Published online Apr 21, 2007. doi: 10.3748/wjg.v13.i15.2209
Revised: January 15, 2007
Accepted: March 15, 2007
Published online: April 21, 2007
AIM: To explore the relationship between acetylation of histone in total chromatin and p21WAF1 expression regulation in human colorectal carcinoma.
METHODS: We analyzed the expression of tumor suppressor gene p21WAF1 mRNA by RT-PCR or real-time PCR in 33 samples of colorectal cancerous tissue, corresponding para-cancerous tissue and normal colorectal mucosa, and also examined the level of acetylated histone H3 in total chromatin using Western blotting.
RESULTS: The expression level of p21WAF1 mRNA was significantly lower in colorectal cancerous tissue from 33 patients than in para-cancerous tissue and normal colorectal mucosa (2377.95 ± 865.80 vs 3216.58 ± 1149.42 and 3541.61 ± 1433.17 respectively, P < 0.01). In addition, when p21WAF1 mRNA expression was undectectable or at very low level (50% less than that in adjacent tissue and normal colorectal mucosa) in all tissues, the level of acetylated histone H3 in colorectal cancerous tissue was significantly lower than that in corresponding para-cancerous tissue and normal colorectal mucosa in five of seven (71.43%) cases. The transcriptional level of p21WAF1 in colorectal carcinoma might not be associated with its biological behaviors.
CONCLUSION: The down-regulation of p21WAF1 transcription is involved in the tumorigenesis and development of colorectal carcinoma. The down-expression of p21WAF1 mRNA in colorectal carcinoma might be associated with histone hypoacetylation in chromatin but not with biological behaviors.
-
Citation: Chen YX, Fang JY, Lu R, Qiu DK. Expression of
p 21WAF1 is related to acetylation of histone H3 in total chromatin in human colorectal cancer. World J Gastroenterol 2007; 13(15): 2209-2213 - URL: https://www.wjgnet.com/1007-9327/full/v13/i15/2209.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i15.2209
Cell cycle progression is regulated by interactions between cyclins and cyclin-dependent kinases (CDKs)[1]. p21WAF1 is one of the CIP/KIP protein family members known to inhibit CDK activity. Increased p21WAF1 expression may play an important role in the growth arrest of transformed cells[2]. Although stability of p21WAF1 mRNA could be altered by different signals such as differentiation and various influencing factors, a recent study suggested that inactive chromatin induced by histone deacetylation may be a likely candidate mechanism for p21WAF1 inactivation[3].
In recent years, some studies indicated that p21WAF1 expression is regulated by gene-associated histone acetylation in tumors[3-5]. We have previously reported that the level of p21WAF1 gene promoter-associated histone acetylation is decreased in human colon cancer cell line, SW1116[6]. However, these investigations were carried out in vitro experiments using certain cell lines, and very few data are available regarding p21WAF1 expression and histone acetylation in excised human carcinoma tissues. Furthermore, whether acetylation of histone in total chromatin affects p21WAF1 gene regulation in human colon cancer is unknown.
In this paper, we provide further evidence that the acetylation of histone in total chromatin is a mechanism of down-regulation of p21WAF1 mRNA in patients with colorectal cancer.
Thirty-three patients with colorectal carcinoma underwent resection at Shanghai Renji Hospital between May 2001 and July 2002. All patients gave their informed consent and none of them received radiation or chemotherapy before surgery. Fresh samples taken from the tumor and its corresponding adjacent tissue (obtained from areas less than 5 cm away from the margin of carcinoma) and normal colorectal mucosa (obtained from areas at least 10 cm away from the margin of carcinoma) were frozen immediately in liquid nitrogen, fixed in formalin solution and embedded in paraffin blocks for pathological diagnosis. Clinicopathological factors, tumor histology and disease stage were assigned according to the general rules for clinical and pathological studies on cancer of colon, rectum, anus and Dukes’ stage[7]. Fifteen cases were identified as stage B, 11 cases as stage C, and 7 cases as stage D. There were 23 cases of tubular adenocarcinoma, 2 cases of mucinous adenocarcinoma and 8 cases of tubular-papillary adenocarcinoma. Twenty-one patients were males and 12 were females, and their mean age was 65.36 years (SD 13.00 years, range 39-89 years). The protocol was approved by the Ethics Committee of Shanghai Second Medical University and the research was carried out according to the Helsinki Declaration in 1975.
