context of the maintenance of fibrosis, one relevant function of TGF-β1 is related to its self-induction ability[58-62]. This auto-induction, mainly triggered by the transcription factor AP-1, probably constitutes one of the first fibrogenic activation loops contributing to fibrosis maintenance by persistent extracellular matrix production and continuous induction of the fibrogenic differentiation of mesenchymal cells (Figure 4A).
CCN2 (also called CTGF) is another relevant growth factor involved in the maintenance of the fibrogenic differentiation and in the control of the extracellular matrix remodeling. In normal tissue, CCN2 is absent or expressed at extremely low concentration, whereas it is highly and specifically expressed in established fibrotic tissue[63] including Crohn disease[64] and intestinal radiation-induced fibrosis[33]. As TGF-β1, CCN2 exhibits auto-induction properties constituting another chronic activation loop particularly relevant for the maintenance of fibrosis as it seems restricted to fibrosis-derived cells[65] (Figure 4A). The cooperation between CCN2 and TGF-β1 constitutes an additional chronic activation loop: TGF-β1 is one of the primary inductor of CCN2[66,67] which in return enhances TGF-β1 binding to the TGF-β-type II receptor and increases Smad pathway activation[68] (Figure 4A). Yet, the mechanisms involved in the sustained and constitutive expression of CCN2 found in long-term established fibrosis is rather obscure. Thereby, in delayed radiation enteropathy[33] and scleroderma[69,70], a paradoxal situation occurs as in-situ TGF-β1 deposition is low, whereas CCN2 is highly expressed and correlates with the severity of the pathology. To explain this paradox, Grotendorst et al[71] proposed that transient TGF-β1 induction might trigger long-term mesenchymal cell differentiation and CCN2 expression, that perpetuates in time without TGF-β1, but additional cooperative signals between the cells and their microenvironment might be involved.
First, the mechanical stress produced by wound contraction triggered the chronic production of CCN2 by direct transactivation of the CCN2 gene expression[72] via the stretch-responsive element located in its promoter. This mechanical stress-induced CCN2 activation occurs through Rho/Rho kinase (ROCK) pathway activation and stress fibers polymerization[73] thus generating novel mechanical stress and subsequent CCN2 activation (Figure 4B). Second, CCN2 triggers fibronectin over-secretion by fibrosis-derived cells[74]. In return, fibronectin has a crucial role in the maintenance of fibrosis, acting in combination with CCN2 to sustain CCN2 own expression[75], controlling the extracellular matrix sequestration of TGF-β1 through its latent complex LTBP-1/TGF-β1[76] and playing an essential structural role in collagen network formation (i.e. polymerization of the fibrillar collagens type I and III).[77,78] Beyond these direct fibrogenic actions, fibronectin binding to the α5β1 and αvβ3 integrins activates the Rho pathway[79], thus increasing the proliferation and differentiation of the smooth muscle cells[80] and regulating the cytoskeleton polymerization (Figure 4C). These structural actions of fibronectin rely on its integrity, that might be damaged by the reactive oxygen species produced upon irradiation (H2O2) and might thus triggered the fibrogenic differentiation of human lung fibroblasts[81,82].
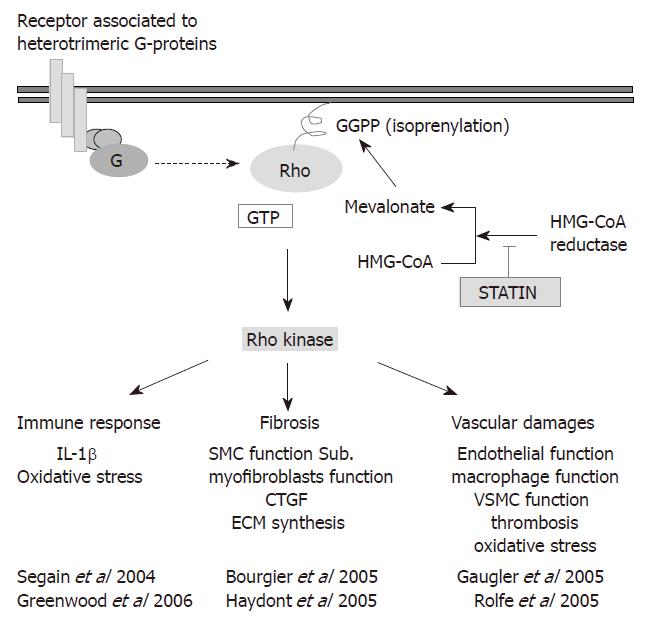
Interestingly these various chronic activation loops involved in the sustained expression of CCN2, depend upon the Rho pathway. Furthermore, despite the high constitutive expression of CCN2 in fibrosis-derived cells[46,53,56], the canonical TGF-β/Smad3/4 pathway is only poorly activated after stimulation with TGF-β1 in dermal myofibroblasts[83] and intestinal smooth muscle cells isolated from radiation fibrosis[56]. Indeed the Smad3/4 pathway is activated in cells derived from normal tissue, whereas in the fibrosis-derived cells the TGF-β1 signal is mainly transduced by the Rho/ROCK pathway[56]. The Rho proteins are small GTPases (from “Ras homologous”) acting as molecular switches to control a wide range of cellular functions like cell adhesion, formation of stress fibers, and cellular contractility through the reorganization of actin-based cytoskeletal structures. These functions are accomplished specifically via their effectors, the ROCKs[84-87], after Rho anchorage to the cell membrane by prenylation[88]. The anchorage allows cycling between the inactivated GDP-bound form to the activated GTP-bound, also controlled by specific activators/inhibitors: the GEFs (Guanosine nucleotide exchange factor), the GDI (Guanine dissociation inhibitors) and the GAP (GTPase Activating Protein) (for review[84,89,90]). Thus, Rho activity might be controlled by inhibitors of HMG-CoA reductase including the statins, that might provide safe and efficient tools for the development of anti-fibrotic strategies.
The development of curative anti-fibrotic strategy is nowadays highly expected by both patients and physicians[8]. Indeed, the high efficacy of the current anti-cancer treatments increases patient’s overall survival, but also increases late complications occurrence especially in the gut[2]. The development of high-throughput biological approaches highlighted by the recent concept of cellular plasticity helps answering this difficult question by the identification of biologically-based therapeutic targets. Thus, targeting one central pathway involved in vascular, immune, and stromal pathogenic response would provide an efficient anti-fibrotic strategy and thus we propose that targeting the Rho/ROCK pathway may help to achieve this aim (Figure 5)[53,56,91,94].
The Rho pathway is known to control vascular functions[94] mediating endothelial barrier functions, inflammation and transendothelial leukocyte migration, platelet activation, thrombosis, and oxidative stress, as well as the homeostasis of vascular smooth muscle cells[93,95-97]. It also controls immune functions[92] as pharmacological inhibitors of Rho including the statins modulate the TH1/TH2 balance thus interfering with chronic inflammation[92,98]. Consistently, statins (lovastatin) displays an anti-fibrotic efficacy in a mice model of radiation-induced pulmonary fibrosis[99]. In addition, reversion of the fibrogenic phenotype of intestinal smooth muscle cells isolated from human radiation enteropathy was shown using Rho (pravastatin)[56] and ROCK (Y-27632) inhibitors[53]. These observations open new perspective for anti-fibrotic therapies by specific inhibition of the Rho/ROCK pathway.
