Published online Apr 28, 2007. doi: 10.3748/wjg.v13.i16.2319
Revised: December 3, 2006
Accepted: January 30, 2007
Published online: April 28, 2007
AIM: To enrich putative hepatic progenitors from the developing human fetal liver using CD34 as a marker.
METHODS: Aborted fetuses of 13-20 wk were used for the isolation of liver cells. The cells were labeled with anti CD34; a marker used for isolating progenitor population and the cells were sorted using magnetic cell sorting. The positive fractions of cells were assessed for specific hepatic markers. Further, these cells were cultured in vitro for long term investigation.
RESULTS: Flow cytometric and immunocytochemical analysis for alphafetoprotein (AFP) showed that the majority of the enriched CD34 positive cells were positive for AFP. Furthermore, these enriched cells proliferated in the long term and maintained hepatic characteristics in in vitro culture.
CONCLUSION: The study shows that aborted human fetal liver is a potential source for isolation of hepatic progenitors for clinical applications. The study also demonstrates that CD34 can be a good marker for the enrichment of progenitor populations.
- Citation: Nyamath P, Alvi A, Habeeb A, Khosla S, Khan AA, Habibullah C. Characterization of hepatic progenitors from human fetal liver using CD34 as a hepatic progenitor marker. World J Gastroenterol 2007; 13(16): 2319-2323
- URL: https://www.wjgnet.com/1007-9327/full/v13/i16/2319.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i16.2319
A shortage of donors for liver transplantation means that hepatocyte transplantation has been explored as a useful bridge or alternative therapy for the treatment of various liver diseases. Hepatocyte transplantation has become a conceptually appealing alternative to liver transplantation in patients suffering from fulminant and chronic liver failure. The potential of hepatocyte transplantation for supporting an actively injured liver by providing metabolic support has been suggested[1,2]. The scarcity of human donor livers and the absence of proliferation in cultured hepatocytes are major limiting factors for the success of hepatocyte therapy. Hepatic progenitors are highly expandable in vitro/in vivo. Studies in rodents have demonstrated the presence of bipotential stem cell/progenitors using different phenotypic markers[3]. These cells are capable of extensive proliferation and subsequent differentiation into biliary ductal cells and hepatocytes. The developing liver progenitor population has been demonstrated to be high and they differentiate from the common cell compartment, the hepatoblasts[4]. Furthermore, these cells reconstituted bile duct and hepatocyte structures following transplantation into retrosine treated liver[5]. Human fetal liver offers important sources for the hepatic progenitor for the clinical application. At 5 wk of gestation, hematopoiesis starts to shift from the yolk sac to the fetal liver[6]. The fetal liver is the main site of hematopoiesis in the fetus until development of the bone marrow. The first trimester human fetal liver contains the full hierarchy of several primitive progenitors detectable by their functional end points. These include hepatic and hematopoietic stem cells (HSCs)[7-9]. During this gestational period, hepatic cells express hepatocyte markers, for example, albumin, alphafetoprotein (AFP), 1 microglobulin, glycogen, glucose-6-phosphatase (G-6-P) and biliary markers; for example, gamma glutamyl transpeptidase (GGT), dipeptidyl peptidase IV (DPPIV), cytokeratin (CK)-19 and Das-1-monoclonal antibody-reactive antigen[10,11]. Human hepatoblasts express these markers throughout the second trimester (20-24 wk), despite significant developments of the fetal liver, which offers opportunities to isolate and study large numbers of progenitor cells. We have previously reported on the use of human fetal hepatocytes for the treatment of fulminant hepatic failure patients[12]. The isolation of hepatic progenitor cells from the developing liver can be ideal because of their morphological and functional attributes. Several investigators have isolated hepatic progenitor cells from human fetal liver and demonstrated their ability to proliferate greatly; and although these progenitor cells proliferated for several months they retained their karyotypes[4].
In several studies hematopoietic markers like CD34, Thy-1, and c-kit, mRNA for the flt-3 receptor, although restricted to hematopoietic stem cells, have been used for the identification/isolation of hepatic progenitors. These cells were characterized by the presence of hepatic markers like AFP, albumin, liver specific cytokeratins (CK-18, CK-19) and liver transcription factors. Hepatic stem cells were enriched using certain physical methods like counter flow elutriation and also by using certain stem cell specific markers. The enrichment of fetal liver stem/progenitor cells was first reported by Reid et al[5], using panning and fluorescence activated cell sorting methods with monoclonal antibodies, reacting with cell surface proteins and expressed on oval cells and bile duct epithelial cells. Fetal liver cells have been sorted by Suzuki et al[13] for those that are positive for expression of 6 α (CD49f) integrin and β1 (CD29) integrin, which are known to be present on hepatocytic cells but not on hematopoietic cells, and negative for CD45 and Ter119, which are specific markers for hematopoietic cells. Interestingly, cells showing the greatest enrichment for liver progenitor cells in a colony forming assay were negative for c-kit, which is a positive sorting marker for hematopoietic stem cells and is also detected in liver 'oval cells'. Strain and colleagues have used CD34 for the positive selection of hepatic stem cells to enrich for human liver stem-like progenitor cells that can differentiate in culture into biliary epithelial cells[14]. Recently, Fiegel et al[15] used CK-18 (hepatic marker) and Thy-1 (hematopoietic marker) for enrichment of potential hepatic progenitor population from developing rat liver. In the present study, hepatic progenitors were sorted using anti-CD34 by magnet activated cell sorting and were cultured for more than a month.
Human fetal liver (FL) tissues were obtained from aborted fetuses at 13-20 wk gestation in accordance with the Institute’s ethical guidelines. The fetuses were collected within 2 h of abortion. Single cell suspension was prepared from the liver by the two-step collagenase perfusion method and the cells were passed through a 70 μ-nylon mesh. The viability of the cells was assessed by the Trypan blue dye exclusion test. All the donors had been serologically screened for syphilis, toxoplasmosis, rubella, hepatitis B and C, human immunodeficiency virus 1, cytomegalovirus, parvovirus, and herpes simplex types 1 and 2.
Isolated human fetal liver cells were suspended in 1 mL of PBS with 5 mmol/L EDTA and labeled with anti CD34 magnetic labeled beads. CD34+ hepatic cells were separated by magnetic cell sorting (MACS) using the human direct CD34-progenitor kit (Miltenyi Biotech; Germany) according to the manufacturer’s instructions. The flow-through fraction was used as a source of CD34- cells. The enriched cells were analyzed for AFP (hepatic lineage marker) and CD45 (leukocyte lineage marker) using CD34PE/AFP FITC and CD45 PE on Flowcytometer (FACS Caliber Becton & Dickinson USA). To assess the nonspecific binding appropriate isotype controls were included.
Enriched CD34+ positive cells were cultured for a long term duration in a serum free medium. Two million cells were seeded on collagen typeI(Sigma Co.) coated 6 well plates (Nunclon). The medium DMEM (Sigma) was supplemented with 50 mg/L L-glutamine (Sigma Co.), 50 mg/L essential amino acids, penicillin, streptomycin, amphotericin B (Hi Media) and 10% heat inactivated fetal bovine serum (Gibco BRL), 50 ng/mL hepatocyte growth factor (HGF) (Sigma Co.), 20 ng/mL of epidermal growth factor (Gibco) and 10 ng/mL basic fibroblast growth factor (bFGF). Non-adherent cells were removed after 24 h and subsequently the medium was changed daily for 3 d. Cultured cells were analyzed for liver specific genes on the following days of culture: d 0, 7, 14, 21, 28 and 35. Cells were trypsinized with trypsin-EDTA diluted 1:10 in PBS for 5-7 min at 37°C on each occasion. For sub passaging, near-confluent cultures were split 1:3 using half-strength trypsin-EDTA for 6-10 min at 37°C.
The trypsinized cells were collected by washing with phosphate-buffered saline. Smears were prepared using cytospin (Shandon) on glass slides. The smears were fixed with methanol at -20°C for 5 min and acetone at 4°C for 15 s. The smears were washed with PBS and blocked with 1% bovine serum albumin (Sigma Co.) in PBS for one hour at room temperature, cells were incubated with mouse anti human-AFP (Santa Cruz) diluted 1:100 (in PBS) at 4°C for two hours and then stained with FITC conjugated rabbit anti mouse secondary antibody, diluted 1:1000 in PBS at 4°C for 30 min. After the incubation the cells were washed with PBS twice and the cells were analyzed under fluorescence and visible light.
For each slide, four randomly chosen areas were analyzed. The total cell number and the number of positive cells were assessed. For morphologic analysis the cells were split into two categories: small cells were round shaped cells with one nucleus and a small rim of cytoplasm. Large cells showed irregular cell shape with a prominent cytoplasm. For each area the total number of cells and the number of small cells were counted. The ratio of positive to total cells and the ratio of small to total cells were calculated as percentages.
Four million cells from each group were spun at 3000 rpm and 3 mL of 0.2% Triton x-100 was added to the pellet. Cells were lysed by freezing and thawing three times, and then the lysate was spun at 15 000 r/min for 45 min at 4°C. The supernatant was used for the estimation of AFP (Figure 1). 20 μL of the supernatant was used for AFP activity using ELISA in accordance with the protocol provided in the kit (Pathozyme).
Total RNA was extracted from CD34+ sorted cultured cells using Trizol (Invitrogen Life technology). Liver specific transcripts were analyzed using complementary DNA, which was amplified later for 35 cycles using primer pair specific for AFP, albumin and CK-18. PCR products were separated in 1.5% agarose gel and visualized under UV light. Primer pairs used in the study are listed in Table 1.
| Gene ID | Primer sequence | Productsize |
| AFP | Sense TGCAGCCAAAGTGAAGAGGGAAGA | 216 bp |
| Antisense CATAGCGAGCAGCCCAAAGAAAGAAA | ||
| Albumin | Sense TGCTTGAATGTGCTGATGACAGGG | 161 bp |
| Antisense AAGGCAAGTCAGCAGGCATCTCATC | ||
| CK-18 | Sense ATGGGCGAGCAGAACCGGAA | 326 bp |
| Antisense CCATGAGCCGCTGGTACTCC |
Flowcytometer analysis was done with freshly isolated or cultured cells. Cell number was assessed by using flow cytometer (BD). Mean values and standard deviations (SD) of cell numbers were calculated. Statistical analysis was performed using the two way Anova.
The viability of isolated cells ranged from 85%-94%. Primary cell isolation yield increased with increases in gestation (13-20 wk) from 39 ± 2.3 × 106 to 90 ± 3.4 × 106. There was no loss in the viability following magnetic cell sorting; viability ranged from 85%-90%.
Analysis of enriched CD34 positive cells showed two populations of cells CD34+/CD45+ and CD34+/CD45-. The majority of cells were CD34+/CD45-, and the CD 45 marker was used to exclude for lymphoid and myeloid lineage cells. The data revealed that CD34+/CD45+ (CD34 and CD45 positive cells) were 5.5% + 0.56% in group-I, 4.8% + 0.72% in group-II and 4.5% + 0.73% in group-III. Whereas CD34+/CD45- (CD45 negative cells) ranged from 4.1% + 0.82% in group I, 4.2% + 0.9% in group II and 3.9% + 0.8% in group-III, CD 45 + cells ranged from 0.3% to 0.5% (Table 2).
| Gestation(wk) | Total cell yield | % of CD34+/CD45+ | % of CD34+/CD45- |
| 13-15 | 39 + 2.3 × 106 | 5.5 + 0.56 | 4.1 + 0.82 |
| 16-18 | 65 + 2.9 × 106 | 4.8 + 0.72 | 4.2 + 0.9 |
| 19-20 | 90 + 3.4 × 106 | 4.5 + 0.82 | 3.9 + 0.8 |
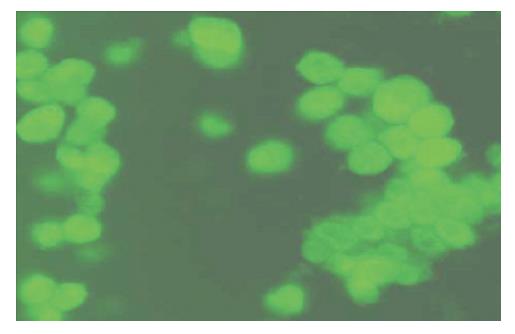
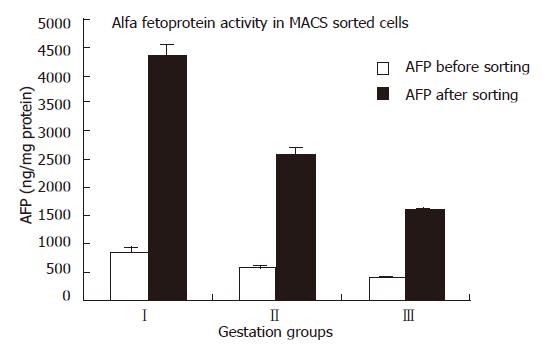
Of the enriched cells, when analyzed after staining with AFP, more than 70% ± 1.5% were observed to be positive for AFP and had a thin rim of cytoplasm with a big nucleus (Figure 1). AFP estimation in CD34 positive enriched cells increased four to five fold in comparison with non-enriched cells. (Table 3, Figure 2).
| Group | AFP before sorting(ng/mg protein) | AFP after sorting(ng/mg protein) | Folds increase |
| I | 835 + 16 | 4336 + 12 | 5.2 + 1.2 |
| II | 554 + 15 | 2557 + 14 | 4.9 + 1.3 |
| III | 384 + 13 | 1572 + 15 | 4.5 + 1.2 |
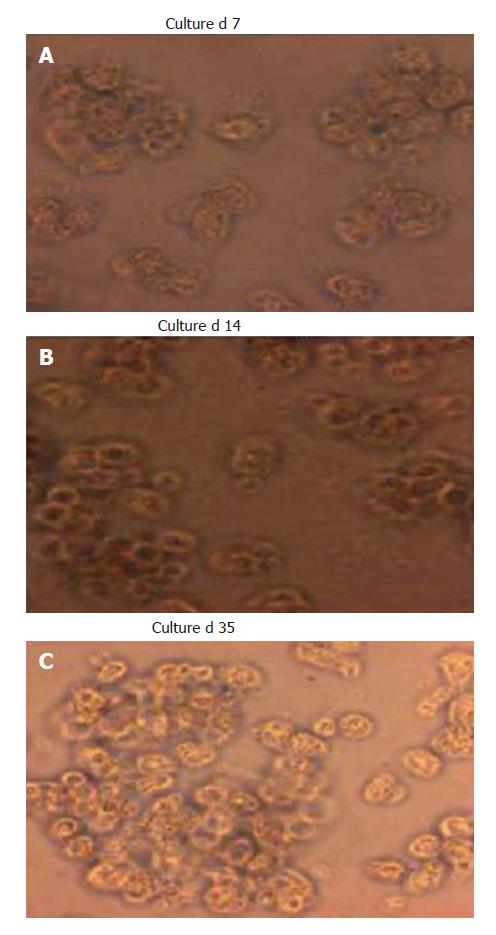
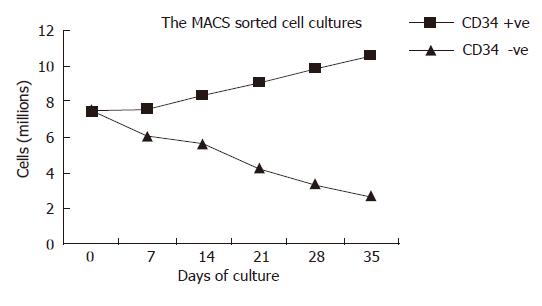
Culture of the CD34 positive cells showed increasing numbers with a significant rise in cell count from 7.4 ± 1.3 × 106/plate at d 0 (Figure 3A and B) to 8.6 ± 1.4 × 106/plate at d 35 of culture (Figure 3C). The cells, which were negative for CD34 when cultured, showed a decrease in cell number from 7.6 ± 0.9 × 106 cells at d 0 to 2.7 ± 0.08 × 106 cells at d 35. At d 28, two populations of cells were found in cultures of CD34+ cells. Cell numbers of cultured CD34+ cells increased significantly (P < 0.05) after d 7 when compared with the numbers of cultured CD34- cells (Figure 4).
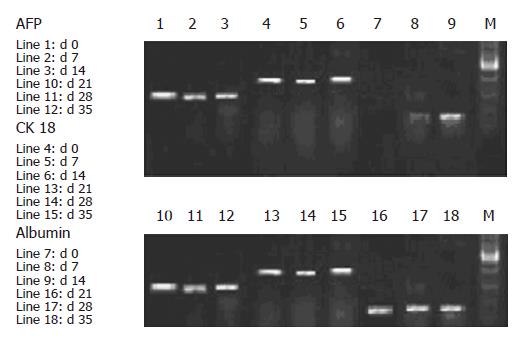
To confirm that the sorted cells were of hepatic lineage, cultured cells were further analyzed for the expression of liver specific mRNA transcript from d 0 to d 35 of culture (Figure 5). AFP and CK-18 expression was observed in the sorted culture cells, which revealed that these cells were capable of differentiating into hepatic and biliary lineages, suggesting the cellular bipotency of progenitor cells. Expression of albumin was noted only after 8 d of culture, indicating that the cells were in the process of maturation.
In our study, we found that cells enriched by haematopoietic stem cell marker CD34 expressed hepatocyte specific markers, but the majority of these cells were negative for leukocyte markers CD45. In the present study we have used two markers, AFP (hepatic marker) and CD45 (common leukocyte marker) in order to identify the CD34 positive cells of hepatic lineage. The flowcytometric analysis data showed two populations of cells CD34+/CD45+ and CD34+/CD45-. The majority of cells were CD34+/CD45- and the CD 45 marker was used to exclude the cells of lymphoid and myeloid lineage.
In the present study CD34+ positive cells were separated by MACS from human fetal liver cells. The data generated by magnetic cell sorting of progenitor cell population using CD34 marker increased AFP activity up to four to five fold in enriched cells when compared with the non sorted cells. As AFP is a marker for proliferation, increases in the AFP activity in the enriched population demonstrates that sorted cells have higher proliferation than the unsorted cells. Our findings also correlate with those of Craig et al and Strain et al who sorted hepatic progenitor cell population by labeling the cells with anti-CD34. The present study also revealed that the AFP activity of the cells before and after sorting with CD34 was more in groupI. This indicates that both CD34 in combination with AFP are good markers to enrich hepatic progenitors. Sorted cells were cultured to study the proliferative activity of the cells. There were increases in cell numbers. This shows that the cells negative for CD34 were matured cells and had low proliferative rates. The sorted CD34 positive cells in cultures showed very high proliferative rates, which indicates that the sorted cells are immature, progenitor cells. The AFP activity indicates that these cells belong to the hepatic lineage. The gene expression analysis in cultures on different days was carried out to study the hepatic markers, AFP, albumin and CK-18. The data reveal that the sorted CD34 positive cells showed AFP expression till 35 d of culture, which shows that the cells are in proliferative states even for 35 d. There was no expression of albumin on d 0, as the sorted cells are immature. With increases over time the expression of albumin started as the cells underwent maturation. The CK-18 expression further confirms that the cells are of epithelial lineage. The culture study revealed that the CD34 positive cells were able to proliferate and differentiate into cells of hepatic lineage.
In conclusion, the above study reveals that CD34 is a good marker for enrichment of hepatic progenitor cells from human fetal livers. This was confirmed by the liver specific marker, AFP which increased after sorting, as CD34 sorting leads to the enrichment of the progenitor population. The expression of hepatic markers in cultured cells concludes that the cells were able to proliferate and differentiate for several days.
S- Editor Liu Y L- Editor Roberts SE E- Editor Zhou T
| 1. | Sutherland DE, Numata M, Matas AJ, Simmons RL, Najarian JS. Hepatocellular transplantation in acute liver failure. Surgery. 1977;82:124-132. |
| 2. | Sommer BG, Sutherland DE, Matas AJ, Simmons RL, Najarian JS. Hepatocellular transplantation for treatment of D-galactosamine-induced acute liver failure in rats. Transplant Proc. 1979;11:578-584. |
| 3. | Farber E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3'-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956;16:142-148. |
| 4. | Malhi H, Irani AN, Gagandeep S, Gupta S. Isolation of human progenitor liver epithelial cells with extensive replication capacity and differentiation into mature hepatocytes. J Cell Sci. 2002;115:2679-2688. |
| 5. | Sigal SH, Brill S, Reid LM, Zvibel I, Gupta S, Hixson D, Faris R, Holst PA. Characterization and enrichment of fetal rat hepatoblasts by immunoadsorption ("panning") and fluorescence-activated cell sorting. Hepatology. 1994;19:999-1006. |
| 6. | Migliaccio G, Migliaccio AR, Petti S, Mavilio F, Russo G, Lazzaro D, Testa U, Marinucci M, Peschle C. Human embryonic hemopoiesis. Kinetics of progenitors and precursors underlying the yolk sac----liver transition. J Clin Invest. 1986;78:51-60. |
| 7. | Rowley PT, Ohlsson-Wilhelm BM, Farley BA. Erythroid colony formation from human fetal liver. Proc Natl Acad Sci USA. 1978;75:984-988. |
| 8. | Hann IM, Bodger MP, Hoffbrand AV. Development of pluripotent hematopoietic progenitor cells in the human fetus. Blood. 1983;62:118-123. |
| 9. | Roy V, Miller JS, Verfaillie CM. Phenotypic and functional characterization of committed and primitive myeloid and lymphoid hematopoietic precursors in human fetal liver. Exp Hematol. 1997;25:387-394. |
| 10. | Haruna Y, Saito K, Spaulding S, Nalesnik MA, Gerber MA. Identification of bipotential progenitor cells in human liver development. Hepatology. 1996;23:476-481. |
| 11. | Badve S, Lôgdberg L, Sokhi R, Sigal SH, Botros N, Chae S, Das KM, Gupta S. An antigen reacting with das-1 monoclonal antibody is ontogenically regulated in diverse organs including liver and indicates sharing of developmental mechanisms among cell lineages. Pathobiology. 2000;68:76-86. |
| 12. | Habibullah CM, Syed IH, Qamar A, Taher-Uz Z. Human fetal hepatocyte transplantation in patients with fulminant hepatic failure. Transplantation. 1994;58:951-952. |
| 13. | Suzuki A, Zheng Y, Kondo R, Kusakabe M, Takada Y, Fukao K, Nakauchi H, Taniguchi H. Flow-cytometric separation and enrichment of hepatic progenitor cells in the developing mouse liver. Hepatology. 2000;32:1230-1239. |
| 14. | Crosby HA, Kelly DA, Strain AJ. Human hepatic stem-like cells isolated using c-kit or CD34 can differentiate into biliary epithelium. Gastroenterology. 2001;120:534-544. |
| 15. | Fiegel HC, Lioznov MV, Cortes-Dericks L, Lange C, Kluth D, Fehse B, Zander AR. Liver-specific gene expression in cultured human hematopoietic stem cells. Stem Cells. 2003;21:98-104. |