Total RNA was extracted from clinical specimens using Trizol reagent (Gibco BRL, Gaithersburg, MD), following the manufacturer’s recommendations. After DNAse I (Promega, UK) digest, the pellet was vacuum dried and resuspended in 20 μL sterile distilled water containing 1 U/μL RNasin (Promega, UK).
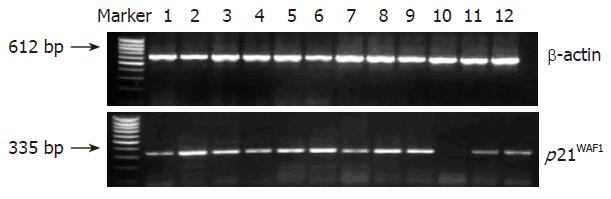
RT-PCR was performed as described previously[8]. The primer sequence and amplification profiles are shown in Table 1. The control consisted of an amplified 612 bp fragment of β-actin cDNA. The density of the bands in RT-PCR was quantitated by using a molecular dynamics phosphor-imager (Nucleo Tech Inc., San Mateo, CA), normalized in each lane to the amount of total RNA as determined by the density of β-actin band from RT-PCR[9].
| Primers | Sense (5’ -> 3’) | Antisense (5’ -> 3’) | Size and PCR condition | GenBank accession number | ||
| β-actin | GGC | ATC | GCT | GGA | 94°C 5 min; 92°C 40 s; | BC023204 |
| GTG | ATG | AGG | TGG | 58°C 40 s; 72°C 50 s, 30X | ||
| GAC TCC G | ACA GCG A | |||||
| p21WAF1 | CAG | GGG | GGG | CGG | 94°C 5 min; 94°C 1 min; | NM_000389 |
| ACA | GCA | CCA | GGG | 58°C 1 min; 72°C 1 min, 35X | ||
| GAG GAA GA | TAT GTA C | |||||
The expression of the p21WAF1 gene in 13 of 33 colorectal carcinoma specimens was detected using a real-time quantitative PCR system. Gene-specific TaqMan probes and PCR primers were designed using Primer Express software (PE Biosystems, Foster City, CA). The sequence for forward and reverse primers and the probe are shown in Table 2. Triplicate PCR was prepared for each cDNA sample. PCR consisted of 40 cycles of 95°C denaturation at 95°C for 15 s and annealing/extension at 60°C for 1 min. Thermal cycling and fluorescent monitoring were performed using an ABI 7700 sequence analyzer (PE Biosystems). The point at which the PCR product was first detected above a fixed threshold, termed cycle threshold (Ct), was determined for each sample, and the average Ct of triplicate samples was calculated. The ratio of p21WAF1 mRNA levels in colorectal carcinoma specimens relative to those in non-neoplastic mucosa was calculated.
| Gene | Primer (forward) (5’ -> 3’) | Primer (reverse) (5’ -> 3’) | Probe | GenBank No. |
| p21WAF1 | CTG GAG ACT | GGA TTA GGG | ACG GCG GCA | NM_078467 |
| CTC AGG GTC | CTT CCT CTT | GAC CAG CAT GA | ||
| GAA | GGA | |||
| β-actin | CTG GCA CCC | GGA CAG CGA | ATC ATT GCT | BC016045 |
| AGC ACA ATG | GGC CAG GAT | CCT CCT GAG |
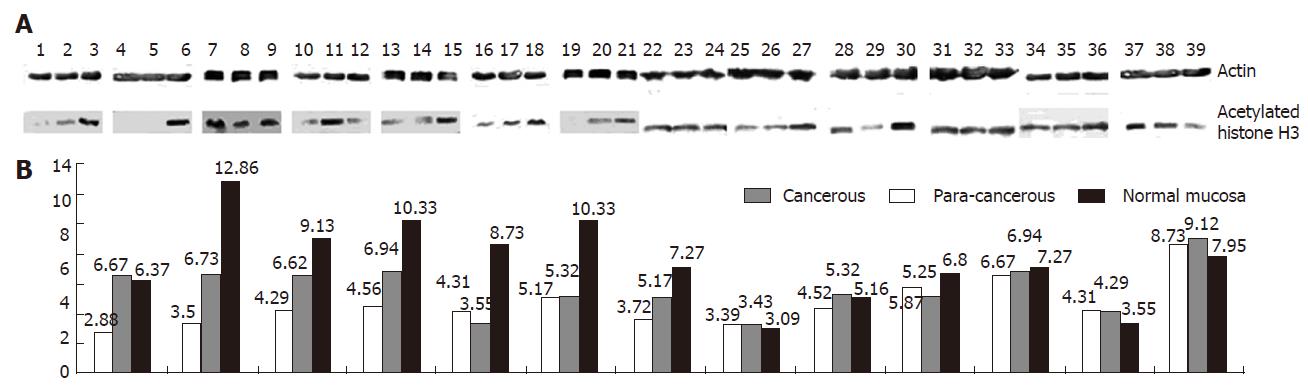
Seven powdered colorectal cancerous tissue sections in which p21WAF1 mRNA expression was undetectable or at very low levels (50% less than that in adjacent tissue and normal colorectal mucosa) (100 mg) and six such sections in which p21WAF1 mRNA expression was comparable to that in non-neoplastic mucosa were suspended in 500 μL Radio-immunoprecipitation assay buffer (150 mmol/L NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mmol/L Tris-HCl pH 8.0, 0.2 mmol/L PMSF, 1 μg/μL aprotinin/ leupeptin) for 15 min with rotation at 4°C, then the soluble protein was collected by centrifugation.
Tissue extracts (200 μg) were boiled in loading buffer (125 mmol/L Tris-HCl, pH 6.8, 4% SDS, 20% glycerol, 0.005% bromphenol blue) for 5 min and then loaded onto a 15% SDS-polyacryamide gel. After electrophoresis, proteins were transferred onto nitrocellulose membranes. The membranes were blocked with 5% nonfat dry milk in PBS for 1 h at room temperature, incubated overnight at 4°C with the first antibody (Upstate Biotechnology, Lake Placid, NY) against acetylated histone H3, and incubated for 4 h at 4°C with secondary antibodies against rabbit IgG-conjugated AP, and then exposed to Kodak BioMax film for 1 min. Antibody against β-actin (Sigma, St Louis, MO) was used as a control for protein concentration.
The level of tumor-related gene expression in cancerous and adjacent tissues and normal colorectal mucosa was compared by variance analysis or Student’s t-test using SPSS 6.0. Variables associated with the expression of two tumor suppressor genes as well as the correlation to various clinicopathological variables were examined by the χ2 method and correlation analysis. P < 0.05 was considered statistically significant.
As shown in Figure 1 and Table 3, the expression level of p21WAF1 mRNA in colorectal cancerous tissue was significantly lower than that in adjacent tissue and normal colorectal mucosa (P < 0.01). However, there was no significant difference in the expression level of p21WAF1 between the samples of adjacent tissue and normal mucosa (P > 0.05). Of the 33 tumor samples, 23 (69.70%) showed varying degrees of decrease in p21WAF1 expression. In seven cases, p21WAF1 mRNA expression was undetectable or at very low levels (50% less than that in adjacent tissue and normal colorectal mucosa). The data were consistent with the result from real-time RT-PCR (Figure 2).
Futher study of the seven specimens with undetectable or very low level p21WAF1 mRNA expression revealed that in five of seven (71.43% ) cases (Figure 2), the level of acetylated histone H3 in cancerous tissue was significantly lower than that in corresponding para-cancerous tissue and normal colorectal mucosa. The level of acetylated core histone H3 in tumor specimen with p21WAF1 mRNA expression was comparable to that in non-neoplastic mucosa specimen. However, it was lower than that in two of six (33.33%) non-neoplastic mucosa specimens (Figure 2).
We examined the relationship between p21WAF1 expression in cancer tissue and the clinicopathological characteristics of colorectal cancer patients. There was no significant correlation between p21WAF1 expression and tumor size, extent of local tumor invasion, lymphatic invasion, Duke’s stage or histological subtype (data not shown).
The initiation and progression of colorectal carcinoma involve unregulated epithelial cell proliferation associated with a series of accumulated genetic alterations[10]. The molecular pathogenesis of human cancer is due to structural and/or functional alterations of specific genes whose normal function is to control cellular growth and differentiation. The development of colorectal cancer is associated with activation of oncogenes and/or inactivation of tumor suppressor genes.
Defects in the mechanisms controlling cell cycle are critical triggers in cell transformation and/or tumor progression. p21WAF1 is a known inhibitor of cyclin-dependent kinases, which can block tumor progression through the cell cycle[11]. The discovery of the p21WAF1 gene has illuminated the importance of down-regulation of cell cycle as an inhibitor of cyclin-cdk complexes. The p21WAF1 gene causes G1 arrest of the cell cycle, and inhibits growth of human tumor cells by blocking G1/S transition through interference with cyclin-cdk function. In this study, we showed that p21WAF1 mRNA expression was constantly and significantly suppressed in tumor tissues compared to that in the corresponding adjacent tissue and normal colorectal mucosa. We also found that p21WAF1 expression was decreased in 69.70% of the tumor samples, which was higher than that in colorectal cancer detected by immunohistochemistry[12], and lower than that detected by Northern blot analysis[13]. The different results may be due to the geographical and racial factors. Our results suggest that tumor cell cycles are disturbed by reduction in p21WAF1 mRNA expression, leading to uncontrolled cell proliferation in human colorectal carcinoma. That is to say, tumor cells may acquire more rapid growth activity and more malignant potential as p21WAF1 expression decreases.
It was reported that two histone deacetylation inhibitors, trichostatin A and sodium butyrate, cause accumulation of acetylated histone H3 and H4[14]. This action most likely occurs in vivo because in rats fed with a high-fiber diet, high butyrate levels are correlated with histone hyperacetylation in colonic epithelial cells[15]. Moreover, accumulation of acetylated histones in total cellular chromatin and hyperacetylation of p21WAF1 gene-associated histones H3 and H4 could activate the expression of p21WAF1 mRNA and protein in SW1116 cells[16]. However, the in vivo status of histone acetylation in human colorectal carcinoma tissue is not well understood. In this study, we investigated colorectal cancerous tissues where p21WAF1 mRNA expression was undetectable or at very low levels, and found that the level of acetylated histone H3 in total chromatin was significantly lower in cancerous tissue than in corresponding para-cancerous tissue and normal colorectal mucosa. Also, acetylated core histone H3 level in tumor specimens where p21WAF1 mRNA expression was comparable to that in non-neoplastic mucosa specimens was lower than that in non-neoplastic mucosa specimens. These results suggest that the down-expression of p21WAF1 mRNA in colorectal carcinoma might be associated with histone hypoacetylation in total chromatin. It is possible that nucleosome conformation was altered due to histone H3 hypoacetylation and that access of transcriptional regulatory proteins to chromatin might be reduced in colorectal cancer. Thus, we hypothesize that p21WAF1 over-expression may be relevant to the possible therapeutic effects of anticancer drugs.
However, the data from this study indicate that none of the clinicopathological parameters correlates with p21WAF1 expression, including different tumor size, tumor depth, lymph node metastasis, Dukes’ classification and histological stage. These results suggest that the expression of p21WAF1 detected by RT-PCR cannot be used per se as a predictive indicator for clinicopathological behaviors in colorectal carcinoma. To date, the potential correlation between tumor-related genes and biological behaviors remains controversial. For example, one recent report[17] demonstrated that allelic deletion on chromosome 18q is associated with poor survival of patients with stage II colorectal cancer, while another study[18] showed that this phenomenon does not provide any prognostic information, suggesting that construction of composite genetic profiles of tumor tissue, with inclusion of several tumor markers, may be premature[14].
It is necessary to further investigate how histone modifications impact the neoplastic process, and to study the means for prevention and treatment of colorectal carcinoma, such as chromatin structure alteration and activation of specific tumor suppressor genes.
Thanks are given to Wei-Qi Gu for performing the real-time PCR, and Hong Yin Zhu for his assistance in Western blotting.
S- Editor Liu Y L- Editor Wang XL E- Editor Chen GJ
| 1. | Marx J. How cells cycle toward cancer. Science. 1994;263:319-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Kim JS, Lee S, Lee T, Lee YW, Trepel JB. Transcriptional activation of p21(WAF1/CIP1) by apicidin, a novel histone deacetylase inhibitor. Biochem Biophys Res Commun. 2001;281:866-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Archer SY, Hodin RA. Histone acetylation and cancer. Curr Opin Genet Dev. 1999;9:171-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 194] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 4. | Shin JY, Kim HS, Park J, Park JB, Lee JY. Mechanism for inactivation of the KIP family cyclin-dependent kinase inhibitor genes in gastric cancer cells. Cancer Res. 2000;60:262-265. [PubMed] |
| 5. | Fang JY, Lu YY. Effects of histone acetylation and DNA methylation on p21( WAF1) regulation. World J Gastroenterol. 2002;8:400-405. [PubMed] |
| 6. | Fang JY, Chen YX, Lu J, Lu R, Yang L, Zhu HY, Gu WQ, Lu LG. Epigenetic modification regulates both expression of tumor-associated genes and cell cycle progressing in human colon cancer cell lines: Colo-320 and SW1116. Cell Res. 2004;14:217-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Japanese Society for Cancer of Colon and Rectum. General Rules for Clinical Pathological Studies on Cancer of Colon, Rectum and Anus, Ed.5. Tokyo: Kanehara Public Co 1994; . |
| 8. | Giannini CD, Roth WK, Piiper A, Zeuzem S. Enzymatic and antisense effects of a specific anti-Ki-ras ribozyme in vitro and in cell culture. Nucleic Acids Res. 1999;27:2737-2744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Fang JY, Mikovits JA, Bagni R, Petrow-Sadowski CL, Ruscetti FW. Infection of lymphoid cells by integration-defective human immunodeficiency virus type 1 increases de novo methylation. J Virol. 2001;75:9753-9761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3455] [Cited by in RCA: 3346] [Article Influence: 115.4] [Reference Citation Analysis (0)] |
| 11. | Gu Y, Turck CW, Morgan DO. Inhibition of CDK2 activity in vivo by an associated 20K regulatory subunit. Nature. 1993;366:707-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 568] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 12. | Viale G, Pellegrini C, Mazzarol G, Maisonneuve P, Silverman ML, Bosari S. p21WAF1/CIP1 expression in colorectal carcinoma correlates with advanced disease stage and p53 mutations. J Pathol. 1999;187:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Matsushita K, Kobayashi S, Kato M, Itoh Y, Okuyama K, Sakiyama S, Isono K. Reduced messenger RNA expression level of p21 CIP1 in human colorectal carcinoma tissues and its association with p53 gene mutation. Int J Cancer. 1996;69:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Donadelli M, Costanzo C, Faggioli L, Scupoli MT, Moore PS, Bassi C, Scarpa A, Palmieri M. Trichostatin A, an inhibitor of histone deacetylases, strongly suppresses growth of pancreatic adenocarcinoma cells. Mol Carcinog. 2003;38:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Boffa LC, Lupton JR, Mariani MR, Ceppi M, Newmark HL, Scalmati A, Lipkin M. Modulation of colonic epithelial cell proliferation, histone acetylation, and luminal short chain fatty acids by variation of dietary fiber (wheat bran) in rats. Cancer Res. 1992;52:5906-5912. [PubMed] |
| 16. | Chen YX, Fang JY, Zhu HY, Lu R, Cheng ZH, Qiu DK. Histone acetylation regulates p21WAF1 expression in human colon cancer cell lines. World J Gastroenterol. 2004;10:2643-2646. [PubMed] |
| 17. | Carethers JM, Hawn MT, Greenson JK, Hitchcock CL, Boland CR. Prognostic significance of allelic lost at chromosome 18q21 for stage II colorectal cancer. Gastroenterology. 1998;114:1188-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Chung DC. Molecular prognostic markers and colorectal cancer: the search goes on. Gastroenterology. 1998;114:1330-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |