Published online Apr 21, 2007. doi: 10.3748/wjg.v13.i15.2193
Revised: March 3, 2007
Accepted: March 22, 2007
Published online: April 21, 2007
AIM: To evaluate the efficacy of 5 compared to 10 granulocyteaphaeresis sessions in patients with active steroid-dependent ulcerative colitis.
METHODS: In this pilot, prospective, multicenter randomized trial, 20 patients with moderately active steroid-dependent ulcerative colitis were randomized to 5 or 10 granulocyteaphaeresis sessions. The primary objective was clinical remission at wk 17. Secondary measures included endoscopic remission and steroid consumption.
RESULTS: Nine patients were randomized to 5 granulocyteaphaeresis sessions (group 1) and 11 patients to 10 granulocyteaphaeresis sessions (group 2). At wk 17, 37.5% of patients in group 1 and 45.45% of patients in group 2 were in clinical remission. Clinical remission was accompanied by endoscopic remission in all cases. Eighty-six percent of patients achieving remission were steroid-free at wk 17. Daily steroid requirements were significantly lower in group 2. Eighty-nine per cent of patients remained in remission during a one year follow-up. One serious adverse event, not related to the study therapy, was reported.
CONCLUSION: Granulocyteaphaeresis is safe and effective for the treatment of steroid-dependent ulcerative colitis. In this population, increasing the number of aphaeresis sessions is not associated with higher remission rates, but affords a significant steroid-sparing effect.
- Citation: Ricart E, Esteve M, Andreu M, Casellas F, Monfort D, Sans M, Oudovenko N, Lafuente R, Panés J. Evaluation of 5 versus 10 granulocyteaphaeresis sessions in steroid-dependent ulcerative colitis: A pilot, prospective, multicenter, randomized study. World J Gastroenterol 2007; 13(15): 2193-2197
- URL: https://www.wjgnet.com/1007-9327/full/v13/i15/2193.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i15.2193
Ulcerative colitis (UC) is a chronic relapsing inflammatory disorder. Corticosteroids are the mainstay of treatment to induce remission. However, long-term corticosteroid use is not recommended due to both toxicity and lack of efficacy. In addition, steroid dependency and resistance occur frequently in patients with UC[1]. In these patients, immune modulators (azathioprine/6-mercaptopurine)[2] and more recently the chimeric monoclonal antibody against tumor necrosis factor, infliximab, have shown therapeutic efficacy[3], but a proportion of patients are intolerant or suffer adverse events related to these medications. Therefore, other therapies are needed to provide non-surgical and safe alternatives for patients who fail to respond to conventional treatment.
In vitro and in vivo studies have suggested that UC relapses are associated with an influx of granulocytes and monocytes/macrophages into the mucosal tissue, and these cell types are regarded as key mediators of mucosal damage[4]. Reducing granulocytes and monocytes by apheresis has the potential to reduce bowel inflammation and to improve patient status. Several controlled and uncontrolled studies have assessed the safety and efficacy of granulocyteaphaeresis (GCAP) in steroid-dependent UC[5-8], steroid-refractory UC[9-11], or as first-line therapy in naïve UC patients[12,13]. However, the optimal treatment scheme is still to be established. In a study by Hanai et al[9], almost 40% of patients with steroid-dependent UC entered into remission after 5 sessions of GCAP compared to 80% after 10 sessions. However, in this study the number of aphaeresis sessions was not established randomly but based on clinical demand, and the question whether a higher number of aphaeresis increases the efficacy of this treatment remains open. We report the results of a small pilot prospective, multicenter, randomized study comparing the efficacy of 5 GCAP and 10 GCAP sessions to induce remission in steroid-dependent UC. The primary end point of the study was clinical remission at wk 17.
This was a pilot open, prospective, multicenter, randomized study evaluating the efficacy and safety of 5 compared to 10 GCAP sessions in patients with moderately active steroid-dependent UC. The study was conducted in five centers in Spain according to the Declaration of Helsinki and the Good Clinical Practice guidelines. The study was approved by the Institutional Review Board of each participating center and written informed consent was obtained from all patients.
Patients were included in the study if they were between 18 and 75 years of age and had clinical and endoscopic evidence of moderately active UC defined as a clinical activity index (CAI) ≥ 6 and ≤ 12 and an endoscopic activity index (EAI) > 4[14]. Additional inclusion criteria were as follows: body weight ≥ 40 kg, use of ≥ 400 mg of prednisone (or equivalent) within 4 wk before study start and at least one unsuccessful attempt to taper corticosteroids, affected colon length ≥ 25 cm, negative stool culture and no cytomegalovirus infection at screening as assessed by immunohistochemistry of colonic tissue samples.
Exclusion criteria included severe disease activity (CAI > 12), aminosalicylates started or increased in dosage within the prior 4 wk, or immune modulators started within 2 mo prior the study entry; increased steroid dose surpassing the baseline dose during the study period; severe local or systemic infection; viral infection within 4 wk of study entry; hypotension (systolic blood pressure < 80 mm Hg and/or diastolic blood pressure < 50 mm Hg) or hypertension (systolic blood pressure > 180 mm Hg and/or diastolic blood pressure > 120 mm Hg); serious renal, hepatic or cardiovascular disease; significant laboratory abnormalities such as absolute neutrophil count < 1 × 109/L, platelet count < 100 × 109/L , haemoglobin < 8 g/L, AST or ALT > 3 times upper normal range, bilirubin > 2.5 upper normal range, serum creatinine > 1.8 mg/dL, partial thromboplastin time > 20% upper normal range, prothombrin time < 50%; pregnancy; breast-feeding; or known allergy to heparin.
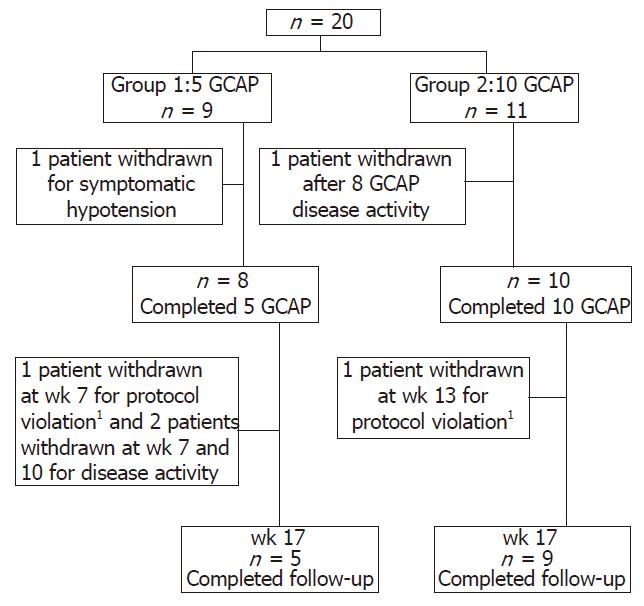
Patients were randomly assigned to receive 5 (group 1) or 10 (group 2) GCAP sessions over 5 or 10 consecutive weeks, respectively, in an out-patient setting (Figure 1). Aphaeresis sessions lasted for 60 min at a flow rate of 30 mL/h. The aphaeresis system used consisted of a pump with an integrated monitor (Adamonitor®, Otsuka Electronics, Japan) and a single use polycarbonate column (Adacolumn®, JIMRO, Japan) with a capacity of 335 mL filled with about 35 000 pieces of cellulose acetate beads of 2 mm in diameter bathed in physiological saline and steam sterilized (JIMRO, Japan). During the sessions, sodium heparin was continuously infused at a rate of 25 UI/min. All adverse events during the study period were registered.
Clinical and endoscopic assessment was performed according to the Rachmilewitz Index (CAI and EAI) (14). CAI was measured at baseline, and thereafter on a weekly basis until wk 17 (primary endpoint). Endoscopic assessment of disease severity was performed at screening and at wk 17. IBDQ was used to assess quality of life. The primary endpoint was clinical remission at wk 17 defined by a CAI ≤ 4. Secondary endpoints included changes in quality of life (IBDQ), endoscopic remission, and steroid consumption. Endoscopic remission was defined as an EAI ≤ 4. Steroid dose could be tapered at the investigator's discretion. In the case of worsening disease activity, steroid dose could be increased without surpassing the baseline dose.
Analysis of the results were performed on an intention to treat (ITT) basis. All patients who completed at least one aphaeresis session were considered in the analysis. Results are presented as mean ± SD or median values. For quantitative parameters statistical significance in comparison to baseline within groups was calculated using the Wilcoxon Signed Rank Test. Differences in categorical variables were assessed using Fisher’s exact test. The Mann-Whitney U-test was used for comparison of results between groups. Statistical analysis was done by Systat® 1.1.
A total of 20 patients were included in the study. Nine patients were randomized to group 1 and 11 patients to group 2. One patient in group 1 was excluded from the study because of symptomatic hypotension at the time of venopuncture before initiating the first aphaeresis treatment.
Demographics and baseline disease characteristics are shown in Table 1. Disease duration was longer in group 2 compared to group 1 (78 ± 42.8 mo and 36 ± 32.3 mo, respectively) but the difference was not statistically significant (P = 0.4). All other parameters were similar between the two groups. The mean number of relapses during the year prior to study entry was 2.33 ± 0.71 and 2.22 ± 1.09 for groups 1 and 2, respectively. Fifty-five per cent of patients in group 1 and 64% of patients in group 2 were previously treated with immune modulators (azathioprine, methotrexate). One patient in group 1 and 3 patients in group 2 had required cyclosporine for previous steroid refractory flares. All patients in both groups were active in spite of receiving high steroid dose (43 ± 10.31 and 35 ± 14.9 mg/d of prednisone for group 1 and 2, respectively).
| Group 1 (n = 9) | Group 2 (n = 11) | |
| Age (yr) | 38.1 ± 13.5 | 44.6 ± 16.6 |
| Gender (male/female) | 6/3 | 6/5 |
| Smoking habit (smoker/non- smoker/former-smoker) | 0/7/2 | 0/4/7 |
| Steroid dose at study entrance (mg/d) | 43 ± 10.31 | 35 ± 14.91 |
| Previous medications | ||
| 5-ASA | 9 | 11 |
| Azathioprine | 5 | 6 |
| Methotrexate | 0 | 1 |
| Cyclosporin A | 1 | 3 |
| Disease duration (mo) | 36 ± 32.3 | 78 ± 42.8 |
| Nº of previous flares in the last 12 mo | 2.33 ± 0.71 | 2.22 ± 1.09 |
| Nº of previous admissions to hospital | 1.33 ± 1.22 | 1.18 ± 1.25 |
| Site of disease | ||
| Proctosigmoiditis | 1 | 4 |
| Left sided colitis | 4 | 4 |
| Extensive colitis | 2 | 1 |
| Pancolitis | 2 | 2 |
| CAI | 8.0 ± 1.0 | 7.9 ± 1.2 |
| EAI | 8.7 ± 3.7 | 8.8 ± 2.2 |
| IBDQ | 106 (230-70) | 147 (224-100) |
| CRP (mg/L) | 8 (23.1-2.5) | 4.6 (13.6-0.5) |
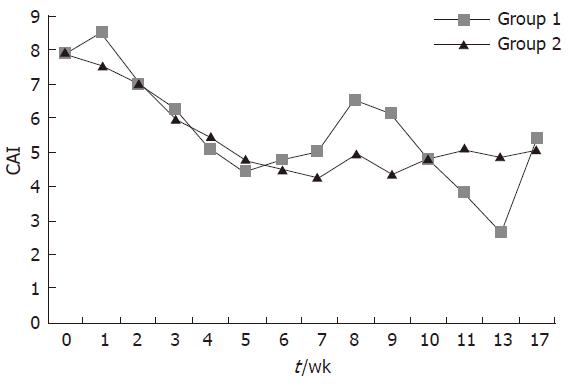
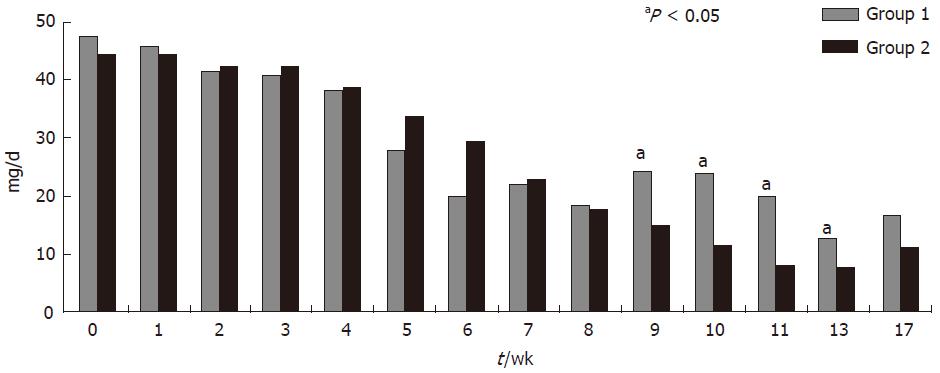
At wk 17, 3 of 8 patients (37.5%) in group 1 and 5 of 11 patients (45.45%) in group 2 were in clinical remission. CAI decreased from 8 ± 1 at baseline to 5.4 ± 3.0 at wk 17 in group 1, and from 7.9 ± 1.2 at baseline to 5.3 ± 5.0 at wk 17 in group 2; the reduction in CAI score was of similar magnitude in both groups (P: NS) (Figure 2). All patients who entered into clinical remission in both groups were also in endoscopic remission. Median C reactive protein (CRP) values showed a trend to reduction in group 2 [median 4.6 mg/L (13.6-0.5) at wk 1 and 2.6 mg/L (31.4-0.3) at wk 17] but not in group 1 [median 8.0 mg/L (13.8-2.5) at wk 1 and 10.4 mg/L (15.8-5.9) at wk 17]. The steroid dose was reduced in both groups. When compared with baseline, the dose of steroid was significantly reduced (P < 0.05) since wk 4 in group 2 and since wk 5 and in group 1. Intergroup comparison showed that daily steroid requirements were significantly lower in group 2 during the period comprised between wk 9 and 13 but not at wk 17 (P = 0.066) (Figure 3). All but one of the patients who had clinical remission were free of steroids at wk 17. There was an improvement in IBDQ scores between wk 0 and wk 17 in both groups, but the differences were not statistically significant[median 106 (230-70) at wk 0 and 110 (157-101) at wk 17 in group 1; median 147 (224-100) at wk 0 and 178 (224-100) at wk 17 in group 2]. Granulocyteaphaeresis with Adacolumn® was very well tolerated and no major side effects were observed during the study. A total of 11 non-serious adverse events and one serious adverse event were reported in both groups. The serious adverse event was a community acquired pneumonia that was judged as not related to the study therapy and resolved with an oral antibiotic.
Seven patients entered into the one year long-term follow-up. Six out of these 7 patients were in clinical remission and remained steroid free without any changes in UC therapy during this period.
A growing body of evidence suggests that GCAP can be a therapeutic alternative for UC. Most of the published studies are non-controlled, include a heterogeneous UC population, and use different GCAP regimens. The only sham-controlled study published so far, included a UC population of similar clinical characteristics to the one selected for the current study, and demonstrated significantly higher response rates in patients receiving active treatment[15]. In that study, 7 sessions of sham or active aphaeresis were performed. In another recent study, Hanai et al[9] reported that a 10 GCAP regimen was associated with higher remission rates in steroid dependent UC patients compared to 5 GCAP, but in this study the number of aphaeresis sessions was based on clinical judgment of the attending physician. No formal comparison of various treatment strategies using aphaeresis has been made so far in order to delineate an optimal treatment scheme. Our group decided to conduct a small pilot randomized controlled trial to determine if there is a therapeutic gain by performing 10 as opposed to 5 aphaeresis sessions for induction of remission in patients with steroid-dependent active UC. Patients recruited in this study represent a UC population that is difficult to treat, 60% were under immune modulator treatment with azathioprine or methotrexate, and 20% had been previously treated with cyclosporine for steroid refractory UC. None of the patients included had been previously treated with infliximab because infliximab was not authorized for UC treatment at the time the study was performed.
Our results show that GCAP induced clinical and endoscopic remission in an important proportion of the patients. At wk 17, all but one of the responding patients were free of steroid treatment. Furthermore, all responding patients but one completed a one year long-term follow up without requiring steroids or the introduction of any other therapy. These results are in keeping with the findings reported by Domenech et al[8] and support the steroid sparing effect of GCAP in steroid dependent UC patients. Interestingly, steroid consumption in our study was lower in group 2, showing statistically significant difference in a period starting when group 1 patients were no longer receiving aphaeresis treatment, and group 2 was still under active aphaeresis treatment. Although the study failed to show any difference in terms of clinical remission between 5 and 10 GCAP treatments, the aforementioned differences in steroid requirement support the notion that GCAP is effective for the treatment of UC.
Comparison of the efficacy of diverse treatment modalities across various studies is subject to numerous pitfalls, but quite often it is the only indirect information on the relative efficacy of various therapeutic options, since head to head comparisons are disappointingly scarce. The population included in the current study consisted of patients with steroid-dependent course and active disease in spite of use of steroids for two weeks, which represents a population particularly difficult to treat. The overall 42% clinical and endoscopic remission rates obtained in the present study compare favorably with those of recent studies using infliximab[3,16].
Although the study has important limitations due to sample size, it is necessary to point out that to our knowledge, this is the first randomized comparison of two different GCAP regimens in a strictly defined UC population. Even though the results show a trend towards lower steroid requirements in group 2 (10 GCAP), in our opinion these data do not support the recommendation of this regimen over a 5 GCAP alternative. Our study did not address the issue of intensification of GCAP (2-3 GCAP per week). A randomized small study in patients with active UC showed that an intensified aphaeresis regimen had more rapid but not superior therapeutic effects than a one GCAP per week schedule, as was performed in our study[17].
In conclusion, GCAP seems to be an effective, safe, and well tolerated therapy that allows steroid withdrawal in steroid dependent moderately active UC patients. However, 10 weekly GCAP treatments do not provide an advantage in this patient population as compared to 5 treatments in terms of clinical or endoscopic remission but afford a significant steroid-sparing effect. This information may be of significant value both in clinical practice and in the design of new clinical trials using this treatment modality.
S- Editor Liu Y L- Editor Lutze M E- Editor Chen GJ
| 1. | Faubion WA, Loftus EV, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 792] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 2. | Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut. 2002;50:485-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 459] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 3. | Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2744] [Cited by in RCA: 2885] [Article Influence: 144.3] [Reference Citation Analysis (2)] |
| 4. | Lügering N, Kucharzik T, Stoll R, Domschke W. Current concept of the role of monocytes/macrophages in inflammatory bowel disease--balance of proinflammatory and immunosuppressive mediators. Ital J Gastroenterol Hepatol. 1998;30:338-344. [PubMed] |
| 5. | Sawada K, Muto T, Shimoyama T, Satomi M, Sawada T, Nagawa H, Hiwatashi N, Asakura H, Hibi T. Multicenter randomized controlled trial for the treatment of ulcerative colitis with a leukocytapheresis column. Curr Pharm Des. 2003;9:307-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 128] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Hanai H, Watanabe F, Yamada M, Sato Y, Takeuchi K, Iida T, Tozawa K, Tanaka T, Maruyama Y, Matsushita I. Adsorptive granulocyte and monocyte apheresis versus prednisolone in patients with corticosteroid-dependent moderately severe ulcerative colitis. Digestion. 2004;70:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Sawada K, Hiwatashi N, Munakata A. A multicentre randomized controlled study of safety and efficacy of adsorptive granulocyte and monocyte apheresis in patients with active ulcerative colitis. Gastroenterology. 2004;126:A462. |
| 8. | Domènech E, Hinojosa J, Esteve-Comas M, Gomollón F, Herrera JM, Bastida G, Obrador A, Ruiz R, Saro C, Gassull MA. Granulocyteaphaeresis in steroid-dependent inflammatory bowel disease: a prospective, open, pilot study. Aliment Pharmacol Ther. 2004;20:1347-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Hanai H, Watanabe F, Takeuchi K, Iida T, Yamada M, Iwaoka Y, Saniabadi A, Matsushita I, Sato Y, Tozawa K. Leukocyte adsorptive apheresis for the treatment of active ulcerative colitis: a prospective, uncontrolled, pilot study. Clin Gastroenterol Hepatol. 2003;1:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 159] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Naganuma M, Funakoshi S, Sakuraba A, Takagi H, Inoue N, Ogata H, Iwao Y, Ishi H, Hibi T. Granulocytapheresis is useful as an alternative therapy in patients with steroid-refractory or -dependent ulcerative colitis. Inflamm Bowel Dis. 2004;10:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Kruis W, Mongersten J, Dignass A. Leucocytapheresis in steroid refractory ulcerative colitis: results of a multicenter pilot study. Gut. 2004;53:A228. |
| 12. | Suzuki Y, Yoshimura N, Saniabadi AR, Saito Y. Selective granulocyte and monocyte adsorptive apheresis as a first-line treatment for steroid naïve patients with active ulcerative colitis: a prospective uncontrolled study. Dig Dis Sci. 2004;49:565-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Yamamoto T, Umegae S, Kitagawa T, Yasuda Y, Yamada Y, Takahashi D, Mukumoto M, Nishimura N, Yasue K, Matsumoto K. Granulocyte and monocyte adsorptive apheresis in the treatment of active distal ulcerative colitis: a prospective, pilot study. Aliment Pharmacol Ther. 2004;20:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989;298:82-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 808] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 15. | Sawada K, Kusugami K, Suzuki Y, Bamba T, Munakata A, Hibi T, Shimoyama T. Leukocytapheresis in ulcerative colitis: results of a multicenter double-blind prospective case-control study with sham apheresis as placebo treatment. Am J Gastroenterol. 2005;100:1362-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Järnerot G, Hertervig E, Friis-Liby I, Blomquist L, Karlén P, Grännö C, Vilien M, Ström M, Danielsson A, Verbaan H. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology. 2005;128:1805-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 774] [Cited by in RCA: 768] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 17. | Sakuraba A, Naganuma M, Hibi T. Intensive therapy of granulocyte and adsorption apheresis induces rapid remission in patients with ulcerative colitis. Gastroenterology. 2003;124 Suppl 1:A522. |