Published online Apr 21, 2007. doi: 10.3748/wjg.v13.i15.2145
Revised: March 10, 2006
Accepted: March 12, 2007
Published online: April 21, 2007
There are four steps in the interaction between intestinal microbes and mucosal inflammation in genetically predisposed individuals from the viewpoints of basic and clinical aspects of inflammatory bowel disease (IBD). The first step is an interaction between intestinal microbes or their components and intestinal epithelial cells via receptors, the second step an interaction between macrophages and dendritic cells and mucosal lymphocytes, the third step an interaction between lymphocytes and vascular endothelial cells, and the fourth step an interaction between lymphocytes and granulocytes producing proinflammatory cytokines or free radicals and mucosal damage and repair. Recent therapeutic approaches for IBD aim to block these four steps in the intestinal inflammation of patients with IBD.
- Citation: Asakura H, Suzuki K, Honma T. Recent advances in basic and clinical aspects of inflammatory bowel disease: Which steps in the mucosal inflammation should we block for the treatment of inflammatory bowel disease? World J Gastroenterol 2007; 13(15): 2145-2149
- URL: https://www.wjgnet.com/1007-9327/full/v13/i15/2145.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i15.2145
Ulcerative colitis and Crohn’s disease are the nonspecific inflammatory bowel diseases (IBD) with unknown etiology. Ulcerative colitis was described by Wilks and Moxon in 1859, and Crohn’s disease by Crohn, Ginzburg, and Oppenheimer in 1932 with their clinical and pathological analyses[1]. Ulcerative colitis was not a common disease before 1945 with an average annual incidence rate of 8.4 per 100 000 population, but after 1945 it showed a gradual rise in average annual incidence rate[2]. In Europe, a steep rise in the incidence of Crohn’s disease began after 1945 and a high incidence of Crohn’s disease and a low incidence of ulcerative colitis were reported in the 1980s. After 1990 the incidence of Crohn’s disease increased from 5.2 per 100 000 population in 1988-1990 to 6.4 per 100 000 population in 1997-1999, and that of ulcerative colitis decreased from 4.2 to 3.5 per 100 000 population, respectively[3]. However, in Asia, Eastern Europe, and South America, the annual incidence rate of ulcerative colitis and Crohn’s disease was low before 1990, but it has been steadily increasing over last 10 years[4-7]. These changes in incidence rates may be influenced by various environmental factors, because the etiopathogenesis of IBD is thought to be caused by the mutual reactions among host susceptibility genes (CARD15/NOD2, HLA-class II), environmental factors including enteric flora and food antigens, and abnormal immune balance[8,9].
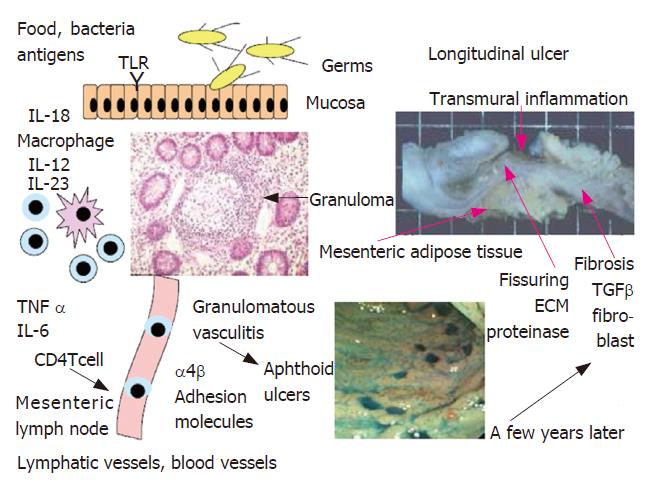
Human and murine studies of Crohn’s disease have shown an increased expression of T-helper 1 (Th1) cytokines by intestinal lamina propria lymphocytes characterized by excessive production of interleukin (IL)-12/IL-23, interferon γ and tumor necrosis factor (TNF) α. These immune responses may be induced by defects in the epithelial barrier, an increased intestinal permeability, adherence of bacteria, and decreased expression of defen-sins[10]. Intestinal epithelial cells have toll-like receptors (TLR) to exert direct antibacterial effects via secretion of antimicrobial peptides and play an important role in the interrelation between the innate and adaptive immunity of the intestine. Moreover, a single nucleotide polymorphism of the nucleotide-binding oligomerization domain 2 (NOD2), which activates nuclear factor κB (NF-κB), is one of candidates for the susceptibility genes of Crohn’s disease, because CARD15/NOD2 is expressed in intestinal epithelial cells and triggers human beta-defensin (HBD)-2 transcription[11]. In Crohn’s disease patients with a mutation in the NOD2 gene, which is an intracellular peptidoglycan receptor, the ileal Paneth cell defensins, human defensin (HD)-2 and HD-3, which are antimicrobial peptides are diminished[12,13]. NOD2 mutant macrophages were reported to produce larger amounts of IL-12 in response to stimulation with microbial components than wild-type cells. Therefore, defects in the innate immune response, which is important for immunological protection against intestinal microbes, investigated by Podolsky’s group, may contribute to the deve-lopment of Crohn’s disease, especially of the ileal type. However, Japanese and Korean patients with Crohn’s disease have no mutations in the CARD15/NOD2 gene. Therefore, there be many routes between intestinal microbes and intestinal epithelial cells and lamina propria antigen-presenting cells leading to the development of Crohn’s disease.
When experimental mice which spontaneously developing spontaneously severe colitis were raised under specific pathogen-free conditions, they developed mild gastrointestinal inflammation[14]. Alteration of the intestinal microflora by antibiotic or probiotic therapy may induce and main-tain remission in colitis mice. Oral administration of Lactobacillus GG induced and maintained remission of some patients with Crohn’s disease. In addition, the VSL#3 probiotic-mixture containing 3600 billion bacteria good for the intestine induced remission in patients with active ulcerative colitis[15]. One of the reasons under lying this mechanism is that bacterial flagellin is a dominant antigen in Crohn’s disease[16]. When the intestinal epithelial cells were exposed to flagellin, they produced chemokines that induced subsequent migration of immature dendritic cells, probably via TLR5. There are many papers stressing that probiotics and prebiotics were effective for the treatment of IBD. Ewaschuk et al[17] demonstrated that Bacteroides spp, Enterococcus faecalis, Enterobacter cloacae, intestinal Helicobacter spp, Fusobacterium spp, adherent/invasive E. coli strains, Eubacterium, and Peptostreptococcus spp were aggressive intestinal microbes and that the beneficial intestinal microbes were Lactobacillus spp, Bifidobacterium spp, Streptococcus salivarius, Saccharomyces boulardii, Clostridium butyricum, Ruminococci, and E coli Nissle 1917. Altering the composition of the intestinal microflora using probiotics and prebiotics is one of the promising therapies for ameliorating chronic intestinal inflammation and may be preventives against IBD in people with disease susceptibility genes.
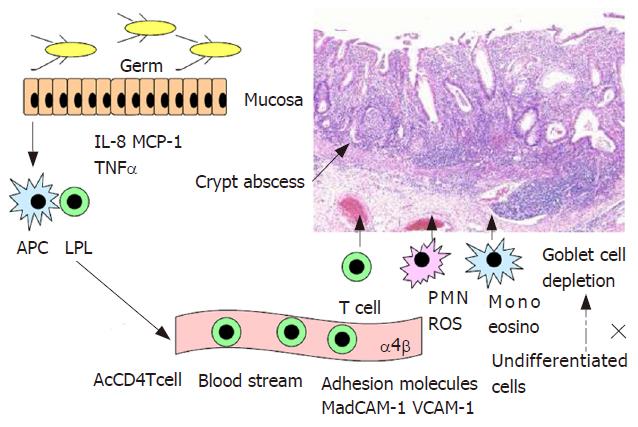
There are numerous macrophages and dendritic cells in the subepithelial space of mucosal lamina propria. Intestinal dendritic cells in active Crohn’s disease patients are matured and activated by antigens, and then have an enhanced expression of TLR2 and TLR4. Toll-like receptors in mucosal dendritic cells will recognize invading microbes and their components, because the permeability of interepithelial spaces is increased in the intestinal mucosa in Crohn’s disease. Dendritic cells will be activated by Toll-like receptor signaling, via MyD88-dependent or independent pathways, and produce interferon γ, TNF-α, IL-6 and IL-12/18. Thus, T cells activated by antigens may undergo distinct developmental pathways, gaining effector functions and properties.
T-helper cells are thought to differentiate into Th1 and Th2, and recently Th17 cells producing IL-17 have been found to induce autoimmunity and regulatory T cells[18]. Intestinal dendritic cells produce IL-12 and IL-6. Antibodies against IL-12 and -6 are effective for the treatment of human Crohn’s disease. Intestinal macrophages will be abnormally differentiated in the subsets, producing excessive IL-12 and IL-23 in response to bacteria in IL-10-deficient conditions[19]. On the other hand, human and murine studies of ulcerative colitis have shown an increased expression of atypical Th2 cytokines or natural killer (NK) T cells by lamina propria lymphocytes characterized by excessive production of IL-13[20]. Intestinal inflammation may be induced by an imbalance between the memory (effector) T cells inducing inflammation and regulatory T cells suppressing inflammation, probably due to loss of oral tolerance. In the animal model of colitis, IL-7 exacerbated chronic colitis with expansion of memory IL-7Rhigh CD4+ mucosal T cells[21]. Kanai and Watanabe reported that naturally arising CD4+CD25+ regulatory T cells suppressed the expansion of colitogenic CD4+CD44high CD62L-effector memory T cells[22]. It was reported that colonic CD4+CD25 positive regulatory T cells increased with disease activity in patients with active ulcerative colitis[23]. A balance between effector T cells and regulatory T cells seems to be very important for induction of colonic inflammation, because it was also reported that CD8 regulatory T cells were decreased in the colonic lamina propria of patients with IBD[24]. The Th1 and Th2 T cell responses that underlie IBD may depend on NF-κB transcriptional activity. NF-κB proteins are regulators of innate and adaptive immunity, inflammatory stress, and proliferative and apoptotic responses of cells to a number of different stimuli. Proinflammatory cytokines such as TNF α and IL-6 are induced by the immuno-competent cells after activation of NF-κB. NF-κB is thought to provide a mechanistic link between inflammation and cancer. In addition, the p38 mitogen-activated protein kinase (MAPK) regulates the expression of proinflammatory cytokines such as IL-8. Inhibitors of intracellular transcriptional factors will be useful for the treatment of IBD in the near future[25]. After NF-κB activation, anti-proinflammatory cytokine antibody therapy such as infliximab, adalimumab and anti-IL-6 monoclonal antibody (MRA) have been used for the treatment of Crohn’s disease and ulcerative colitis[26-29].
Intestinal dendritic cells in active Crohn’s disease patients were matured and activated upon exposure to intestinal microbes or their components. Memory T cells sensitized by antigens may proliferate and produce proinflammatory cytokines, and then are transported in the mesenteric lymph nodes via intestinal lymphatics where they will be modulated. They home again into the intestinal mucosa via the thoracic duct and this homing of lymphocytes is induced by a mutual interaction between the receptors L-selectin and α4β integrin on CD4 T cells and endothelial cell receptors. Anti-adhesion molecule antibody against α4 integrin is effective for the treatment of Crohn’s disease with a few complications such as progressive multifocal leukoencephalopathy[30]. Intestinal lesions may occur macroscopically and microscopically in a spotted and regional manner in Crohn’s disease and diffusely in ulcerative colitis. The pathologic features of Crohn’s disease may occur in association with vascular injury, focal arteritis, fibrin deposition, and arterial occlusion mainly at the level of the lamina propria, resulting in multifocal gastrointestinal tissue infarction and lastly longitudinal ulcers in a few years after occurrence of multiple aphthoid ulcers (Figure 1). The majority of granulomas in Crohn’s disease may be formed within or near the walls of blood and lymphatic vessels, suggesting granulomatous vasculitis as an early element in the path-ogenesis of Crohn’s disease[31,32]. Blockade of intestinal lymphatic flow and mesenteric lymph nodes are also very important factors in the pathogenesis of Crohn’s disease, because Crohn’s disease reveals dilated lymphatic vessels and edema in the mucosa and submucosa, resulting in intestinal protein loss and albuminemia[33,34].
Intestinal inflammation occurring in ulcerative colitis is characterized by the mucosal and submucosal infiltration of numerous lymphocytes and granulocytes and the depletion of goblet cells with crypt abscess in most of moderate and severe cases (Figure 2). Goblet cell depletion is thought to be induced by disturbed transformation of undifferentia-ted cells into goblet cells under an environment exposed to proinflammatory cytokines. Recently, granulocytes including neutrophils have again attracted attention in the pathophysiology of IBD, because leukopheresis is effective for the treatment of active ulcerative colitis and Crohn’s disease[35-37]. In experimental colitis induced by dextran sulfate sodium (DSS), deletion of neutrophils by administration of antibody against neutrophils decreased severity of colonic inflammation and production of reactive oxygen species[38]. IL-8 may induce mucosal infiltration of neutrophils at first and then lymphocytes. Mucosal IL-8 is produced by macrophages, colonic epithelial cells and neutrophils when they were activated by antigens and proinflammatory cytokines[39]. The mucosal levels of IL-8 were closely correlated with levels of luminol-dependent chemiluminescence and myeloperoxidase in the mucosa of patients with ulcerative colitis[40]. On the other hand, lower neutrophil accumulation in the intestine and lower production of IL-8 were found in Crohn’s disease than in ulcerative colitis[41]. Production of reactive oxygen species by polymor-phonuclear cells was not increased in Crohn’s disease when compared with that of controls[42]. Activation of neutrophils was induced in contact with activated platelets via P-selectin, resulting in neutrophil-mediated tissue injury via excessive production of free radicals[43]. Therefore, active chronic inflammation is a very important factor for the pathogenesis of ulcerative colitis.
Oxidative stress-induced intestinal epithelial cell injury may be induced via Rho/ROK/PKC pathway activation[44]. However, precise mechanisms of free radicals-induced cell injury have not been clarified in human ulcerative colitis. There is a clinical trial reporting that lecithinized superoxide dismutase was effective for the treatment of ulcerative colitis[45]. However, in transgenic mice overex-pressing human CuZn-SOD, the severity of colitis and the levels of myeloperoxidase in DSS colitis were worsened, implying divergent roles of superoxide and nitric oxide[46].
The mesenchymal cells including fibroblasts, endo-thelial cells involved in angiogenesis, and platelets may play an important role in the pathophysiology of IBD. Fibroblasts and platelets are very important factors for ulcer healing and tissue remodeling[47]. Microvessels having strongly positive staining of αV β3 integrins were increased in density in the colonic mucosa of IBD. Activated platelets released CD40L which was involved in the CD40/CD40L system and reactive oxygen species from granulocytes when they contacted granulocytes of patients with active IBD[48]. Reconstitution of damaged tissues and epithelial cells is one of targets for wound healing. Watanabe's group found that bone-marrow derived cells could promote the regeneration of damaged epithelia in human gastrointestinal tract[49,50]. In bone -marrow transplantion recipients, epithelial cells of donor origin were distributed throughout the gastrointestinal tract. Bone-marrow transplantation has been tried clinically as one of the treatment for Crohn's disease[51].
There are four steps in the interaction between intestinal microbes and mucosal inflammation in individuals genetically pre-disposed to IBD. The first step is an interaction between intestinal microbes or their components and intestinal epithelial cells via receptors including Toll-like receptors, the second step an interaction between macrophages and dendritic cells and mucosal lymphocytes, the third step an interaction between lymphocytes and vascular endothelial cells, and the fourth step an interaction between lymphocytes and granulocytes producing proin-flammatory cytokines or free radicals and mucosal damage and repair. Recent therapeutic approaches for IBD are to block these four steps in the intestinal inflammation of patients with IBD.
S- Editor Zhu LH L- Editor Zhu LH E- Editor Liu Y
| 1. | Kirsner JB. Inflammatory bowel disease at the University of Chicago--the first 50 years: some personal reflections. Am J Gastroenterol. 1985;80:219-226. [PubMed] |
| 2. | Sedlack RE, Nobrega FT, Kurland LT, Sauer WG. Inflammatory colon disease in Rochester, Minnesota, 1935-1964. Gastroenterology. 1972;62:935-941. [PubMed] |
| 3. | Molinié F, Gower-Rousseau C, Yzet T, Merle V, Grandbastien B, Marti R, Lerebours E, Dupas JL, Colombel JF, Salomez JL. Opposite evolution in incidence of Crohn's disease and ulcerative colitis in Northern France (1988-1999). Gut. 2004;53:843-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 180] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 4. | Lakatos PL. Recent trends in the epidemiology of inflammatory bowel diseases: up or down. World J Gastroenterol. 2006;12:6102-6108. [PubMed] |
| 5. | Yang SK, Hong WS, Min YI, Kim HY, Yoo JY, Rhee PL, Rhee JC, Chang DK, Song IS, Jung SA. Incidence and prevalence of ulcerative colitis in the Songpa-Kangdong District, Seoul, Korea, 1986-1997. J Gastroenterol Hepatol. 2000;15:1037-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 124] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Ouyang Q, Tandon R, Goh KL, Ooi CJ, Ogata H, Fiocchi C. The emergence of inflammatory bowel disease in the Asian Pacific region. Curr Opin Gastroenterol. 2005;21:408-413. [PubMed] |
| 7. | Zheng JJ, Zhu XS, Huangfu Z, Gao ZX, Guo ZR, Wang Z. Crohn's disease in mainland China: a systematic analysis of 50 years of research. Chin J Dig Dis. 2005;6:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Timmer A. Environmental influences on inflammatory bowel disease manifestations. Lessons from epidemiology. Dig Dis. 2003;21:91-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Zheng CQ, Hu GZ, Zeng ZS, Lin LJ, Gu GG. Progress in searching for susceptibility gene for inflammatory bowel disease by positional cloning. World J Gastroenterol. 2003;9:1646-1656. [PubMed] |
| 10. | Cobrin GM, Abreu MT. Defects in mucosal immunity leading to Crohn's disease. Immunol Rev. 2005;206:277-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 149] [Article Influence: 7.5] [Reference Citation Analysis (2)] |
| 11. | Hisamatsu T, Suzuki M, Reinecker HC, Nadeau WJ, McCormick BA, Podolsky DK. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology. 2003;124:993-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 429] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 12. | Mueller T, Podolsky DK. Nucleotide-binding-oligomerization domain proteins and toll-like receptors: sensors of the inflammatory bowel diseases' microbial environment. Curr Opin Gastroenterol. 2005;21:419-425. [PubMed] |
| 13. | Wehkamp J, Fellermann K, Stange EF. Human defensins in Crohn's disease. Chem Immunol Allergy. 2005;86:42-54. [PubMed] |
| 14. | Schultz M, Tonkonogy SL, Sellon RK, Veltkamp C, Godfrey VL, Kwon J, Grenther WB, Balish E, Horak I, Sartor RB. IL-2-deficient mice raised under germfree conditions develop delayed mild focal intestinal inflammation. Am J Physiol. 1999;276:G1461-G1472. [PubMed] |
| 15. | Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, De Simone C, Sartor RB. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005;100:1539-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 491] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 16. | Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, Fort M, Hershberg RM. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 586] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 17. | Ewaschuk JB, Dieleman LA. Probiotics and prebiotics in chronic inflammatory bowel diseases. World J Gastroenterol. 2006;12:5941-5950. [PubMed] |
| 18. | Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1048] [Cited by in RCA: 1048] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 19. | Kamada N, Hisamatsu T, Okamoto S, Sato T, Matsuoka K, Arai K, Nakai T, Hasegawa A, Inoue N, Watanabe N. Abnormally differentiated subsets of intestinal macrophage play a key role in Th1-dominant chronic colitis through excess production of IL-12 and IL-23 in response to bacteria. J Immunol. 2005;175:6900-6908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 20. | Targan SR, Karp LC. Defects in mucosal immunity leading to ulcerative colitis. Immunol Rev. 2005;206:296-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 136] [Article Influence: 6.8] [Reference Citation Analysis (2)] |
| 21. | Okada E, Yamazaki M, Tanabe M, Takeuchi T, Nanno M, Oshima S, Okamoto R, Tsuchiya K, Nakamura T, Kanai T. IL-7 exacerbates chronic colitis with expansion of memory IL-7Rhigh CD4+ mucosal T cells in mice. Am J Physiol Gastrointest Liver Physiol. 2005;288:G745-G754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Kanai T, Tanimoto K, Nemoto Y, Fujii R, Makita S, Totsuka T, Watanabe M. Naturally arising CD4+CD25+ regulatory T cells suppress the expansion of colitogenic CD4+CD44highCD62L- effector memory T cells. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1051-G1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Holmén N, Lundgren A, Lundin S, Bergin AM, Rudin A, Sjövall H, Ohman L. Functional CD4+CD25high regulatory T cells are enriched in the colonic mucosa of patients with active ulcerative colitis and increase with disease activity. Inflamm Bowel Dis. 2006;12:447-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 148] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 24. | Brimnes J, Allez M, Dotan I, Shao L, Nakazawa A, Mayer L. Defects in CD8+ regulatory T cells in the lamina propria of patients with inflammatory bowel disease. J Immunol. 2005;174:5814-5822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 25. | Schreiber S, Feagan B, D'Haens G, Colombel JF, Geboes K, Yurcov M, Isakov V, Golovenko O, Bernstein CN, Ludwig D. Oral p38 mitogen-activated protein kinase inhibition with BIRB 796 for active Crohn's disease: a randomized, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2006;4:325-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 26. | Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2987] [Cited by in RCA: 3055] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 27. | Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, Panaccione R, Wolf D, Pollack P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323-333; quiz 591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1194] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 28. | Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2744] [Cited by in RCA: 2885] [Article Influence: 144.3] [Reference Citation Analysis (2)] |
| 29. | Ito H, Takazoe M, Fukuda Y, Hibi T, Kusugami K, Andoh A, Matsumoto T, Yamamura T, Azuma J, Nishimoto N. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn's disease. Gastroenterology. 2004;126:989-996; discussion 947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 471] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 30. | Ghosh S, Goldin E, Gordon FH, Malchow HA, Rask-Madsen J, Rutgeerts P, Vyhnálek P, Zádorová Z, Palmer T, Donoghue S. Natalizumab for active Crohn's disease. N Engl J Med. 2003;348:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 617] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 31. | Wakefield AJ, Sawyerr AM, Dhillon AP, Pittilo RM, Rowles PM, Lewis AA, Pounder RE. Pathogenesis of Crohn's disease: multifocal gastrointestinal infarction. Lancet. 1989;2:1057-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 405] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 32. | Wakefield AJ, Sankey EA, Dhillon AP, Sawyerr AM, More L, Sim R, Pittilo RM, Rowles PM, Hudson M, Lewis AA. Granulomatous vasculitis in Crohn's disease. Gastroenterology. 1991;100:1279-1287. [PubMed] |
| 33. | Maconi G, Di Sabatino A, Ardizzone S, Greco S, Colombo E, Russo A, Cassinotti A, Casini V, Corazza GR, Bianchi Porro G. Prevalence and clinical significance of sonographic detection of enlarged regional lymph nodes in Crohn's disease. Scand J Gastroenterol. 2005;40:1328-1333. [PubMed] |
| 34. | Miura S, Yoshioka M, Tanaka S, Serizawa H, Tashiro H, Asakura H, Tsuchiya M. Faecal clearance of alpha 1-antitrypsin reflects disease activity and correlates with rapid turnover proteins in chronic inflammatory bowel disease. J Gastroenterol Hepatol. 1991;6:49-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Sawada K, Muto T, Shimoyama T, Satomi M, Sawada T, Nagawa H, Hiwatashi N, Asakura H, Hibi T. Multicenter randomized controlled trial for the treatment of ulcerative colitis with a leukocytapheresis column. Curr Pharm Des. 2003;9:307-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 128] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 36. | Honma T, Sugimura K, Asakura H, Matsuzawa J, Suzuki K, Kobayashi M, Aoyagi Y. Leukocytapheresis is effective in inducing but not in maintaining remission in ulcerative colitis. J Clin Gastroenterol. 2005;39:886-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Fukuda Y, Matsui T, Suzuki Y, Kanke K, Matsumoto T, Takazoe M, Matsumoto T, Motoya S, Honma T, Sawada K. Adsorptive granulocyte and monocyte apheresis for refractory Crohn's disease: an open multicenter prospective study. J Gastroenterol. 2004;39:1158-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Natsui M, Kawasaki K, Takizawa H, Hayashi SI, Matsuda Y, Sugimura K, Seki K, Narisawa R, Sendo F, Asakura H. Selective depletion of neutrophils by a monoclonal antibody, RP-3, suppresses dextran sulphate sodium-induced colitis in rats. J Gastroenterol Hepatol. 1997;12:801-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 92] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Arai F, Takahashi T, Furukawa K, Matsushima K, Asakura H. Mucosal expression of interleukin-6 and interleukin-8 messenger RNA in ulcerative colitis and in Crohn's disease. Dig Dis Sci. 1998;43:2071-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Anezaki K, Asakura H, Honma T, Ishizuka K, Funakoshi K, Tsukada Y, Narisawa R. Correlations between interleukin-8, and myeloperoxidase or luminol-dependent chemiluminescence in inflamed mucosa of ulcerative colitis. Intern Med. 1998;37:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Marks DJ, Harbord MW, MacAllister R, Rahman FZ, Young J, Al-Lazikani B, Lees W, Novelli M, Bloom S, Segal AW. Defective acute inflammation in Crohn's disease: a clinical investigation. Lancet. 2006;367:668-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 287] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 42. | Kitahora T, Suzuki K, Asakura H, Yoshida T, Suematsu M, Watanabe M, Aiso S, Tsuchiya M. Active oxygen species generated by monocytes and polymorphonuclear cells in Crohn's disease. Dig Dis Sci. 1988;33:951-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Suzuki K, Sugimura K, Hasegawa K, Yoshida K, Suzuki A, Ishizuka K, Ohtsuka K, Honma T, Narisawa R, Asakura H. Activated platelets in ulcerative colitis enhance the production of reactive oxygen species by polymorphonuclear leukocytes. Scand J Gastroenterol. 2001;36:1301-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 44. | Song J, Li J, Lulla A, Evers BM, Chung DH. Protein kinase D protects against oxidative stress-induced intestinal epithelial cell injury via Rho/ROK/PKC-delta pathway activation. Am J Physiol Cell Physiol. 2006;290:C1469-C1476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Mizushima T. Lecithinized superoxide dismutase was effective for ulcerative colitis. Inflam Regen. 2006;26:317. |
| 46. | Krieglstein CF, Cerwinka WH, Laroux FS, Salter JW, Russell JM, Schuermann G, Grisham MB, Ross CR, Granger DN. Regulation of murine intestinal inflammation by reactive metabolites of oxygen and nitrogen: divergent roles of superoxide and nitric oxide. J Exp Med. 2001;194:1207-1218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 202] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 47. | Danese S, Katz JA, Saibeni S, Papa A, Gasbarrini A, Vecchi M, Fiocchi C. Activated platelets are the source of elevated levels of soluble CD40 ligand in the circulation of inflammatory bowel disease patients. Gut. 2003;52:1435-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 199] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 48. | Danese S, Sans M, de la Motte C, Graziani C, West G, Phillips MH, Pola R, Rutella S, Willis J, Gasbarrini A. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology. 2006;130:2060-2073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 335] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 49. | Okamoto R, Yajima T, Yamazaki M, Kanai T, Mukai M, Okamoto S, Ikeda Y, Hibi T, Inazawa J, Watanabe M. Damaged epithelia regenerated by bone marrow-derived cells in the human gastrointestinal tract. Nat Med. 2002;8:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 287] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 50. | Brittan M, Chance V, Elia G, Poulsom R, Alison MR, MacDonald TT, Wright NA. A regenerative role for bone marrow following experimental colitis: contribution to neovasculogenesis and myofibroblasts. Gastroenterology. 2005;128:1984-1995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 51. | Lopez-Cubero SO, Sullivan KM, McDonald GB. Course of Crohn's disease after allogeneic marrow transplantation. Gastroenterology. 1998;114:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 142] [Article Influence: 5.3] [Reference Citation Analysis (0)] |