Published online Apr 21, 2007. doi: 10.3748/wjg.v13.i15.2198
Revised: February 12, 2007
Accepted: March 1, 2007
Published online: April 21, 2007
AIM: To investigate the interactions at a metabolic level between lovastatin, amiodarone and carbon tetrachloride in isolated rat hepatocytes.
METHODS: For cell isolation two-step collagenase liver perfusion was performed. Lovastatin was administered alone in increasing concentrations (1 μmol/L, 3 μmol/L, 5 μmol/L and 10 μmol/L) and in combination with CCl4 (86 μmol/L). The cells were also pretreated with 14 μmol/L amiodarone and then the other two compounds were added.
RESULTS: Lovastatin promoted concentration-dependent significant toxicity estimated by decrease in cell viability and GSH level by 45% and 84%, respectively. LDH-activity increased by 114% and TBARS content by 90%. CCl4 induced the expected severe damage on the examined parameters. CCl4 induced toxicity was attenuated after lovastatin pretreatment, which was expressed in less increased values of LDH activity and TBARS levels, as well as in less decreased cell viability and GSH concentrations. However, the pretreatment of hepatocytes with amiodarone abolished the protective effect of lovastatin.
CONCLUSION: We suggest that the observed cytopro-tective effect was due to interactions between lovastatin, CCl4 and amiodarone at a metabolic level.
-
Citation: Krasteva A, Mitcheva M, Kondeva-Burdina M, Descatoire V.
In vitro study of lovastatin interactions with amiodarone and with carbon tetrachloride in isolated rat hepatocytes. World J Gastroenterol 2007; 13(15): 2198-2204 - URL: https://www.wjgnet.com/1007-9327/full/v13/i15/2198.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i15.2198
The 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, commonly referred to as statins, are widely used in hypercholesterolemia and prevention of cardiovascular events[1]. Statins inhibit the rate-limiting step in the mevalonate pathway, reducing the endogenous de novo synthesis of cholesterol[1]. Statins differ with respect to their pharmacokinetic properties and drug interaction profiles. Lovastatin is intensively metabolized by CYP3A4[2-4].
The most common side effect during statin-therapy is skeletal muscle toxicity (from myalgia to rhabdomyolisis). Some cases of statin-related liver toxicity have also been reported[5-8]. The incidence is heightened, when statins are used in combination with other compounds, fibrates, NSAID, and ethanol-intake[2,9], which is connected to their pharmacokinetics and pharmacodynamics.
However, some recent in vitro data suggest that statins can promote potent systemic antioxidant effects through suppression of oxidation pathways[10-12]. In hypercholesterolic animals Chen et al[13] have found that lovastatin, as well as vitamin E and amlodipine, reduced lipid peroxidation and preserved superoxide dismutase activity. El-Swefy S et al[14] found that lovastatin provided antioxidant protection in a hyperlipidemic-diabetic hamster experimental model.
The precise mechanisms underlying the lovastatin-induced toxicity and its eventual antioxidant protection remain to be clarified.
This study also aims to evaluate the interactions between lovastatin, amiodarone and carbon tetrachloride at a metabolic level in freshly isolated rat hepatocytes. It is well-known that the three compounds are substrates of the CYP P450 enzyme system.
The effects of increasing concentrations of lovastatin were evaluated. The concentration of 10 μmol/L, where the most significant change has been observed, was chosen.
In another experiment the possible antioxidant effect of lovastatin in co-treatment with carbon tetrachloride, a known toxic liver agent, was assessed. To clarify the potential mechanism underlying this protective effect, amiodarone, a substrate of CYP3A4, was used.
Animals: Male Wistar rats (200 ± 20 g b.w.) were used. Rats were housed in Plexiglas cages (3 animals per cage) in a 12/12 light/dark cycle, temperature 20 ± 2°C. Food and water were provided ad libitum. Animals were purchased from the National Breeding Centre, Sofia, Bulgaria. All performed procedures were approved by the Institutional Animal Care Committee and were in accordance with European Union Guidelines for animal experimentation.
Chemicals and reagents: The following chemicals and reagents were used: lovastatin (Sigma Aldrich, Germany); amiodarone (Sigma Aldrich, Germany); Pentobarbital sodium (Sanofi, France); Hepes [N-(2-hydroxuethyl) piperazine-N’-(2-ethanesulfonic acid)] (Sigma Aldrich, Germany); NaCl (Merck, Germany); KCl (Merck, Germany); D-Glucosa (Merck, Germany); NaHCO3 (Merck, Germany); KH2PO4 (Scharlau Chemie S.A., Spain); CaCl2•2H2O (Merck, Germany); MgSO4•7H2O (Fluka AG, Germany); collagenase from Clostridium histolyticum Type IV (Sigma Aldrich, Germany); bovine albumin serum Fraction V, minimum 98% (Sigma Aldrich, Germany); EGTA (ethylene glycol-bis (β-aminoethylether)-N, N, N’, N’-tetraacetic acid) (Sigma Aldrich, Germany); 2-thiobarbituric acid (4, 6-dihydroxypyrimidine-2-thiol) (TBA) (Sigma Aldrich, Germany); trichloroacetic acid (TCA) (Valerus, Bulgaria); carbon tetrachloride (CCl4) (Merck, Germany); 2, 2’-Dinitro-5, 5’-dithiodibenzoic acid (DTNB) (Merck, Germany); lactate dehydrogenase Kit (LD opt.) (Randox, United Kingdom); penicillin G, Williams'E culture medium (Gibco-BRL); trypsin-EDTA (Gibco-BRL); hydrocortisone; fetal bovine serum (Invitrogen Carlsbad, CA); nitrocellulose membrane (Hybond); primary antibodies: CYP3A4 and CYP 2B1 (Polyclonal Chemicon, Eutromedex); rabbit anti-rabbit IgG HRP-conjugated (Dako rabbit immunoglobulins); ECL western blotting detection reagents and analysis system (Amersham, Les Ulis, France).
Isolation and incubation of hepatocytes: Rats were anaesthetized with intraperitoneal application of pentobarbital sodium (0.2 mL/100 g). In situ two-step collagenase liver perfusion and cell isolation were performed as previously described by Fau with our modifications[15].
After portal catheterization, the liver was perfused with 100 mL HEPES buffer (pH 7.85), containing 10 mmol/L HEPES, 142 mmol/L NaCl, 7 mmol/L KCl, 5 mmol/L glucose + 0.6 mmol/L EDTA (pH 7.85), followed by 200 mL HEPES buffer (pH 7.85) only and finally 200 mL HEPES buffer containing collagenase type IV (50 mg/200 mL) and 7 mmol/L CaCl2 (pH 7.85). The liver was excised, minced into small pieces and hepatocytes were dispersed in 50 mL Krebs-Ringer-bicarbonate (KRB) buffer containing 1.2 mmol/L KH2PO4, 1 mmol/L CaCl2, 1.2 mmol/L MgSO4, 5 mmol/L KCl, 5 mmol/L NaHCO3, 4.5 mmol/L glucose and 1% bovine serum albumin. After filtration, the hepatocytes were centrifuged at 500 ×g for 1 min and washed 3 times with KRB buffer.
Cells were counted by light microscopy and viability was estimated by the Trypan blue (0.2%) exclusion test. Only preparations with viability higher than 80% were used. Cells were diluted with KRB buffer (pH 7.35) to make a suspension of approximately 3 × 106 hepatocytes per mL.
Incubations were carried out in 25-mL Erlenmeyer flasks. Each flask contained 3 mL of the cell suspension (i.e. 9 × 106 hepatocytes) with or without various additions, as indicated below. Incubations were performed in a 95% O2 + 5% CO2 atmosphere at 37°C[10]. The following series of experiments were performed: (1) The cells were treated in vitro with lovastatin in increasing concentrations (0.1-10 μmol/L); (2) The cells were pretreated for 15 min with lovastatin and then loaded with 86 μmol/L CCl4; (3) The cells were treated with 14 μmol/L amiodarone.
The cells were pretreated for 15 min with 14 μmol/L amiodarone, then with 10 μmol/L lovastatin for 15 min and at the end 86 μmol/L CCl4 was added. Non-treated hepatocytes were used as controls. Shaking incubations of the samples were performed for 1 h under 95% O2 + 5% CO2 at 37°C. Thus, the metabolic activities of the hepatocytes were best preserved.
After isolation the hepatocytes were cultured at 37°C under a 5% CO2, 95% air atmosphere in Williams E culture medium, supplemented with fetal calf serum and penicillin (1000 U/mL)[16]. After cell attachment (3 h) the medium was replaced by a new, serum-free medium containing hydrocortisone (70 μmol/L) and 31 μmol/L lovastatin[17,18]. After 24 h the cells were recovered and the technique for isolation of microsomes was performed.
After the incubation period the hepatocytes were used for microsome fraction preparation by ultracentrifugation. The cells were homogenized in 3 volumes of ice-cold 0.154 mol/L KCl, 0.01 mol/L sodium potassium phosphate buffer, pH 7.4. The homogenate was firstly centrifuged at 10 000 g for 20 min. The supernatant was then centrifuged at 100 000 ×g for 60 min[19]. The microsomal pellet was resuspended in sodium potassium buffer with 20% glycerol and was stored at -80°C.
At the end of incubation the cells were counted under a microscope and the viability was estimated by a Trypan blue (0.2) exclusion test.
Cell injury was assessed by LDH activity. The level of LDH activity was measured in a suspension of isolated hepatocytes as described by Bergmeyer et al[20]. using a commercially available kit (LD opt., Randox).
At the end of the incubation cells were recovered by centrifugation at 4°C and used to measure intracellular glutathione (GSH). GSH level was assessed by measuring nonprotein sulfhydrils after precipitation of proteins with TCA, followed by measurement of thiols in the supernatant by the DTNB reagent[21]. The absorbance was measured at 412 nm.
Malondialdehyde levels were determined by monitoring thiobarbituric acid reactive substances (TBARS) according to the method of Fau et al[21].
Hepatocyte suspension (1 mL) was taken and added to 0.67 mL of 20% (w/v) TCA. After centrifugation 1 mL of the supernatant was added to 0.33 ml of 0.67% (w/v) TBA and heated at 100°C for 30 min. The absorbance was measured at 535 nm, and the amount of TBARS was calculated using a molar extinction coefficient of 1.56 × 105 mol/L-1 cm-1.
The BCA protein assay was performed to obtain protein concentrations.
Isolated microsomes (10 μg of protein) were separated by 10% SDS-polyacrylamide gel electrophoresis, transferred to nitrocellulose paper and exposed to rabbit monoclonal IgG antibody (CYP2B1 rabbit-monoclonal IgG) at a dilution recommended by the supplier. The membrane was then washed, and primary antibody was detected with the rabbit anti-rabbit-IgG conjugated to horseradish peroxidase. The bands were visualized with enhanced chemiluminescence (Amersham, Les Ulis, France). The films were scanned and the figures were created using the program Adobe Photoshop version CS2.
Statistical analysis was performed and analyzed by one way ANOVA at a significance level of P < 0.05. All data (n = 12) were expressed by mean ± SD. A correlation analysis was also applied by inclusion of all obtained values (software: Statistica 5.0).
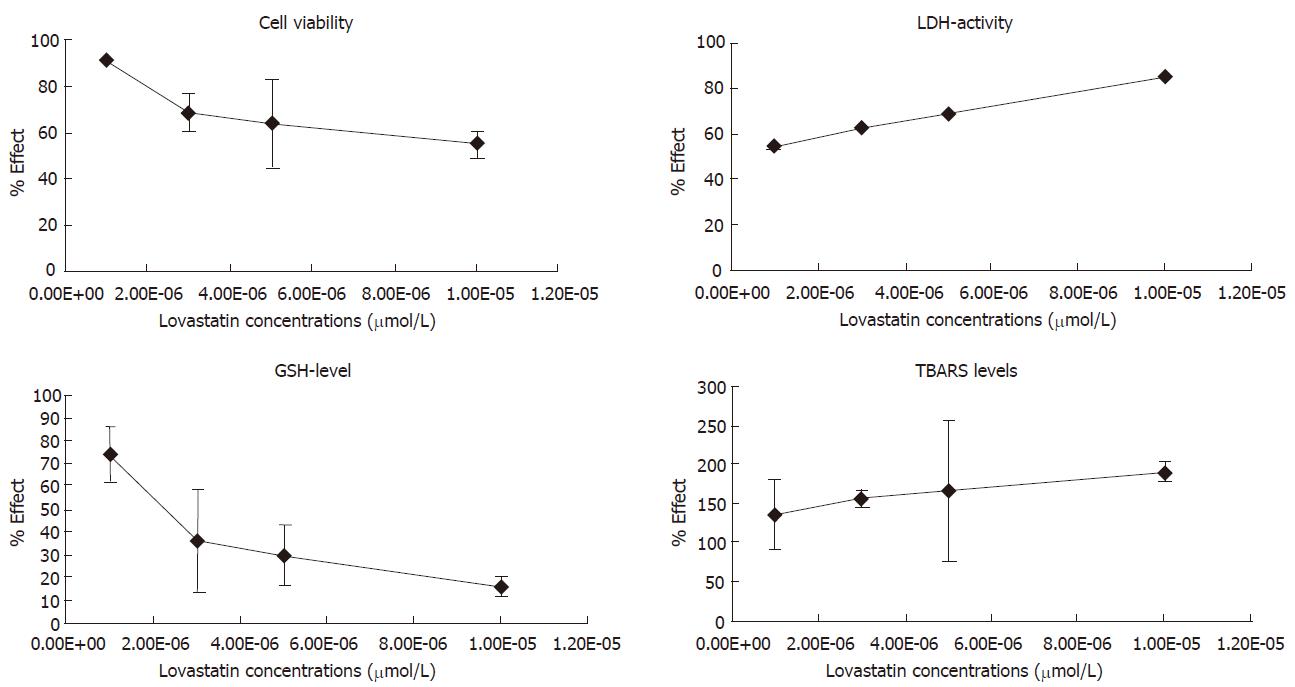
The assessed parameters after incubation of hepatocytes with lovastatin in four increasing concentrations are shown in Table 1.
| Groups | Cell viability % | LDH nmol/min/106 cells | TBARSnmol/106 cells | GSH nmol/106 cells |
| Control | 75 ± 5.97 | 0.157 ± 0.096 | 0.102 ± 0.018 | 13.0 ± 2.53 |
| 1 μmol/L Lovastatin | 68 ± 0.051b | 0.214 ± 0.084 | 0.138 ± 0.061 | 9.63 ± 1.19 |
| 3 μmol/L Lovastatin | 52 ± 4.28d | 0.248 ± 0.063b | 0.158 ± 0.016b | 4.68 ± 1.06b |
| 5 μmol/L Lovastatin | 48 ± 9.19d | 0.272 ± 0.057b | 0.169 ± 0.067b | 3.93 ± 0.51b |
| 10 μmol/L Lovastatin | 41 ± 2.36d | 0.336 ± 0.007b | 0.194 ± 0.022d | 2.05 ± 0.042d |
The low concentrations of lovastatin to 1 μmol/L did not show significant toxic effects.
The addition of 1-10 μmol/L lovastatin promoted a concentration-dependent significant decrease of cell viability and GSH concentration (Table 1, Figure 1). LDH activity and the amount of TBARS also increased significantly in a concentration-dependent manner. At the highest concentration of lovastatin (10 μmol/L) the following alterations expressed as percentage were recorded: cell viability decreased by 45% (P < 0.001) and GSH concentration by 84% (P < 0.001); LDH activity increased by 114% (P < 0.01) and TBARS content by 90% (P < 0.001) (Table 1, Figure 1). Our results are in accordance with previous findings of hepatotoxicity of lovastatin in the concentration range of 1-100 μmol/L[22].
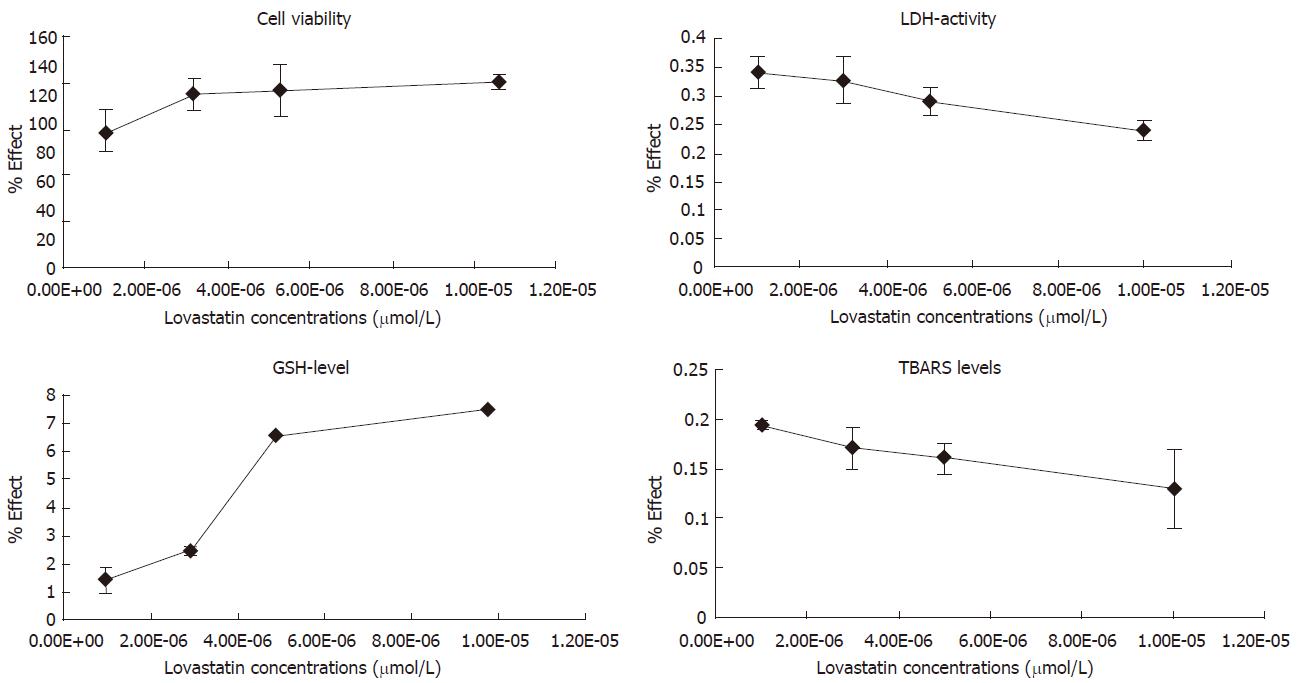
The assessed parameters after incubation of hepatocytes with increasing lovastatin concentrations and 86 μmol/L CCl4 are shown in Table 2.
| Group | Cell viability(%) | LDH nmol/min/106 cells | TBARS nmol/106 cells | GSHnmol/106 cells |
| Control | 75 ± 5.97 | 0.157 ± 0.096 | 0.102 ± 0.018 | 13.0 ± 2.53 |
| CCl4 | 35 ± 6.59f | 0.563 ± 0.098f | 0.304 ± 0.056f | 1.24 ± 0.43f |
| 1 μmol/L Lovastatin + CCl4 | 33 ± 4.99f | 0.342 ± 0.028da | 0.193 ± 0.004da | 1.40 ± 0.49f |
| 3 μmol/L Lovastatin + CCl4 | 42 ± 4.50d | 0.328 ± 0.041a | 0.170 ± 0.020a | 2.49 ± 0.10f |
| 5 μmol/L Lovastatin + CCl4 | 43 ± 7.77d | 0.291 ± 0.025a | 0.160 ± 0.014a | 6.55 ± 0.005fb |
| 10 μmol/L Lovastatin + CCl4 | 45 ± 1.52d | 0.239 ± 0.016a | 0.130 ± 0.041a | 7.50 ± 0.008db |
CCl4 administration markedly decreased cell viability by 53% (P < 0.001) and the level of reduced glutathione by 90% (P < 0.001). Treatment with CCl4 significantly increased LDH activity by 259% (P < 0.001) and TBARS amount by 198% (P < 0.001) (Table 2).
The combination of lovastatin and CCl4 was also very toxic to the cells. The toxicity was highest in the group treated with 1 μmol/L lovastatin-CCl4: cell viability and GSH level decreased by 56% (P < 0.001) and by 89% (P < 0.001), respectively versus control group. The LDH-activity and TBARS content increased by 118% (P < 0.01) and 89% (P < 0.01), respectively versus the control group (Table 2, Figure 2).
On the other hand, the combination 10 µmol/L lovastatin-CCl4 preserved the cell viability by 29% (P < 0.001) and GSH level by 505% (P < 0.001), versus the group treated with CCl4 only; LDH leakage was also less since it decreased by 68% (P < 0.01); and TBARS levels decreased by 57% (P < 0.01) (Table 2, Figure 2).
The assessed parameters after incubation of hepatocytes with amiodarone, lovastatin and CCl4 are shown in Table 3.
| Group | Cellviability% | LDHnmol/min/106cells | TBARSnmol/106cells | GSHnmol/106cells |
| Control | 75 ± 5.97 | 0.157 ± 0.096 | 0.102 ± 0.018 | 13.0 ± 2.53 |
| 86 μmol/L CCl4 | 35 ± 6.59j | 0.563 ± 0.098j | 0.304 ± 0.056j | 1.24 ± 0.43j |
| 10 μmol/L Lovastatin | 41 ± 2.36j | 0.336 ± 0.007f | 0.194 ± 0.022j | 2.05 ± 0.042h |
| 10 μmol/L Lovastatin + 86 μmol/L CCl4 | 45 ± 1.52bj | 0.239 ± 0.016a | 0.130 ± 0.041af | 7.50 ± 0.008d |
| 14 μmol/L amiodarone | 65 ± 2.71dj | 0.229 ± 0.046b | 0.156 ± 0.072ah | 5.51 ± 1.06fj |
| 14 μmol/L amiodarone + 86 μmol/L CCl4 | 39 ± 6.63j | 0.270 ± 0.032af | 0.162 ± 0.059ah | 4.80 ± 0.284dj |
| 14 μmol/L amiodarone + 10 μmol/L lovastatin | 52 ± 4.58dfj | 0.294 ± 0.032hl | 0.166 ± 0.063ah | 5.42 ± 1.04bjl |
| 14 μmol/L amiodarone + 10 μmol/L lovastatin + 86 μmol/L CCl4 | 35 ± 2.22j | 0.286 ± 0.052f | 0.151 ± 0.077af | 2.94 ± 1.61j |
Hepatocyte incubation with amiodarone (14 μmol/L) resulted in significant reduction of cell viability by 13% (P < 0.001), increased LDH leakage by 46% (P < 0.05), depletion of GSH by 58% (P < 0.001) and increased TBARS by 53% (P < 0.01) compared to the control (Table 3).
The co-treatment of hepatocytes with amiodarone and lovastatin resulted in the following alterations: cell viability and GSH level decreased by 31% (P < 0.001) and 58% (P < 0.001), respectively; LDH activity increased by 114% (P < 0.01) and TBARS levels by 63% (P < 0.01) vs control group.
The same results assigned to the group treated with lovastatin only, had the following alterations: Cell viability increased by 27 (P < 0.01); LDH-activity decreased by 13% (P < 0.01); GSH level rose by 164% (P < 0.01); TBARS levels didn’t change significantly.
The combination of amiodarone and carbon tetrachloride showed a significant reduction in cell viability-by 48% (P < 0.001) and GSH amount-by 63% (P < 0.001); LDH leakage and TBARS levels rose by 72% (P < 0.05) and 59% (P < 0.01), respectively vs control group.
Pre-incubation of the hepatocytes with amiodarone abolished the protective effect of lovastatin on CCl4-induced injury. Cell viability decreased by 22% (P < 0.05), GSH level by 61% (P < 0.01) and LDH activity and TBARS level increased by 20% (P > 0.05) and 16% (P > 0.05), respectively vs the group treated with lovastatin and CCl4.
The correlation coefficient (r) values were between -0.73 and -0.90 for the following correlation pairs: cell viability/LDH activity; cell viability/TBARS content; LDH activity/GSH content; TBARS content/GSH content. The correlation coefficients between cell viability/GSH content and LDH-activity/TBARS content amounted to +0.83 and +0.75, respectively.
All correlation coefficient values were highly significant (P < 0.001).
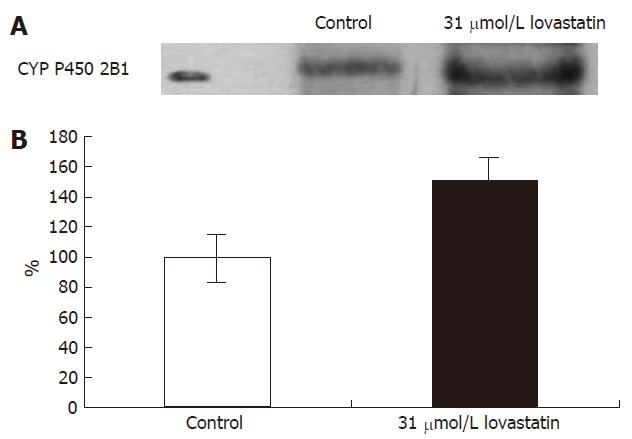
After incubation of hepatocytes with 31 μmol/L lovastatin, CYP 2B1 protein was quantified by Western blot and expressed as a percentage of the mean value of control cells (Figure 3). Considering absolute values, there was a significant increase (49%) in protein expression by the cells treated with lovastatin.
Isolated hepatocytes provide an opportunity to evaluate the effects of direct interactions of the studied compounds with endogenous factors. The present study showed considerable alterations in isolated hepatocytes under the influence of the tested compounds lovastatin, CCl4 and amiodarone. We chose CCl4 not only as a known toxic agent, but also as a substrate of CYP P450 isoenzymes in order to evaluate the precise mechanism of interactions at a metabolic level with lovastatin.
After oral ingestion, lovastatin, which is an inactive lactone, is readily hydrolyzed to the corresponding β-hydroxyacid form and its 6’-hydroxy, 6’-hydroxymethyl, and 6’-hydroxymethylene derivates. The principle metabolite (β-hydroxyacid form) is a specific inhibitor of HMG-CoA-reductase[2] which is pharmacologically active. Previous studies have shown that cytochrome P450 3A enzymes are primarily responsible for the metabolism of lovastatin in rat and human liver microsomes[4,22]. Kocarek et al[17] demonstrated that lovastatin induces several varieties of cytochrome P450, including CYP2B1/2, CYP3A1/2.
It is well known that CCl4 causes hepatic injury after metabolization by CYP 2E1, CYP 2B1 and to a smaller extend by CYP 3A[18,23]. The formed radicals covalently bind to lipid membranes and other cellular macromolecules[24,25]. CCl4-induced lipid peroxidation is highly dependent on its bioactivation to the trichloromethyl and trichloromethyl peroxy radicals. Consequently, unsaturated fatty acids in hepatocyte membranes undergo perturbations, resulting in production of lipid peroxides[24,25].
In this study the administration of lovastatin alone exerted toxic effects on isolated hepatocytes estimated by cell viability, LDH activity, the amount of TBARS and GSH level (Table 1). The unfavorable consequences increased gradually with the rising lovastatin concentrations. The correlation is significant and negative. This finding is not surprising, as there are some episodic data pointing at hepatotoxicity due to statin therapy[3,26-29]. The precise mechanism underlying hepatotoxicity has not been fully elucidated. Kornbrust et al[30] have found that the toxicity of high doses of lovastatin to rabbits is a consequence of a highly exaggerated pharmacologic action in blocking mevalonate synthesis. Rousseau et al[3] discovered that lovastatin decreased ubiquinone tissue level in the hypercholesterolemic hamster. In another study Willis et al[4] demonstrate that lovastatin decreased tissue ubiquinone levels in rats. It is well known that ubiquinone acts primarily by preventing the formation of lipid peroxyl radicals[31]. Kubota et al[9] have found that statins (1-100 μmol/L) cause toxicity in cultured human hepatocytes.
CCl4 induced the expected severe damage demonstrated by markedly elevated levels of LDH activity and decreased cell viability. The increased TBARS content suggests oxidative stress promoting an expressed GSH depletion. In this study CCl4 toxicity was obviously attenuated after lovastatin pretreatment (Table 2). Recent data suggest that lovastatin prevents hepatic GSH depletion induced by acetaldehyde[32,33]. Jeon et al[34] have found that lovastatin exhibited an inhibitory effect on the plasma and hepatic lipid peroxidation and increased the hepatic catalase activity in high-cholesterol fed rabbits. Chen et al[13] stated that lovastatin reduced lipid peroxidation and preserved superoxide dismutase in vivo in rabbits fed cholesterol diet. These suggestions explain and confirm the biological protective effect of lovastatin. Our data indicating improvement in TBARS and GSH values after lovastatin pretreatment are in accordance with the above investigations.
These phenomena could also be ascribed to an interaction at a metabolic level between lovastatin and CCl4. As previously mentioned, lovastatin and CCl4 are metabolized by identical CYP isoenzymes, namely CYP 3A4 and CYP 2B1[17,18]. Our results show that lovastatin induces expression of CYP 2B1 in cultured rat hepatocytes, assessed by Western blot analysis (Figure 1).
We can suggest that in combination both compounds compete for the same isoforms. Thus, the metabolism of lovastatin is inhibited and the active metabolite is less formed. This probably led to the reduced toxicity observed in hepatocytes (Table 2).
To test this hypothesis we pretreated hepatocytes with amiodarone, a substrate and inhibitor of CYP3A4[35]. Amiodarone at a concentration of 14 μmol/L[27] exerted toxic effects (Table 3), manifested by a decrease of cell viability, GSH level and by an increase of LDH leakage and TBARS level.
The pretreatment of hepatocytes with amiodarone, a substrate of CYP 3A4, reduced the toxic effect of lovastatin. We can suggest that an inhibition of its metabolism takes place. Probably amiodarone interferes with the metabolism of lovastatin by the CYP3A4 metabolic pathway, thus the metabolites of lovastatin are less produced and there is no severe toxicity.
In combination with CCl4, amiodarone is a smaller protector as in the group with lovastatin. The pretreatment of hepatocytes with amiodarone affected the metabolism of CCl4 by the CYP 3A4 metabolic pathway, which can explain the significantly smaller toxic effect. LDH activity and TBARS levels are less expressed than in the CCl4 group. Reduced GSH is also preserved. We can speculate that the pretreatment of hepatocytes with amiodarone inhibited the formation of intermediate reactive metabolites and there was no severe damage as observed in the group treated with CCl4 only.
The combination of the three substrates-amiodarone, lovastatin and CCL4 resulted in significant toxicity. The applied concentration of amiodarone is probably not sufficient to inhibit at the same time the metabolism of lovastatin and CCl4. This might explain the lack of protection of lovastatin.
In conclusion, our data show the following phenomena: Lovastatin is hepatotoxic in a concentration-dependent manner; Lovastatin exerts some protective effect on CCl4-induced injury, probably through an interaction of cytochrome P450 on a metabolic level by CYP3A4 and CYP 2B1. The observed protective effect of lovastatin on CCl4-induced toxicity is abolished after pretreatment with amiodarone, substrate and inhibitor of CYP3A4.
Many patients with hyperlipidemia present the concomitant medical problems, such as diabetes, hypertension, and coronary artery disease. Statins are very often used in combination with other medications. This raises the potential for adverse drug-drug interactions, which can have serious clinical consequences. Our data suggest that the combined intake of lovastatin with other substrates of different isoforms of cytochrome P450 (drugs, biological active agents) can result in interactions on a biotransformation level. In these cases, clinically, drug-drug interactions may lead to severe toxicity and may force discontinuation of needed pharmacotherapy.
S- Editor Wang J L- Editor Alpini GD E- Editor Chen GJ
| 1. | Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3933] [Cited by in RCA: 4001] [Article Influence: 114.3] [Reference Citation Analysis (0)] |
| 2. | Beaird SL. HMG-CoA reductase inhibitors: assessing differences in drug interactions and safety profiles. J Am Pharm Assoc (Wash). 2000;40:637-644. [PubMed] |
| 3. | Rousseau G, Veronneau M, Des Rosiers C, Varin F. Effects of lovastatin and pravastatin on ubiquinone and 4-hydroxynonenal tissue levels in the hypercholestrolemic hamster. Curr Ther Res. 1999;60:87-104. |
| 4. | Willis RA, Folkers K, Tucker JL, Ye CQ, Xia LJ, Tamagawa H. Lovastatin decreases coenzyme Q levels in rats. Proc Natl Acad SciUSA. 1990;87:8928-8930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Ballaré M, Campanini M, Catania E, Bordin G, Zaccala G, Monteverde A. Acute cholestatic hepatitis during simvastatin administration. Recenti Prog Med. 1991;82:233-235. [PubMed] |
| 6. | Bruguera M, Joya P, Rodés J. Hepatitis associated with treatment with lovastatin. Presentation of 2 cases. Gastroenterol Hepatol. 1998;21:127-128. [PubMed] |
| 7. | Grimbert S, Pessayre D, Degott C, Benhamou JP. Acute hepatitis induced by HMG-CoA reductase inhibitor, lovastatin. Dig Dis Sci. 1994;39:2032-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Hartleb M, Rymarczyk G, Januszewski K. Acute cholestatic hepatitis associated with pravastatin. Am J Gastroenterol. 1999;94:1388-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Kubota T, Fujisaki K, Itoh Y, Yano T, Sendo T, Oishi R. Apoptotic injury in cultured human hepatocytes induced by HMG-CoA reductase inhibitors. Biochem Pharmacol. 2004;67:2175-2186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Shishehbor MH, Brennan ML, Aviles RJ, Fu X, Penn MS, Sprecher DL, Hazen SL. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation. 2003;108:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 293] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 11. | Wassmann S, Laufs U, Müller K, Konkol C, Ahlbory K, Bäumer AT, Linz W, Böhm M, Nickenig G. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2002;22:300-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 393] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 12. | Yang S, Zhu H, Li Y, Lin H, Gabrielson K, Trush MA, Diehl AM. Mitochondrial adaptations to obesity-related oxidant stress. Arch Biochem Biophys. 2000;378:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 263] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 13. | Chen L, Haught WH, Yang B, Saldeen TG, Parathasarathy S, Mehta JL. Preservation of endogenous antioxidant activity and inhibition of lipid peroxidation as common mechanisms of antiatherosclerotic effects of vitamin E, lovastatin and amlodipine. J Am Coll Cardiol. 1997;30:569-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 153] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | El-Swefy S, Schaefer EJ, Seman LJ, van Dongen D, Sevanian A, Smith DE, Ordovas JM, El-Sweidy M, Meydani M. The effect of vitamin E, probucol, and lovastatin on oxidative status and aortic fatty lesions in hyperlipidemic-diabetic hamsters. Atherosclerosis. 2000;149:277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Mitcheva M, Kondeva M, Vitcheva V, Nedialkov P, Kitanov G. Effect of benzophenones from Hypericum annulatum on carbon tetrachloride-induced toxicity in freshly isolated rat hepatocytes. Redox Rep. 2006;11:3-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Berson A, Renault S, Lettéron P, Robin MA, Fromenty B, Fau D, Le Bot MA, Riché C, Durand-Schneider AM, Feldmann G. Uncoupling of rat and human mitochondria: a possible explanation for tacrine-induced liver dysfunction. Gastroenterology. 1996;110:1878-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Kocarek TA, Reddy AB. Regulation of cytochrome P450 expression by inhibitors of hydroxymethylglutaryl-coenzyme A reductase in primary cultured rat hepatocytes and in rat liver. Drug Metab Dispos. 1996;24:1197-1204. [PubMed] |
| 18. | Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33:105-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 1149] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 19. | Robin MA, Maratrat M, Loeper J, Durand-Schneider AM, Tinel M, Ballet F, Beaune P, Feldmann G, Pessayre D. Cytochrome P4502B follows a vesicular route to the plasma membrane in cultured rat hepatocytes. Gastroenterology. 1995;108:1110-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Bergmeyer HU, Gawehn K, Grassil M. Methods of enzymatic analysis 3th ed. In: Verlag Chemie, Weinheim, H.U. Bergmeyer (editors). 1974;1:481-482. |
| 21. | Fau D, Berson A, Eugene D, Fromenty B, Fisch C, Pessayre D. Mechanism for the hepatotoxicity of the antiandrogen, nilutamide. Evidence suggesting that redox cycling of this nitroaromatic drug leads to oxidative stress in isolated hepatocytes. J Pharmacol Exp Ther. 1992;263:69-77. [PubMed] |
| 22. | Cohen LH, van Leeuwen RE, van Thiel GC, van Pelt JF, Yap SH. Equally potent inhibitors of cholesterol synthesis in human hepatocytes have distinguishable effects on different cytochrome P450 enzymes. Biopharm Drug Dispos. 2000;21:353-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Kitamura S, Shimizu Y, Shiraga Y, Yoshida M, Sugihara K, Ohta S. Reductive metabolism of p,p'-DDT and o,p'-DDT by rat liver cytochrome P450. Drug Metab Dispos. 2002;30:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Kim HJ, Chun YJ, Park JD, Kim SI, Roh JK, Jeong TC. Protection of rat liver microsomes against carbon tetrachloride-induced lipid peroxidation by red ginseng saponin through cytochrome P450 inhibition. Planta Med. 1997;63:415-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Tirkey N, Pilkhwal S, Kuhad A, Chopra K. Hesperidin, a citrus bioflavonoid, decreases the oxidative stress produced by carbon tetrachloride in rat liver and kidney. BMC Pharmacol. 2005;5:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 157] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Heuer T, Gerards H, Pauw M, Gabbert HE, Reis HE. Toxic liver damage caused by HMG-CoA reductase inhibitor. Med Klin (Munich). 2000;95:642-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Huchzermeyer H, Münzenmaier R. Lovastatin-induced acute cholestatic hepatitis. Dtsch Med Wochenschr. 1995;120:252-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Ohyama K, Nakajima M, Suzuki M, Shimada N, Yamazaki H, Yokoi T. Inhibitory effects of amiodarone and its N-deethylated metabolite on human cytochrome P450 activities: prediction of in vivo drug interactions. Br J Clin Pharmacol. 2000;49:244-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 144] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Raveh D, Arnon R, Israeli A, Eisenberg S. Lovastatin-induced hepatitis. Isr J Med Sci. 1992;28:101-102. [PubMed] |
| 30. | Kornbrust DJ, MacDonald JS, Peter CP, Duchai DM, Stubbs RJ, Germershausen JI, Alberts AW. Toxicity of the HMG-coenzyme A reductase inhibitor, lovastatin, to rabbits. J Pharmacol Exp Ther. 1989;248:498-505. [PubMed] |
| 31. | Ernster L, Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta. 1995;1271:195-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 838] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 32. | Lluis JM, Colell A, Garcia-Ruiz C, Coll O, Kaplowitz N, Fernandez-Checa JC. Acetaldehyde sensitizes HepG2 cells to tumor necrosis factor by imparing mitochondrila GSH transport through reticulum endoplasmic stress. Gastroenterology. 2007;In press. |
| 33. | Lluis JM, Colell A, García-Ruiz C, Kaplowitz N, Fernández-Checa JC. Acetaldehyde impairs mitochondrial glutathione transport in HepG2 cells through endoplasmic reticulum stress. Gastroenterology. 2003;124:708-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 115] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Jeon SM, Bok SH, Jang MK, Lee MK, Nam KT, Park YB, Rhee SJ, Choi MS. Antioxidative activity of naringin and lovastatin in high cholesterol-fed rabbits. Life Sci. 2001;69:2855-2866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 153] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 35. | Ha HR, Bigler L, Binder M, Kozlik P, Stieger B, Hesse M, Altorfer HR, Follath F. Metabolism of amiodarone (part I): identification of a new hydroxylated metabolite of amiodarone. Drug Metab Dispos. 2001;29:152-158. [PubMed] |