Published online Apr 21, 2007. doi: 10.3748/wjg.v13.i15.2250
Revised: February 10, 2007
Accepted: March 8, 2007
Published online: April 21, 2007
We describe our experience of treatment for a giant esophageal malignant fistula, which has not been reported previously. A 36-year-old woman who was diagnosed as having massive esophageal small cell carcinoma with metastases was treated with chemoradiotherapy. However, a giant esophagomediastinal fistula appeared due to shrinkage of the massive tumor, and all anti-cancer treatment was suspended. However, chemoradiotherapy was restarted at the request of the patient despite the presence of the fistula. After restarting treatment, the giant esophageal fistula was naturally closed despite intensive chemoradiotherapy, and the patient became able to eat and drink. Although the patient finally died, her QOL and prognosis seemed to be improved by the chemoradiotherapy. Anti-cancer treatment could be safely performed despite the presence of a giant fistula. The giant fistula closed while intensive chemotherapy was administered to the patient. Therefore, the presence of a fistula may not be a contraindication for curative chemoradiotherapy. Completion of treatment with proper management and maintenance of patients would be of benefit to patients with fistula.
- Citation: Nomiya T, Teruyama K, Wada H, Nemoto K. Chemoradiotherapy for a patient with a giant esophageal fistula. World J Gastroenterol 2007; 13(15): 2250-2254
- URL: https://www.wjgnet.com/1007-9327/full/v13/i15/2250.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i15.2250
There are many causes of esophageal fistula or perforation, including trauma, congenital, infection, tuberculosis, malignancy, and iatrogenic causes[1-5] In cases of advanced esophageal malignancy that has invaded adjacent organs, there is a high risk of fistula formation[1,2,6]. And in the treatment of advanced esophageal malignancy, there is a high risk of an esophageal fistula in the course of treatment or after treatment due to shrinkage of the tumor rather than before treatment.
Among various treatments, including esophagectomy, stent insertion, bypass surgery and radiotherapy, none has been tried for esophageal malignancy with a fistula, and no standard treatment has been established. Several studies, however, have suggested that bypass surgery and radiotherapy may be more effective than other treatments[1,2,7-10]. In radiotherapy for advanced esophageal malignancies, continuation and completion of the radiotherapy is important regardless of fistula formation[1,7].
However, the treatable limit for fistula size is not clear, and there has been no report on treatment for a giant esophageal malignant fistula. In this report, we describe out experience of treatment for a patient with a giant esophageal malignant fistula and the course of events.
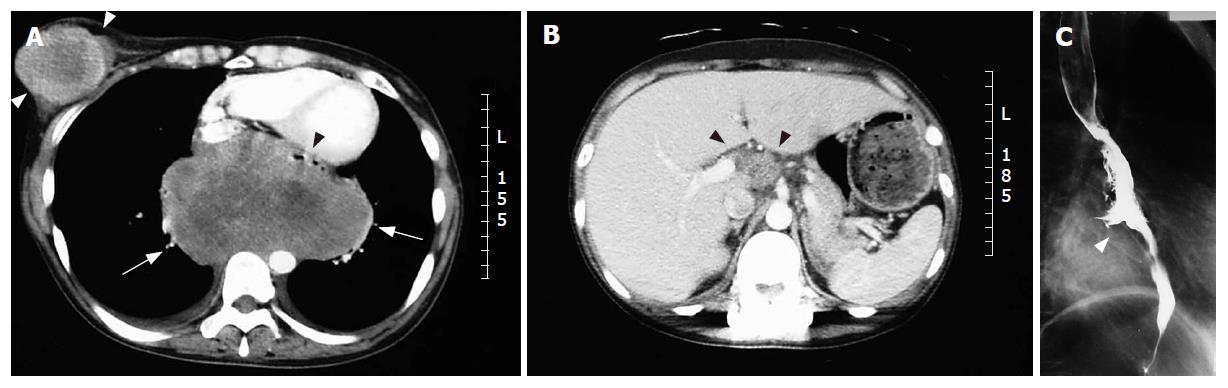
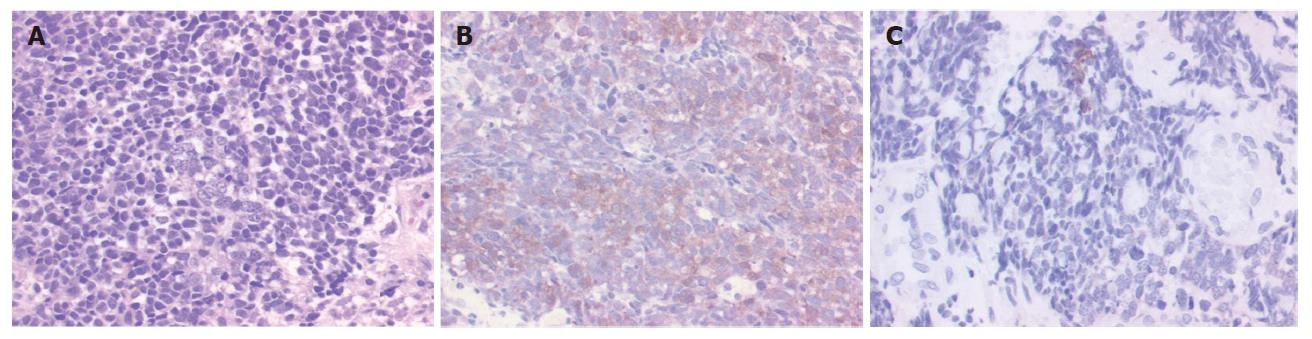
A 36-year-old woman visited our hospital with chief complaints of a palpable tumor in the right breast and a sense of chest incompatibility. An elastic hard, round tumor of 6 cm in maximum diameter in the right breast was palpable in clinical examination. Computed tomography (CT) revealed a solid massive tumor of 13 cm × 9 cm located in the middle to lower mediastinum (Figure 1A) and celiac artery lymphadenopathy appeared to be metastases (Figure 1B). A barium esophagogram showed leftward shift of the esophagus and ulceration in the lower esophagus, but dysphagia and obstruction were not observed (Figure 1C). In examination of tumor markers, serum NSE neuron-specific enolase (NSE) level was elevated to 130 ng/mL (normal: < 12.0 ng/mL) and serum CA125 level was also elevated to 59.9 U/mL (normal: < 35 U/mL). Serum carcinoembryonic antigen (CEA), CA19-9 (carbohydrate antigen 19-9), soluble IL-2R (interleukin 2 receptor), and squamous cell carcinoma antigen (SCC) levels were within normal ranges. In cytology, the tumor consisted of abnormal cells containing little cytoplasm and round-shaped nuclei, a finding that is similar to that in malignant lymphoma (Figure 2A). Surface marker analyses showed CD20 negative, CD45RO negative, CD56 positive and CD38 negative, suggesting that the tumor was derived from cells other than hematogenous cells. Immunohistochemical staining for NSE was positive (Figure 2B), for keratin-wide was weakly positive (Figure 2C), and for chromogranin A was negative (not shown).
Based on the results of cytopathology, analyses of surface antigen, immunohistochemistry and serum tumor marker levels, the malignancy was histopathologically diagnosed as small cell carcinoma with characteristics of a neuroendocrine tumor. The patient was clinically diagnosed as having esophageal small cell carcinoma (ESCC) with right breast and multiple lymph nodes metastases.
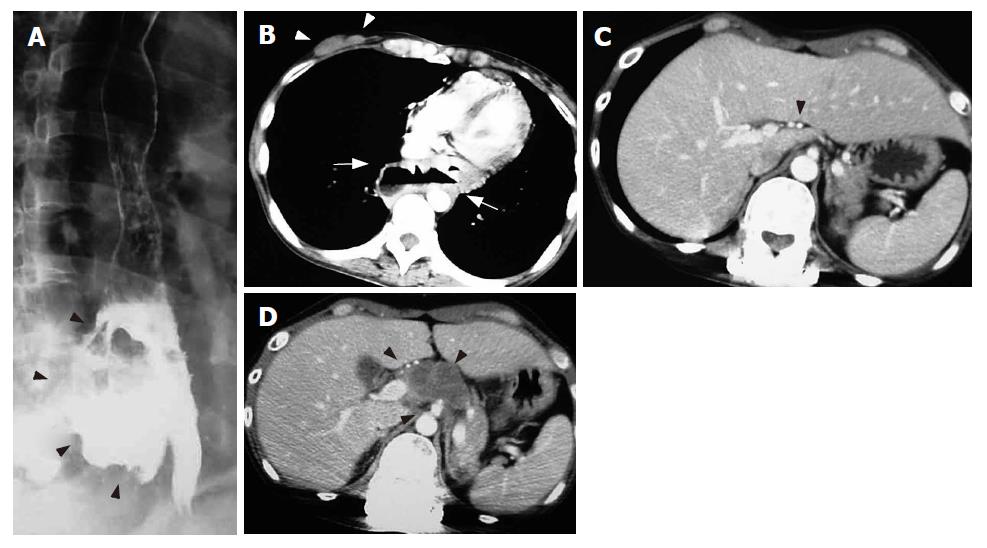
CDDP (cis-dichlorodiammineplatinum II) at 90 mg/body and irinotecan (CPT-11) at 100 mg/body were used in initial chemotherapy. Radiotherapy for the massive esophageal tumor (60 Gray in 50 fractions, 1.2 Gray/fraction, twice a day, 10 mega-volt photon) and for the right breast tumor (60 Gray in 50 fractions, 1.2 Gray/fraction, twice a day, 4 mega-volt photon) was performed concomitantly. After the completion of 3 courses of chemotherapy and radotherapy for the esophageal tumor and breast tumor (3 mo after the start of initial treatment), both the massive esophageal tumor and right breast tumor almost disappeared (Figure 3B and 3C). The formation of a giant esophagomediastinal fistula was observed, and all anti-cancer treatments were suspended (Figure 3A).
The abdominal lymph node metastasis shrank and started to regrow after the suspension of treatment, and opioid analgesics were administered for abdominal cancerous pain. Furthermore, a tumor in the neck appeared to be a metastasis. The giant esophageal fistula was still present at this time, but restarting anti-cancer treatment was suggested, and the patient agreed and was transferred to the Department of Radiation Oncology, where anti-cancer treatment was restarted.
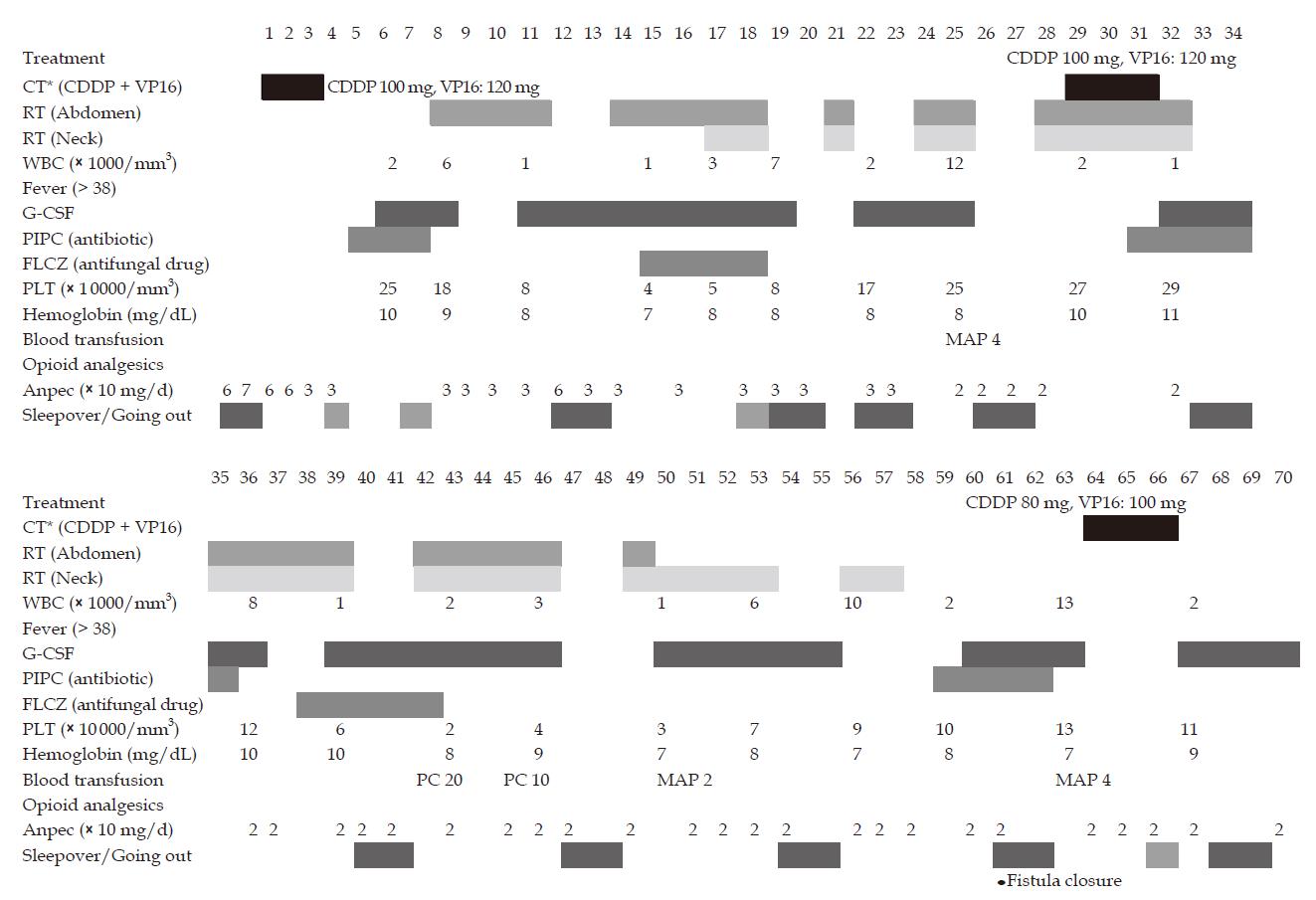
Radiotherapy and chemotherapy were restarted 5 mo after the start of initial treatment. The course of treatment is shown in Figure 4. Second chemotherapy was started with CDDP at 100 mg/body and VP16 at 120 mg/body. Radiotherapy was planned for abdominal lymph node metastasis, which was causing abdominal cancerous pain, and for neck lymph node metastasis. Prescribed dose for both lesions was 60 Gy/50 fractions (b.i.d.) with limited irradiation fields as far as possible.
Treatments were started sequentially in order to relieve the patient's sense of anxiety. Granulocyte colony stimulating factor (G-CSF), mannitol, adenine and phosphate added red cell product (MAP) and platelet concentrate (PC) were used for leukopenia, anemia and thrombocytopenia by chemotherapy. Antibiotics and antifungal drug were prophylactically used for a short term at minimal doses. Feeding and drinking were stopped, and nutrition was administered by intravenous hyperalimentation. If the WBC count was more than 2000/mm3, sleepover at home at the weekend was permitted despite the presence of the fistula. No special treatment for the esophageal fistula was performed other than the above-mentioned treatment.
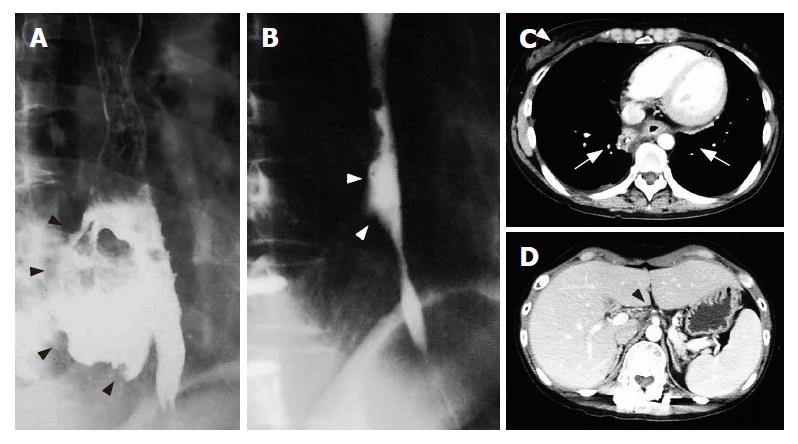
Gastrografin esophagography performed on d 60 (60 d after restarting treatment) showed that the giant esophageal fistula had completely disappeared, and only a mucosal notch was observed (Figure 5A and 5B). CT revealed disappearance of abdominal lymph node metastasis and neck lymph node metastasis and obstruction of the esophageal fistula without recurrence (Figure 5C and 5D). Furthermore, the serum NSE and CA125 levels were within normal ranges, and the patient was diagnosed as having a complete response (CR: no visible tumor with normalized tumor markers). The patient gradually became able to eat and to drink, and activity of daily life (ADL) was maintained at a good level.
At d 120 after restarting treatment (9 mo after the initial treatment), multiple lung nodules that were thought to be pulmonary metastases appeared in the course of adjuvant chemotherapy, indicating that cells were resistant to the anti-cancer agents. The anti-cancer agents were then altered, but response was poor. Furthermore, myelosuppression due to long-term chemotherapy impeded continuation of intensive chemotherapy, and multiple pulmonary metastases could not be improved. At d 360 after the initial treatment, the patient died of respiratory insufficiency due to multiple pulmonary metastases.
It has been reported that continuing and completing a course of treatment for malignancy seems to be better than suspending treatment even if a malignant fistula appears in the course of treatment[1,2,7]. However, there has been no report on the size limit of a treatable fistula and the propriety of treatment with the presence of a giant fistula.
In the present case, it was possible to perform radical treatment and intensive chemoradiotherapy safely in spite of the presence of a giant esophageal fistula. The presence of the esophageal fistula had no adverse effect on continuation of chemoradiotherapy. Intensive chemoradiotherapy caused no deterioration of the esophageal fistula. Although the risk of mediastinal infection seems to be higher than that in normal patients, it is thought that chemoradiotherapy is not a contraindication even for a patient with a giant esophageal fistula as long as informed consent has been obtained.
The giant fistula in the present case improved in an unexpectedly short period despite intensive chem-oradiotherapy. CT and esophagography showed that the fistula was closed because of not only progression of malignancy but also of regrowth of normal tissues. Although the site of the fistula was completely included in the irradiation field and was irradiated with 60 Gy/50 fr. in the initial treatment, the giant fistula showed unexpected improvement. This suggests that normal esophageal mucosal tissue has a potential restoring force even with irradiation of a total dose of 60 Gy.
It has been reported that an esophageal fistula due to tuberculosis can be closed only by administration of antituberculous drugs[4-6]. Similar to this mechanism, it is presumed that elimination of foreign bodies (abscess, tumor, bacteria, etc.) enables restoration of the perforated mucosa and connective tissues, and it therefore seems that treatment for the cause of the fistula should come before protection of the fistula. This theory is supported by the course of the present case and the course of a previously reported case[7].
No obvious infection was observed only by administration of limited antibiotics and antifungal drugs as prophylactics (several days per month). The patient was not confined to bed but allowed to sleep at home and go out as much as possible despite the presence of a giant esophageal fistula. An appropriate amount of exercise and maintenance of activities of daily life (ADL) might be important for maintaining the patient's stamina.
From the viewpoint of cure of disease, the achievement of systemic CR seemed to be important. However, prognosis of ESCC is extremely poor, and past studies have shown that the median survival of patients with untreated ESCC is several weeks and that even with intensive treatment it is only 6-12 mo[11-13]. Complete cure of ESCC is therefore extremely difficult. However, the fact that a patient with massive ESCC and distant metastases survived for about one year from the initial treatment indicates that curative treatment can significantly contribute to improvement of prognosis.
Relief of abdominal cancerous pain by restarting anti-cancer treatment led to an improvement in the patient's QOL shown by the fact that the patient became able to eat and drink. The patient was able to stay at home with her family every weekend even in the course of treatment. Staying at home seems to have resulted in both improvement of QOL and maintenance of ADL. The patient could walk independently until several days before her death, and her ADL was maintained at a good level for her terminal state (ECOG performance status: 2-3).
The 36-year-old female patient was too young to accept her terminal status and death. However, the patient survived 7 mo with minimum pain from the hopeless situation due to fistula formation and suspension of treatment. From the viewpoint of psycho-oncology, this period that was obtained by restart of intensive treatment seemed to make her enable to accept her death.
In conclusion, although a giant esophageal fistula was present, the fistula improved despite intensive chemoradiotherapy. Our experience suggests that it is possible to perform anti-cancer treatment even for patients with a giant esophageal fistula and that there is no need to wait for closure of the fistula. The giant esophageal fistula in this case was restored during anti-cancer treatment without any special treatment for the fistula.
S- Editor Liu Y L- Editor Ma JY E- Editor Zhou T
| 1. | Arlington A, Bohorquez J. Irradiation of carcinoma of the esophagus containing a tracheoesophageal fistula. Cancer. 1993;71:3808-3812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Gschossmann JM, Bonner JA, Foote RL, Shaw EG, Martenson JA, Su J. Malignant tracheoesophageal fistula in patients with esophageal cancer. Cancer. 1993;72:1513-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Mathisen DJ, Grillo HC, Wain JC, Hilgenberg AD. Management of acquired nonmalignant tracheoesophageal fistula. Ann Thorac Surg. 1991;52:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 124] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Devarbhavi HC, Alvares JF, Radhikadevi M. Esophageal tuberculosis associated with esophagotracheal or esophagomediastinal fistula: report of 10 cases. Gastrointest Endosc. 2003;57:588-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Porter JC, Friedland JS, Freedman AR. Tuberculous bronchoesophageal fistulae in patients infected with the human immunodeficiency virus: three case reports and review. Clin Infect Dis. 1994;19:954-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Rosanowski F, Tigges M, Eysholdt U. Esophagomediastinal fistula and recurrent laryngeal nerve paralysis after radiotherapy of Hodgkin's disease. Laryngorhinootologie. 1995;74:516-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Muto M, Ohtsu A, Miyamoto S, Muro K, Boku N, Ishikura S, Satake M, Ogino T, Tajiri H, Yoshida S. Concurrent chemoradiotherapy for esophageal carcinoma patients with malignant fistulae. Cancer. 1999;86:1406-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Burt M, Diehl W, Martini N, Bains MS, Ginsberg RJ, McCormack PM, Rusch VW. Malignant esophagorespiratory fistula: management options and survival. Ann Thorac Surg. 1991;52:1222-1228; discussion 1228-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 137] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Reed MF, Mathisen DJ. Tracheoesophageal fistula. Chest Surg Clin N Am. 2003;13:271-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 205] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Eleftheriadis E, Kotzampassi K. Endoprosthesis implantation at the pharyngo-esophageal level: problems, limitations and challenges. World J Gastroenterol. 2006;12:2103-2108. [PubMed] |
| 11. | Wu Z, Ma JY, Yang JJ, Zhao YF, Zhang SF. Primary small cell carcinoma of esophagus: report of 9 cases and review of literature. World J Gastroenterol. 2004;10:3680-3682. [PubMed] |
| 12. | Brenner B, Tang LH, Klimstra DS, Kelsen DP. Small-cell carcinomas of the gastrointestinal tract: a review. J Clin Oncol. 2004;22:2730-2739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 206] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 13. | Nemoto K, Zhao HJ, Goto T, Ogawa Y, Takai Y, Matsushita H, Takeda K, Takahashi C, Saito H, Yamada S. Radiation therapy for limited-stage small-cell esophageal cancer. Am J Clin Oncol. 2002;25:404-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |