Published online Apr 14, 2007. doi: 10.3748/wjg.v13.i14.2094
Revised: February 23, 2007
Accepted: March 1, 2007
Published online: April 14, 2007
AIM: To investigate the in vivo effect of beta-casomorphin-7 on the regulation of gastric somatostatin and gastrin messenger RNA in rat gastric mucosa.
METHODS: Somatostatin and gastrin mRNA were quantified by RT-PCR and in situ hybridization (ISH) in 24 rats. The rats were divided into three treatment groups: basal diet + physiological saline (n = 8), basal diet + beta-casomorphin-7 (7.5 × 10-7 mol) (n = 8), and basal diet + poly-Gly-7 (containing equal mol of N with 7.5 × 10-7 mol beta-casomorphin-7) (n = 8). After oral administration for 30 days, rats were killed by exsanguinations.
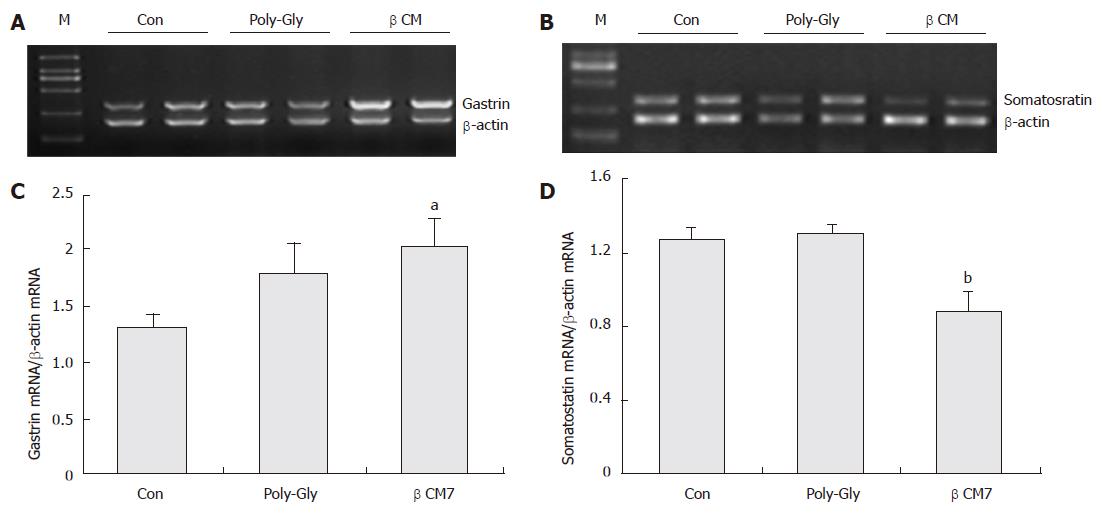
RESULTS: After intra-gastric administration of beta-casomorphin-7 for 30 d, gastrin mRNA increased by 52.8% (P < 0.05, n = 8), and somatostatin mRNA levels decreased by 30.7% compared with the controls (P < 0.01, n = 8). No significant differences in the expression of the two genes were observed in the poly-Gly-treated group, although gastrin mRNA expression was elevated by 35.6% as against the control group (P = 0.15, n = 8). The long-term oral administration of a casomorphin solution significantly decreased the even gray of D-cells, but did not lower the number of D-cells both in the antrum and fundus. Interestingly, the number of G-cells increased in the antrum and fundus, but its average density was augmented only in the antrum.
CONCLUSION: Beta-casomorphin-7 is capable of modulating gene expression of the regulatory peptides from G and D cells. Data from in situ hybridization studies indicate that beta-casomorphin-7 affects gastrin gene expression indirectly by means of the paracrine action of somatostatin, and depends on its intrinsic molecular function.
- Citation: Zong YF, Chen WH, Zhang YS, Zou SX. Effects of intra-gastric beta-casomorphin-7 on somatostatin and gastrin gene expression in rat gastric mucosa. World J Gastroenterol 2007; 13(14): 2094-2099
- URL: https://www.wjgnet.com/1007-9327/full/v13/i14/2094.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i14.2094
Milk proteins are found with a number of biologically active peptides (BAP) of different biological functions. One particular peptide sequence in β-casein attracts higher research interests than other BAPs. This class of peptides was isolated from β-casein hydrolysate and is referred to as beta-casomorphins. Beta-casomorphins have been detected in vivo in the intestinal chyme of mini-pigs[1] and human[2]. Passive transport of beta-casomorphins across intestinal mucosal membranes does occur in neonates, which may experience various physiological responses, such as an analgesic effect on the nervous system, resulting in calmness and sleep in infants[3]. In contrast, because their absorption in the gut has not been observed in adults, it is generally concluded that the physiological influences of beta-casomorphin are related to their concentration and their transit speed through the gastrointestinal tract. Once the beta-casomorphins are released in the gastrointestinal tract from milk protein or through oral administration, they first come into contact with the luminal mucosa and produce a number of rapid effects on the gastrointestinal system.
Endocrine cells within the stomach respond to lumi-nal acidity and dietary nutrients and, in turn, regulate gastric secretion by the paracrine and hormonal actions of regulatory peptides[4]. The importance of the luminal environment in gastric G and D cell secretory and synthetic function is reflected in the animal’s nutritional status[5,6]. Furthermore, the regulatory peptides and neurotransmitters involved in normal gastric function are associated with gastrin mRNA and somatostatin mRNA[7,8]. Studies have shown that endogenous opioid peptides do contribute to the stimulation of gastric acid secretion[9-11]. There are other studies proving that postprandial release of somatostatin is regulated by endogenous opioid peptides in vivo and in vitro[12,13]. Compatible with the presence of endogenous opioid peptides in the gastrointestinal tract, beta-casomorphins, as exogenous opioid ligands, prolong the gastrointestinal transit time by inhibiting intestinal peristalsis and motility[14], modulate ion transport[15], and regulate various endocrine responses, such as the secretion of insulin[16], somatostatin[17] and gastrin[18,19]. However, these functions have not yet revealed whether exogenous peptides can modulate target endocrine cells in the gastrointestinal tract or if they just correspond to the nitrogen status of the animal.
This study was designed to examine the in vivo effects of intra-gastric beta-casomorphin-7 on gastrin and somatosatin messenger RNA expression in the gastric mucosa. In addition, it aims at determining whether this peptide’s actions on G cell secretory function and gastrin gene expression were related to the paracrine influences of somatostatin.
Adult female Sprague-Dawley rats (n = 24, Shanghai SLAC Laboratory Animal Co., Ltd, China), weighing 220-240 g, were housed indoor under controlled lighting (12 h per day), constant temperature (20 ± 3°C) and humidity (50% ± 3%). The Institutional Animal Care and Use Committee of Nanjing Agricultural University approved the animal care and use protocol. The rats were fed with a commercially available basic diet for 7 d and then randomly assigned to one of three treatment groups: basal diet + physiological saline, basal diet + beta-casomorphin-7 (7.5 × 10-7 mol), and basal diet + poly-Gly-7 (containing equal mol of N with 7.5 × 10-7 mol beta-casomorphin-7). After treated with beta-casomorphin-7, physiological saline and poly-Gly-7 by gastric tube for 30 d, the rats were killed by exsanguinations. The stomachs were removed, cut open along the greater curvature, rinsed with ice-cold 0.9% saline, and then dissected. The gastric tissue was cut open in parallel to the greater curvature. The moiety of the stomach tissue was fixed for 6 h in 4% paraformaldehyde in 0.1 mol/L PBS, and soaked overnight in 20% sucrose, prior to sectioning at 10 μm. Sections were collected onto poly (L-lysine) slides and left overnight to dry at 37°C. Other gastric tissues were flattened on a cold (4°C) glass plate with the mucosa directed upwards, and the mucosal lining was scraped off with a scalpel, and stored at -70°C until analyzed.
Total RNA was extracted from the mucosal samples by the acid guanidinium thiocyanate-phenol-chloroform method, and the RNA concentration was quantified with a photometer (Eppendorf Biophotometer). Both RT and PCR were performed in a Gene Amp PCR System 9600 (Perkin Elmer, USA). The PCR primers for gastrin, somatostatin, and β-actin were designed using Primer Premier 5.0 (Premier Biosoft International, USA) and synthesized with an instrument made by Haojia Biotech (China). The nucleotide sequences of these primers and PCR conditions set for respective genes are shown in Table 1.
| Target genes | GenBank accession | PCR products | Primer sequences | PCR conditions |
| Gastrin | M38653 | 315 bp | F: 5'- CCTACTGCCACAACAGTTAA -3' | 94°C, 30 s; 52°C, 30 s; 72 °C, 60 s; 32 cycles |
| R: 5'- CATCCATCCGTATGCTTC -3' | ||||
| Somatostatin | M25890 | 330 bp | F: 5'- ATGCTGTCCTGCCGTCTC -3' | 94°C, 30 s; 60°C, 30 s; 72 °C, 60 s; 30 cycles |
| R: 5'- CAGCCAGCTTTGCGTTCC -3' | ||||
| β-actin | AF122902 | 200 bp | F: 5'- CCCTGTGCTGCTCACCGA -3' | - |
| R: 5'- ACAGTGTGGGTGACCCCGTC -3' |
Each target gene was co-amplified with β-actin in the same reaction. By adjusting the ratio of β-actin primers to primers of the target genes in the reaction system, the overall PCR amplification efficiency of β-actin can be reduced to the level comparable to that of target genes. All samples were included in the same run of RT–PCR and repeated three times. An aliquot (20 μL) of PCR products was analyzed by electrophoresis on 2% agarose gels stained with ethidium bromide and then photographed. The net intensities of individual bands were measured using Kodak Digital Science 1D software (Eastman Kodak, Rochester, NY, USA). The ratios of net intensities of target genes to β-actin were used to represent the relative levels of target genes expressed.
In situ hybridization: Diethyl pyrocarbonate (DEPC)-treated Milli-Q water was used in each step. Sections were immersed in 30% H2O2/methanol (1:50 dilution) solution for 30 min to eliminate endogenous peroxidase activity. After rinsed in 0.1 mol/L phosphate-buffered saline (PBS), sections were treated with pepsin (0.02%) at 37°C for 10 min. Thereafter, sections were fixed in freshly prepared 4% paraformaldehyde in PBS for 20 min at 4°C, rinsed in PBS, and immersed in 0.25% acetic anhydride, 0.1 mol/L triethanolamine and 0.9% NaCl for 10 min at room temperature. The probe for rat somatostatin and gastrin were single stranded oligonucleotide labeled with digoxigenin (Boster Bio-technology Code MK 1751 and MK 1445). Sections were hybridized overnight at 40°C in hybridization buffer containing digoxigenin labeled oligonucleotide probe at a final concentration of 400 mg/L. After hybridization, sections were washed in fresh graded SSC (saline sodium citrate) at 37°C for 1 h, incubated in 5% bovine serum albumin for 30 min at room temperature, and in biotin-anti-digoxigenin for 1 h, conjugated at 37°C and then washed and incubated in SABC for 30 min at 37°C. The anti-digoxigenin antibody bound peroxidase reacts with DAB to generate a brown precipitate at the site of digoxigenin-probe hybridization.
Controls for specificity: The specificity of the dig-labeled probe was tested by: (1) omission of the labeled probe from the hybridization buffer, (2) pretreatment of sections before hybridization with RNase, and (3) staining tissues with non-immune serum and inappropriate antiserum (rabbit anti-prolactin) as the first layer. Negative results were achieved in all these procedures.
Quantitative assay of G and D cells: The number of positive cells was expressed as the mean number per unit area (0.64 × 0.45 = 0.29 mm2) from the sections × 200. Optical densities were quantified by an observer blinded to treatment using a computer image analysis system (ImagePro Plus5.2, Media Cybernetics, Maryland). The individual value for each animal was the average of three sections measured within the areas of interest.
Data are expressed as the mean ± SE. SPSS 13.0 software was used. An F test was performed to separate means and compare among groups, and an analysis of variance, followed by the Duncan’s test, was utilized for unpaired data. A statistical significance was considered if P < 0.05.
As shown in Figure 1, after administration of beta-casomorphin-7 (7.5 × 10-7 mol), the relative abundance of gastrin mRNA was significantly increased by 152.8%, P < 0.05, n = 8) and the mRNA levels of somatostatin were decreased by 30.7% compared with the control group, P < 0.01, n = 8). No significant differences in the poly-Gly-treated group were observed in gastrin and somatostatin mRNA expression, although poly-Gly numerically, but insignificantly, augmented the gastrin mRNA expression level by 35.6% as agaist the control group (P = 0.15, n = 8).
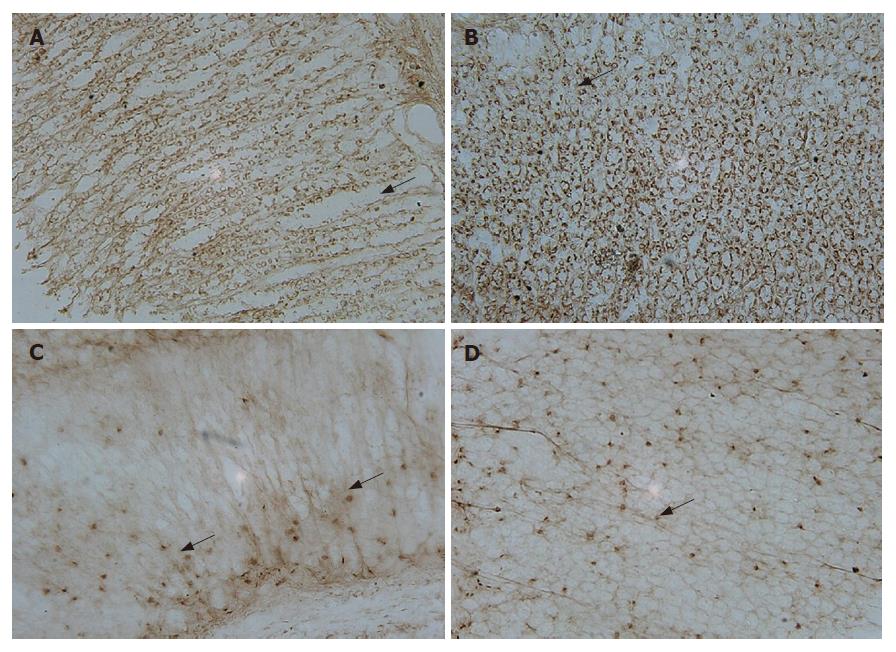
In situ hybridization with digoxigenin-labeled oligonucleotide probes of gastrin and somatostatin detected positive cells in both pyloric and oxyntic glands. The distributions of cells expressing gastrin and somatostatin mRNA fit quite well. Somatostatin-positive cells were present in both pyloric and oxyntic glands (Figure 2C and D). Most of the cells appeared round, elliptical, fusiform, triangular or irregular, and were located along the lateral sides of the middle and basal parts of the glands. Gastrin-positive cells were mainly located in the lower 2/3 of the mucosa and rarely in the upper 1/3. The appearance of G cells with large nuclei was similar to those of D cells (Figure 2A and B). They differed from the typical endocrine cells of the gastroduodenum where they produced long cytoplasms and processed the end with small bulbous expansions on the putative effecter cells.
Intra-gastric beta-casomorphin-7 administration for 30 d resulted in a significant increase in the number of positive cells of gastrin mRNA by 121.9% in oxyntic mucosa and 117.5% in pyloric mucosa, P < 0.05, n = 8), but the number of positive cells of somatostatin mRNA was not significantly different from that in the control rats. Unexpectedly, however, the mean intensity, recorded as the grey value (GV) of signals that corresponded to somatostatin mRNA, was significantly lower in the casomorphin-treated group than in the poly-Gly-treated group and the control group by 22.0% in oxyntic mucosa and 19.5% in pyloric mucosa, P < 0.05, n = 8). In contrast, the antrum G-cell density was increased in the casomorphin-treated and in the poly-Gly-treated groups. The G-cell density was increased by 22.5% (P < 0.01) in rat antrum treated with ploy-Gly solution, although a decrease in D-cell density was not observed. Results are shown in Tables 2 and 3.
This report demonstrates that beta-casomorphin-7 is not only a transmitter substance modulating peptide secretion from D and G cells, but it is also involved in the control of somatostatin and gastrin gene expression. It is indicated that the ability of beta-casomorphin-7 to promote G cell secretion and gastrin messenger RNA levels is indirect and dependent primarily on the paracrine actions of somatostatin. Previous studies on the role of gastric afferents in regulating antral D cell function further suggest that exogenous opioid peptides contribute to feedback mechanisms in the modulation of regulatory peptide gene expression.
In recent years, constant attention has been paid to BAP’s role in regulating regional events within the stomach. Opioid peptide-mediated regulation of gastrointestinal function was well documented; however, less has been reported on its effect on gastrointestinal function of exorphins after luminal administration. Most of the researches on exorphins involved acute responses of isolated organs or tissue preparations. Compatible with the presence of beta-casomorphins in the gastrointestinal tract, opioid effects in gastrointestinal functions have also been shown upon casein digestion. Several studies have demonstrated that beta-casomorphins affect gastrin release from stomach mucosa in rats and piglets in vivo[18,19] and in vitro[20]. The current study also showed that intra-gastric bovine beta-casomorphin-7 administration for 30 days helped evoke gastrin mRNA expression and inhibit somatostatin expression. In contrast, given the effect of beta-casomorphin-7 observed here, poly-Gly treatment yielded no effect on somatostatin gene expression, but only a modest augmentation of gastrin mRNA expression. Food, especially high protein diets, has been observed to increase gastrin release and gene expression. This is considered to be one of the nutritional functions of protein. The authors concluded that their effects on endocrine function of beta-casomorphin-7 and poly-peptides (equal quantity of N with beta-casomorphin-7) were distinct with regard to their intrinsic configuration and their role in N nutrition.
With regard to gastric somatostatin secretion, there were differences among previous studies[17] in dogs and the current results. In the current experiment, each rat was infused with 7.5 × 10-7 mol beta-casomorphin-7 daily by gastric tube for 30 d. The expression of somatostatin mRNA in the gastric mucosa was reduced by 30.7% as compared with the control group. Schusdziarra et al[17] have described a significant increase in the postprandial levels of peripheral vein plasma SLI following the administration of 12 mg beta-casomorphins to conscious dogs. They observed that these peptides were able to rapidly inhibit insulin release, which exerted an influence on the release of somatostatin[16]. These differences might be due to the experimental designs used and the dosage of applied opioid peptides[21].
Reciprocal regulation of gastrin and somatostatin gene expression of these regulatory peptides has provided a basis for understanding negative feedback control in the prevention of acid hypersecretion and, conversely, helped explain hypergastrinemia in hypochlorhydria[22]. Gastrin gene expression in antral explants has been shown to be reduced by both exogenous and endogenous somatostatins[23]. Several studies suggested that somatostatin exerted its inhibitory effects on gene expression[24], in part, by al-tering post-transcriptional processing through stabilization and decreased the turnover of gastrin mRNA. In vitro studies[25] with somatostatin antibody have demonstrated quantitatively greater responses in antral gastrin gene ex-pression. The present study demonstrated that long-term casomorphin solution administered orally significantly decreased D-cell density, but did not affect the number of D-cells both in the antrum and fundus. Interestingly, long-term administration with casomorphin intragastrically has been observed to increase the number of G-cells both in the antrum and fundus, and to augment G-cell density only in the antrum. These differences may be due, in part, to the differential regulation of somatostatin in the antrum and fundus[5,27,28]. Antral D cells were open cells with an apical process in contact with the lumen and controlled by nearby G cells, while corpus D cells were closed cells and regulated directly or indirectly parietal cell function[26]. It remained to be studied how the relevant factors act at a cellular level to control somatostatin mRNA abundance. These factors could include regulation of gene expression or regulation of mRNA stability[25,28], which is similar with the result from the recent studies on gastrin. Changes in somatostatin mRNA abundance might not be directly reflected in changed tissue stores[29]. It was clear, however, that factors which influence gastric somatostatin mRNA were able to act over relatively short periods[30], and that the same factors also rapidly influence somatostatin secretion[31,32], supporting the idea that the two were functionally linked. In addition, the G-cell density was increased in rat antrum treated with ploy-Gly solution, although the decrease in D-cell density was not observed. That is to say, that endocrine function of casomorphin is functionally dependent on its intrinsic configuration.
In conclusion, beta-casomorphin-7 is capable of regulating the mRNA levels of somatostatin and gastrin from D and G cells in an in vivo experimental system. The reciprocal effects on somatostatin and gastrin mRNA abundance of the respective regulatory peptides suggested that casomorphins might be involved in the negative feedback control of G cell function. In situ hybridization studies indicated that beta-casomorphin-7 indirectly affects gastrin gene expression through the paracrine actions of somatostatin, and this action depends on its intrinsic form.
We appreciate the efforts made by William W Riley, PhD, Research Director in Feed & Grow International Co., Ltd., in his critical reading of the manuscript.
Beta casomorphins belong to a family of opioid peptides derived from milk protein. Since the bovine beta casomorphin 7 (Tyr-Pro-Phe-Pro-Gly-Pro-Ile) was first isolated from an enzymatic digest of bovine beta-casein, the effects of beta casomorphins have been found as neurotransmitters and neuromodulators on the central nervous, endocrine, cardiovascular, and gastrointestinal systems. Beta casomorphins, released in gastrointestinal tract from the bovine milk protein or through oral administration, firstly might come into contact with luminal mucosa. To characterize the effect of bovine beta-casomorphin 7 in gastrointestinal tract is an essential precondition to analyze its physiological value. Gastrin secretes from gastric endocrine cells and regulates the secretion of gastric acid, the motility of the stomach, and the gastric, small and large intestinal mucosa. Endogenous opioid peptides strongly affect gastrointestinal function but without influencing the gastrin release. Beta casomorphins has been reported to affect gastrin release from stomach mucosa in rats and piglet in vivo and in vitro. However, the physiological mechanism of beta casomorphins about release of gastrin is ambiguous.
As a classical bioactive peptide from casein, the main milk proteins, beta casomorphins has been reported to have health enhancing potentials for food and pharmaceutical applications. Opioid-mediated regulation of gastrointestinal function is well documented; however, less has been reported on the effect on gastrointestinal function of exorphins after luminal administration. Most of the studies on exorphins involve acute responses of isolated organs or tissue preparations. Few studies have determined these tissue effects on the digestion passage and nutrient utilization in vivo.
The present study is speculated, for the first time, that the beta-casomorphin-7 indirectly affects gastrin gene expression through the paracrine actions of somatostatin and depends on its intrinsic form.
It is hopeful that beta casomorphins will be applied as potential health enhancing nutraceuticals for food and pharmaceuticals.
The manuscript is of a high standard. The design and concept of this study is good and reproducible.
S- Editor Wang J L- Editors Ma JY E- Editor Liu Y
| 1. | Meisel H. Chemical characterization and opioid activity of an exorphin isolated from in vivo digests of casein. FEBS Lett. 1986;196:223-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 72] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Svedberg J, de Haas J, Leimenstoll G, Paul F, Teschemacher H. Demonstration of beta-casomorphin immunoreactive materials in in vitro digests of bovine milk and in small intestine contents after bovine milk ingestion in adult humans. Peptides. 1985;6:825-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 119] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Sakaguchi M, Koseki M, Wakamatsu M, Matsumura E. Effects of systemic administration of beta-casomorphin-5 on learning and memory in mice. Eur J Pharmacol. 2006;530:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Lloyd KCK, Debas HT. Peripheral regulation of gastric acid secretion. Johnson LR, editor. Physiology of the gastrointestinal tract, vol. 2. New York: Raven Press 1994; 1185. |
| 5. | Sandvik AK, Dimaline R, Forster ER, Evans D, Dockray GJ. Differential control of somatostatin messenger RNA in rat gastric corpus and antrum. Role of acid, food, and capsaicin-sensitive afferent neurons. J Clin Invest. 1993;91:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Li YY. Mechanisms for regulation of gastrin and somatostatin release from isolated rat stomach during gastric distention. World J Gastroenterol. 2003;9:129-133. [PubMed] |
| 7. | Madaus S, Bender H, Schusdziarra V, Kehe K, Munzert G, Weber G, Classen M. Vagally induced release of gastrin, somatostatin and bombesin-like immunoreactivity from perfused rat stomach. Effect of stimulation frequency and cholinergic mechanisms. Regul Pept. 1990;30:179-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Weigert N, Schepp W, Haller A, Schusdziarra V. Regulation of gastrin, somatostatin and bombesin release from the isolated rat stomach by exogenous and endogenous gamma-aminobutyric acid. Digestion. 1998;59:16-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Konturek SJ, Kwiecień N, Obtułowicz W, Swierczek J, Bielański W, Oleksy J, Coy DH. Effect of enkephalin and naloxone on gastric acid and serum gastrin and pancreatic polypeptide concentrations in humans. Gut. 1983;24:740-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Intorre L, Mengozzi G, Vanni E, Grassi F, Soldani G. The role of peripheral opioid receptor subtypes in the modulation of gastric acid secretion and plasma gastrin in dogs. Eur J Pharmacol. 1993;243:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Ishihara S, Minowa S, Tsuchiya S, Horie S, Watanabe K, Murayama T. Gastric acid secretion stimulated by centrally injected nociceptin in urethane-anesthetized rats. Eur J Pharmacol. 2002;441:105-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | McIntosh CH, Jia X, Kowk YN. Characterization of the opioid receptor type mediating inhibition of rat gastric somatostatin secretion. Am J Physiol. 1990;259:G922-G927. [PubMed] |
| 13. | Lippl F, Schusdziarra V, Allescher HD. Effect of endomorphin on somatostatin secretion in the isolated perfused rat stomach. Neuropeptides. 2001;35:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Daniel H, Vohwinkel M, Rehner G. Effect of casein and beta-casomorphins on gastrointestinal motility in rats. J Nutr. 1990;120:252-257. [PubMed] |
| 15. | Tomé D, Ben Mansour A, Hautefeuille M, Dumontier AM, Desjeux JF. Neuromediated action of beta-casomorphins on ion transport in rabbit ileum. Reprod Nutr Dev. 1988;28:909-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Kim TG, Choung JJ, Wallace RJ, Chamberlain DG. Effects of intra-abomasal infusion of beta-casomorphins on circulating concentrations of hyperglycaemic insulin and glucose in dairy cows. Comp Biochem Physiol A Mol Integr Physiol. 2000;127:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Schusdziarra V, Schick R, de la Fuente A, Holland A, Brantl V, Pfeiffer EF. Effect of beta-casomorphins on somatostatin release in dogs. Endocrinology. 1983;112:1948-1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 48] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Tan YF, Chen WH, Zou SX. The effect of beta casomorphin on gastrin level in murine serum. Nanjing Nongye Daxue Xuebao. 2001;24; 63-66. |
| 19. | Zhang YS, Zou SX, Zhao RQ, Chen WH. Effect of Bioactive Peptides Derived from Casein on mRNA Express of Gastrin in Early Weaning Piglet. Nongye Shengwu Jishu Xuebao. 2004;12; 61-65. |
| 20. | Tan YF, Chen WH, Zou SX. The effect of beta Casomorphin on piglets' stomach antrum under superfusion. Nanjing Nongye Daxue Xuebao. 2000;23; 72-75. |
| 21. | Di Scala-Guénot D, McIntosh CH. The effect of met-enkephalin and naloxone on somatostatin and insulin secretion from the isolated, perfused rat pancreas. Diabetes. 1985;34:1283-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Befrits R, Uvnäs-Moberg K, Johansson C. Interactions between antral peptides and prostaglandin biosynthesis in gastric acid regulation in man. Digestion. 1990;45:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | McIntosh CH, Tang CL, Malcolm AJ, Ho M, Kwok YN, Brown JC. Effect of a purified somatostatin monoclonal antibody and its Fab fragments on gastrin release. Am J Physiol. 1991;260:G489-G498. [PubMed] |
| 24. | Karnik PS, Dushkin H, Wolfe MM. Somatostatin inhibition of gastrin gene expression: involvement of pertussis toxin-sensitive and -insensitive pathways. Regul Pept. 1992;38:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Karnik PS, Wolfe MM. Somatostatin stimulates gastrin mRNA turnover in dog antral mucosa. J Biol Chem. 1990;265:2550-2555. [PubMed] |
| 26. | Zavros Y, Fleming WR, Hardy KJ, Shulkes A. Regulation of fundic and antral somatostatin secretion by CCK and gastrin. Am J Physiol. 1998;274:G742-G750. [PubMed] |
| 27. | Holst JJ, Orskov C, Seier-Poulsen S. Somatostatin is an essential paracrine link in acid inhibition of gastrin secretion. Digestion. 1992;51:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Wu SV, Giraud A, Mogard M, Sumii K, Walsh JH. Effects of inhibition of gastric secretion on antral gastrin and somatostatin gene expression in rats. Am J Physiol. 1990;258:G788-G793. [PubMed] |
| 29. | Larsson LI, Houggaard DM. Evidence for paracrine somatostatinergic regulation of gastrin gene expression by double-staining cytochemistry and quantitation. J Histochem Cytochem. 1994;42:37-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Wu SV, Sumii K, Tari A, Mogard M, Walsh JH. Regulation of gastric somatostatin gene expression. Metabolism. 1990;39:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Inui T, Kinoshita Y, Yamaguchi A, Yamatani T, Chiba T. Linkage between capsaicin-stimulated calcitonin gene-related peptide and somatostatin release in rat stomach. Am J Physiol. 1991;261:G770-G774. [PubMed] |
| 32. | Yao YL, Xu B, Zhang WD, Song YG. Gastrin, somatostatin, and experimental disturbance of the gastrointestinal tract in rats. World J Gastroenterol. 2001;7:399-402. [PubMed] |