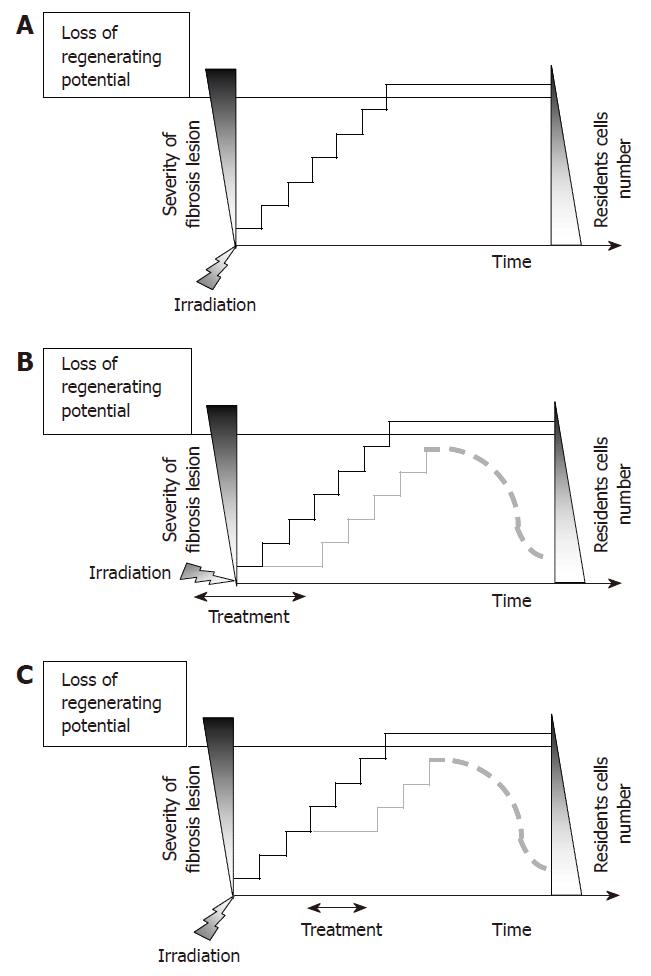
In light of several investigations on the differentiation status of mesenchymal cells during fibrosis, it appeared that the secretory phenotype of pathological cells would be associated to a less maturation compared to such in normal cells. Thus, the biochemical maintenance of radiation fibrosis is a complex process that depends upon continuous and integrated activation loops involving cell differentiation, and crosstalk between the various cellular components of the tissue within the matrix[38]. However, the time and kinetic notion has never been evoked to explain the maintenance of fibrosis. Yet, fibrotic pathogenesis is progressive and results from successive and sequential induction of chronic molecular activation loops. The evolution of the pathology during time correlates with a decrease in the cell number present within the tissue[5] and with a progressive loss of regenerating potential. In this context, when a new molecular loop is activated, the severity of fibrosis increases and correlates to decreased number in the resident cell number (Figure 6A). As a consequence, both preventive (Figure 6B) and curative (Figure 6C) therapeutic strategies might be efficient since inhibition of one or several steps would inhibit fibrosis evolution and preserve tissue-regenerating potential.
VH is fellow of IRSN/region Ile de France. MCVB group is supported by “Association pour la Recherche sur le cancer” grant n°3881.
S- Editor Liu Y L- Editor Glaser SS E- Editor Ma WH
| 1. | Nutting CM, Convery DJ, Cosgrove VP, Rowbottom C, Padhani AR, Webb S, Dearnaley DP. Reduction of small and large bowel irradiation using an optimized intensity-modulated pelvic radiotherapy technique in patients with prostate cancer. Int J Radiat Oncol Biol Phys. 2000;48:649-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 172] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 2. | Andreyev J. Gastrointestinal complications of pelvic radiotherapy: are they of any importance. Gut. 2005;54:1051-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 157] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 3. | Hauer-Jensen M, Wang J, Denham JW. Bowel injury: current and evolving management strategies. Semin Radiat Oncol. 2003;13:357-371. [PubMed] |
| 4. | Graham MF. Pathogenesis of intestinal strictures in Crohn's disease-an update. Inflamm Bowel Dis. 1995;1:220-227. [PubMed] |
| 5. | Delanian S, Lefaix JL. The radiation-induced fibroatrophic process: therapeutic perspective via the antioxidant pathway. Radiother Oncol. 2004;73:119-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 448] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 6. | Delanian S, Porcher R, Rudant J, Lefaix JL. Kinetics of response to long-term treatment combining pentoxifylline and tocopherol in patients with superficial radiation-induced fibrosis. J Clin Oncol. 2005;23:8570-8579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Gothard L, Cornes P, Brooker S, Earl J, Glees J, Hall E, Peckitt C, Tait D, Yarnold J. Phase II study of vitamin E and pentoxifylline in patients with late side effects of pelvic radiotherapy. Radiother Oncol. 2005;75:334-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Anscher MS. The irreversibility of radiation-induced fibrosis: fact or folklore. J Clin Oncol. 2005;23:8551-8552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Hovdenak N, Wang J, Sung CC, Kelly T, Fajardo LF, Hauer-Jensen M. Clinical significance of increased gelatinolytic activity in the rectal mucosa during external beam radiation therapy of prostate cancer. Int J Radiat Oncol Biol Phys. 2002;53:919-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Strup-Perrot C, Mathé D, Linard C, Violot D, Milliat F, François A, Bourhis J, Vozenin-Brotons MC. Global gene expression profiles reveal an increase in mRNA levels of collagens, MMPs, and TIMPs in late radiation enteritis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G875-G885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Augustine AD, Gondré-Lewis T, McBride W, Miller L, Pellmar TC, Rockwell S. Animal models for radiation injury, protection and therapy. Radiat Res. 2005;164:100-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Anthony PP, Ishak KG, Nayak NC, Poulsen HE, Scheuer PJ, Sobin LH. The morphology of cirrhosis. Recommendations on definition, nomenclature, and classification by a working group sponsored by the World Health Organization. J Clin Pathol. 1978;31:395-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 309] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10:76-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 538] [Cited by in RCA: 610] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 14. | Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, Haimovitz-Friedman A, Cordon-Cardo C, Kolesnick R. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 966] [Cited by in RCA: 950] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 15. | Potten CS. Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiat Res. 2004;161:123-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 16. | Rubin P, Casarett G. Clinical radiation Pathology. 1968;193-240. |
| 17. | Law MP. Radiation-induced vascular injury and its relation to late effects in normal tissues. Adv Radiat Biol. 1981;9:37-73. [RCA] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 89] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Hall EJ. Radiobiology for the radiobiologist, Fifth Edition. Philadelphia: Lippincott Williams & Wilkins 2001; . |
| 19. | Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci USA. 2003;100:10623-10628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 578] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 20. | Sartor RB. Current concepts of the etiology and pathogenesis of ulcerative colitis and Crohn's disease. Gastroenterol Clin North Am. 1995;24:475-507. [PubMed] |
| 21. | Denham JW, Hauer-Jensen M, Kron T, Langberg CW. Treatment-time-dependence models of early and delayed radiation injury in rat small intestine. Int J Radiat Oncol Biol Phys. 2000;48:871-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Dörr W, Hendry JH. Consequential late effects in normal tissues. Radiother Oncol. 2001;61:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 291] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 23. | Dörr W, Spekl K, Farrell CL. Amelioration of acute oral mucositis by keratinocyte growth factor: fractionated irradiation. Int J Radiat Oncol Biol Phys. 2002;54:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Terry NH, Brinkley J, Doig AJ, Ma J, Patel N, White RA, Mahajan N, Kang Y. Cellular kinetics of murine lung: model system to determine basis for radioprotection with keratinocyte growth factor. Int J Radiat Oncol Biol Phys. 2004;58:435-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Booth C, Booth D, Williamson S, Demchyshyn LL, Potten CS. Teduglutide ([Gly2]GLP-2) protects small intestinal stem cells from radiation damage. Cell Prolif. 2004;37:385-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Thulesen J, Hartmann B, Hare KJ, Kissow H, Ørskov C, Holst JJ, Poulsen SS. Glucagon-like peptide 2 (GLP-2) accelerates the growth of colonic neoplasms in mice. Gut. 2004;53:1145-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Finch PW, Rubin JS. Keratinocyte growth factor expression and activity in cancer: implications for use in patients with solid tumors. J Natl Cancer Inst. 2006;98:812-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1132] [Cited by in RCA: 1313] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 29. | Bamias G, Martin C, Mishina M, Ross WG, Rivera-Nieves J, Marini M, Cominelli F. Proinflammatory effects of TH2 cytokines in a murine model of chronic small intestinal inflammation. Gastroenterology. 2005;128:654-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 30. | Strieter RM, Keane MP. Innate immunity dictates cytokine polarization relevant to the development of pulmonary fibrosis. J Clin Invest. 2004;114:165-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Grémy O, Benderitter M, Linard C. Caffeic acid phenethyl ester modifies the Th1/Th2 balance in ileal mucosa after gamma-irradiation in the rat by modulating the cytokine pattern. World J Gastroenterol. 2006;12:4996-5004. [PubMed] |
| 32. | Hovdenak N, Fajardo LF, Hauer-Jensen M. Acute radiation proctitis: a sequential clinicopathologic study during pelvic radiotherapy. Int J Radiat Oncol Biol Phys. 2000;48:1111-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 112] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Vozenin-Brotons MC, Milliat F, Sabourin JC, de Gouville AC, François A, Lasser P, Morice P, Haie-Meder C, Lusinchi A, Antoun S. Fibrogenic signals in patients with radiation enteritis are associated with increased connective tissue growth factor expression. Int J Radiat Oncol Biol Phys. 2003;56:561-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Bentzen SM, Overgaard M, Thames HD, Christensen JJ, Overgaard J. Early and late normal-tissue injury after postmastectomy radiotherapy alone or combined with chemotherapy. Int J Radiat Biol. 1989;56:711-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Bentzen SM, Overgaard M. Relationship between early and late normal-tissue injury after postmastectomy radiotherapy. Radiother Oncol. 1991;20:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 143] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Bentzen SM, Overgaard M, Overgaard J. Clinical correlations between late normal tissue endpoints after radiotherapy: implications for predictive assays of radiosensitivity. Eur J Cancer. 1993;29A:1373-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Bourhis J, Lapeyre M, Tortochaux J, Rives M, Aghili M, Bourdin S, Lesaunier F, Benassi T, Lemanski C, Geoffrois L. Phase III randomized trial of very accelerated radiation therapy compared with conventional radiation therapy in squamous cell head and neck cancer: a GORTEC trial. J Clin Oncol. 2006;24:2873-2878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 38. | Barcellos-Hoff MH, Costes SV. A systems biology approach to multicellular and multi-generational radiation responses. Mutat Res. 2006;597:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3466] [Cited by in RCA: 3334] [Article Influence: 119.1] [Reference Citation Analysis (0)] |
| 40. | Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2964] [Cited by in RCA: 3158] [Article Influence: 137.3] [Reference Citation Analysis (0)] |
| 41. | Singer II, Kawka DW, Kazazis DM, Clark RA. In vivo co-distribution of fibronectin and actin fibers in granulation tissue: immunofluorescence and electron microscope studies of the fibronexus at the myofibroblast surface. J Cell Biol. 1984;98:2091-2106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 200] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 42. | Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730-2741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 1031] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 43. | Hinz B, Gabbiani G. Mechanisms of force generation and transmission by myofibroblasts. Curr Opin Biotechnol. 2003;14:538-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 306] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 44. | Gabbiani G, Chaponnier C, Hüttner I. Cytoplasmic filaments and gap junctions in epithelial cells and myofibroblasts during wound healing. J Cell Biol. 1978;76:561-568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 226] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 45. | Zheng H, Wang J, Hauer-Jensen M. Role of mast cells in early and delayed radiation injury in rat intestine. Radiat Res. 2000;153:533-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Haydont V, Bourgier C, Vozenin-Brotons MC. Rho/ROCK pathway as a molecular target for modulation of intestinal radiation-induced toxicity. Br J Radiol. 2007;80 Spec No 1:S32-S40. [PubMed] |
| 47. | Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2373] [Cited by in RCA: 2640] [Article Influence: 125.7] [Reference Citation Analysis (0)] |
| 48. | Gabbiani G. Modulation of fibroblastic cytoskeletal features during wound healing and fibrosis. Pathol Res Pract. 1994;190:851-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 49. | Hendrix JA, Wamhoff BR, McDonald OG, Sinha S, Yoshida T, Owens GK. 5' CArG degeneracy in smooth muscle alpha-actin is required for injury-induced gene suppression in vivo. J Clin Invest. 2005;115:418-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 50. | Bayreuther K, Rodemann HP, Hommel R, Dittmann K, Albiez M, Francz PI. Human skin fibroblasts in vitro differentiate along a terminal cell lineage. Proc Natl Acad Sci USA. 1988;85:5112-5116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 268] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 51. | Rodemann HP, Bamberg M. Cellular basis of radiation-induced fibrosis. Radiother Oncol. 1995;35:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 247] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 52. | Desmoulière A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146:56-66. [PubMed] |
| 53. | Bourgier C, Haydont V, Milliat F, François A, Holler V, Lasser P, Bourhis J, Mathé D, Vozenin-Brotons MC. Inhibition of Rho kinase modulates radiation induced fibrogenic phenotype in intestinal smooth muscle cells through alteration of the cytoskeleton and connective tissue growth factor expression. Gut. 2005;54:336-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 54. | Brittingham J, Phiel C, Trzyna WC, Gabbeta V, McHugh KM. Identification of distinct molecular phenotypes in cultured gastrointestinal smooth muscle cells. Gastroenterology. 1998;115:605-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 55. | Beqaj S, Jakkaraju S, Mattingly RR, Pan D, Schuger L. High RhoA activity maintains the undifferentiated mesenchymal cell phenotype, whereas RhoA down-regulation by laminin-2 induces smooth muscle myogenesis. J Cell Biol. 2002;156:893-903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 56. | Haydont V, Mathé D, Bourgier C, Abdelali J, Aigueperse J, Bourhis J, Vozenin-Brotons MC. Induction of CTGF by TGF-beta1 in normal and radiation enteritis human smooth muscle cells: Smad/Rho balance and therapeutic perspectives. Radiother Oncol. 2005;76:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 57. | Massagué J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1828] [Cited by in RCA: 1866] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 58. | Kim SJ, Denhez F, Kim KY, Holt JT, Sporn MB, Roberts AB. Activation of the second promoter of the transforming growth factor-beta 1 gene by transforming growth factor-beta 1 and phorbol ester occurs through the same target sequences. J Biol Chem. 1989;264:19373-19378. [PubMed] |
| 59. | Kelley J, Shull S, Walsh JJ, Cutroneo KR, Absher M. Auto-induction of transforming growth factor-beta in human lung fibroblasts. Am J Respir Cell Mol Biol. 1993;8:417-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Martin M, Vozenin MC, Gault N, Crechet F, Pfarr CM, Lefaix JL. Coactivation of AP-1 activity and TGF-beta1 gene expression in the stress response of normal skin cells to ionizing radiation. Oncogene. 1997;15:981-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 61. | Martin M, Lefaix J, Delanian S. TGF-beta1 and radiation fibrosis: a master switch and a specific therapeutic target. Int J Radiat Oncol Biol Phys. 2000;47:277-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 501] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 62. | Bonniaud P, Margetts PJ, Ask K, Flanders K, Gauldie J, Kolb M. TGF-beta and Smad3 signaling link inflammation to chronic fibrogenesis. J Immunol. 2005;175:5390-5395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 206] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 63. | Rachfal AW, Brigstock DR. Structural and functional properties of CCN proteins. Vitam Horm. 2005;70:69-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 139] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 64. | Dammeier J, Brauchle M, Falk W, Grotendorst GR, Werner S. Connective tissue growth factor: a novel regulator of mucosal repair and fibrosis in inflammatory bowel disease. Int J Biochem Cell Biol. 1998;30:909-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 123] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 65. | Riser BL, Denichilo M, Cortes P, Baker C, Grondin JM, Yee J, Narins RG. Regulation of connective tissue growth factor activity in cultured rat mesangial cells and its expression in experimental diabetic glomerulosclerosis. J Am Soc Nephrol. 2000;11:25-38. [PubMed] |
| 66. | Kucich U, Rosenbloom JC, Herrick DJ, Abrams WR, Hamilton AD, Sebti SM, Rosenbloom J. Signaling events required for transforming growth factor-beta stimulation of connective tissue growth factor expression by cultured human lung fibroblasts. Arch Biochem Biophys. 2001;395:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 67. | Blom IE, Goldschmeding R, Leask A. Gene regulation of connective tissue growth factor: new targets for antifibrotic therapy. Matrix Biol. 2002;21:473-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 277] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 68. | Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4:599-604. [PubMed] |
| 69. | Leask A. Transcriptional profiling of the scleroderma fibroblast reveals a potential role for connective tissue growth factor (CTGF) in pathological fibrosis. Keio J Med. 2004;53:74-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 70. | Sato S, Nagaoka T, Hasegawa M, Tamatani T, Nakanishi T, Takigawa M, Takehara K. Serum levels of connective tissue growth factor are elevated in patients with systemic sclerosis: association with extent of skin sclerosis and severity of pulmonary fibrosis. J Rheumatol. 2000;27:149-154. [PubMed] |
| 71. | Grotendorst GR, Rahmanie H, Duncan MR. Combinatorial signaling pathways determine fibroblast proliferation and myofibroblast differentiation. FASEB J. 2004;18:469-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 215] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 72. | Schild C, Trueb B. Mechanical stress is required for high-level expression of connective tissue growth factor. Exp Cell Res. 2002;274:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 73. | Chaqour B, Yang R, Sha Q. Mechanical stretch modulates the promoter activity of the profibrotic factor CCN2 through increased actin polymerization and NF-kappaB activation. J Biol Chem. 2006;281:20608-20622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 74. | Frazier K, Williams S, Kothapalli D, Klapper H, Grotendorst GR. Stimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. J Invest Dermatol. 1996;107:404-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 570] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 75. | Chen Y, Abraham DJ, Shi-Wen X, Pearson JD, Black CM, Lyons KM, Leask A. CCN2 (connective tissue growth factor) promotes fibroblast adhesion to fibronectin. Mol Biol Cell. 2004;15:5635-5646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 76. | Dallas SL, Sivakumar P, Jones CJ, Chen Q, Peters DM, Mosher DF, Humphries MJ, Kielty CM. Fibronectin regulates latent transforming growth factor-beta (TGF beta) by controlling matrix assembly of latent TGF beta-binding protein-1. J Biol Chem. 2005;280:18871-18880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 255] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 77. | Velling T, Risteli J, Wennerberg K, Mosher DF, Johansson S. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins alpha 11beta 1 and alpha 2beta 1. J Biol Chem. 2002;277:37377-37381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 291] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 78. | Sottile J, Hocking DC. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol Biol Cell. 2002;13:3546-3559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 475] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 79. | Bourdoulous S, Orend G, MacKenna DA, Pasqualini R, Ruoslahti E. Fibronectin matrix regulates activation of RHO and CDC42 GTPases and cell cycle progression. J Cell Biol. 1998;143:267-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 169] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 80. | Mack CP, Somlyo AV, Hautmann M, Somlyo AP, Owens GK. Smooth muscle differentiation marker gene expression is regulated by RhoA-mediated actin polymerization. J Biol Chem. 2001;276:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 309] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 81. | Skalli O, Schürch W, Seemayer T, Lagacé R, Montandon D, Pittet B, Gabbiani G. Myofibroblasts from diverse pathologic settings are heterogeneous in their content of actin isoforms and intermediate filament proteins. Lab Invest. 1989;60:275-285. [PubMed] |
| 82. | Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM, Thomas PE. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278:12384-12389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 501] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 83. | Reisdorf P, Lawrence DA, Sivan V, Klising E, Martin MT. Alteration of transforming growth factor-beta1 response involves down-regulation of Smad3 signaling in myofibroblasts from skin fibrosis. Am J Pathol. 2001;159:263-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 84. | Kaibuchi K, Kuroda S, Fukata M, Nakagawa M. Regulation of cadherin-mediated cell-cell adhesion by the Rho family GTPases. Curr Opin Cell Biol. 1999;11:591-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 152] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 85. | Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1440] [Cited by in RCA: 1515] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 86. | Palazzo AF, Eng CH, Schlaepfer DD, Marcantonio EE, Gundersen GG. Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science. 2004;303:836-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 341] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 87. | Guan JL. Cell biology. Integrins, rafts, Rac, and Rho. Science. 2004;303:773-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 88. | Wheeler AP, Ridley AJ. Why three Rho proteins RhoA, RhoB, RhoC, and cell motility. Exp Cell Res. 2004;301:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 374] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 89. | Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348 Pt 2:241-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 371] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 90. | Ridley AJ. Rho family proteins: coordinating cell responses. Trends Cell Biol. 2001;11:471-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 572] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 91. | Segain JP, Raingeard de la Blétière D, Sauzeau V, Bourreille A, Hilaret G, Cario-Toumaniantz C, Pacaud P, Galmiche JP, Loirand G. Rho kinase blockade prevents inflammation via nuclear factor kappa B inhibition: evidence in Crohn's disease and experimental colitis. Gastroenterology. 2003;124:1180-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 156] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 92. | Greenwood J, Steinman L, Zamvil SS. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nat Rev Immunol. 2006;6:358-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 530] [Cited by in RCA: 514] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 93. | Gaugler MH, Vereycken-Holler V, Squiban C, Vandamme M, Vozenin-Brotons MC, Benderitter M. Pravastatin limits endothelial activation after irradiation and decreases the resulting inflammatory and thrombotic responses. Radiat Res. 2005;163:479-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 94. | Rolfe BE, Worth NF, World CJ, Campbell JH, Campbell GR. Rho and vascular disease. Atherosclerosis. 2005;183:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 95. | Shi J, Wang J, Zheng H, Ling W, Joseph J, Li D, Mehta JL, Ponnappan U, Lin P, Fink LM. Statins increase thrombomodulin expression and function in human endothelial cells by a nitric oxide-dependent mechanism and counteract tumor necrosis factor alpha-induced thrombomodulin downregulation. Blood Coagul Fibrinolysis. 2003;14:575-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 96. | Shiga N, Hirano K, Hirano M, Nishimura J, Nawata H, Kanaide H. Long-term inhibition of RhoA attenuates vascular contractility by enhancing endothelial NO production in an intact rabbit mesenteric artery. Circ Res. 2005;96:1014-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 97. | Veillard NR, Braunersreuther V, Arnaud C, Burger F, Pelli G, Steffens S, Mach F. Simvastatin modulates chemokine and chemokine receptor expression by geranylgeranyl isoprenoid pathway in human endothelial cells and macrophages. Atherosclerosis. 2006;188:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 98. | Weitz-Schmidt G. Statins as anti-inflammatory agents. Trends Pharmacol Sci. 2002;23:482-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 250] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 99. | Williams JP, Hernady E, Johnston CJ, Reed CM, Fenton B, Okunieff P, Finkelstein JN. Effect of administration of lovastatin on the development of late pulmonary effects after whole-lung irradiation in a murine model. Radiat Res. 2004;161:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |