Published online Apr 14, 2007. doi: 10.3748/wjg.v13.i14.2083
Revised: February 2, 2007
Accepted: February 8, 2007
Published online: April 14, 2007
AIM: To understand the digestive stability and mechanism of release and intestinal uptake of pea ferritin iron in caco-2 cell line model.
METHODS: Pea seed ferritin was purified using salt fractionation followed by gel filtration chromatography. The bioavailability of ferritin iron was assessed using coupled in vitro digestion/Caco-2 cell model in the presence or absence of ascorbic acid and phytic acid. Caco-2 cell ferritin formation was used as a surrogate marker of iron uptake. Structural changes of pea ferritin under simulated gastric pH were characterized using electrophoresis, gel filtration and circular dichroism spectroscopy.
RESULTS: The caco-2 cell ferritin formation was significantly increased (P < 0.001) with FeSO4 (19.3 ± 9.8 ng/mg protein) and pea ferritin (13.9 ± 6.19 ng/mg protein) compared to the blank digest (3.7 ± 1.8 ng/mg protein). Ascorbic acid enhanced while phytic acid decreased the pea ferritin iron bioavailability. However, either in the presence or absence of ascorbic acid, the ferritin content of caco-2 cells was significantly less with pea ferritin than with FeSO4. At gastric pH, no band corresponding to ferritin was observed in the presence of pepsin either on native PAGE or SDS-PAGE. Gel filtration chromatography and circular dichroism spectroscopy revealed a pH dependent loss of quaternary and secondary structure.
CONCLUSION: Under gastric conditions, the iron core of pea ferritin is released into the digestive medium due to acid induced structural alterations and dissociation of protein. The released iron interacts with dietary factors leading to modulation of pea ferritin iron bioavailability, resembling the typical characteristics of non-heme iron.
- Citation: Bejjani S, Pullakhandam R, Punjal R, Nair KM. Gastric digestion of pea ferritin and modulation of its iron bioavailability by ascorbic and phytic acids in caco-2 cells. World J Gastroenterol 2007; 13(14): 2083-2088
- URL: https://www.wjgnet.com/1007-9327/full/v13/i14/2083.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i14.2083
Iron deficiency anemia (IDA) is a serious public health problem worldwide, affecting about two billion people globally[1,2]. In India, the major etiology for the alarming prevalence of iron deficiency is poor density and bio-availability of iron from commonly consumed cereal/pulse based diets[3,4]. Therefore, food fortification is considered as a measure to increase the iron intake of the general population[5,6]. Nevertheless, it is often difficult due to traditional consumption of whole grains such as rice, whose chemical fortification is not possible.
Ferritins are iron storage proteins ubiquitously present in all organisms. Like mammals, plants (particularly legumes) store iron in their seeds in the form of ferritin[7]. Therefore, increasing the expression level of ferritin in plants is thought to increase the iron density of the staple grains and thereby the iron intake of iron deprived populations as well[8]. Nevertheless, iron bioavailability from these sources remains a concern due to the presence of high concentrations of phytates and tannins, which are known to inhibit iron absorption.
Iron bioavailability studies on purified ferritin or soybean, a rich source of ferritin, are conflicting[9-15]. Non-anemic women fed on reconstituted soybean ferritin as the iron source showed improvement in iron status[16]. These observations along with the high stability of ferritin protein against denaturants and complex chemistry of ferritin iron led to the conclusion that either ferritin or its released iron core is directly absorbed by the enterocytes[14,15]. This is in contrast with the reported low bioavailability of iron from legumes[17]. Additionally, the transgenic maize over expressing soybean ferritin gene together with phy-tase resulted in improved bioavailability of iron[18]. Apparent discrepancy between these observations has been ex-plained by various procedures such as iron labeling of ferritin, source of ferritins in vitro or in vivo methods and the physiological and nutritional status of the subjects [16,19]. Thus, there is a need to understand the digestive stability of plant ferritin, the possible mechanism(s) of iron release and the influence of dietary factors on ferritin iron uptake in the gut. These studies are of specific interest in the context of ferritin-mediated biofortification as a strategy to improve bioavailable iron in plant food.
Caco-2 cell based screening methods have been used extensively in recent years to assess iron and carotenoid bioavailability from various foods and meals[20-24]. The model consists of sequentially simulating gastric and intestinal digestion of a test sample and adding it to differentiated cultures of caco-2 cells. Furthermore, ferritin formation in the caco-2 cells exposed to digests containing iron has been used as a surrogate marker of cellular iron status and is therefore, proportional to the bioavailability of iron[23]. The objective of the present study was to evaluate modulation of purified pea ferritin iron bioavailability by ascorbic and phytic acids using coupled in vitro digestion/Caco-2 cell model and its digestive stability, and the possible mechanism of iron release.
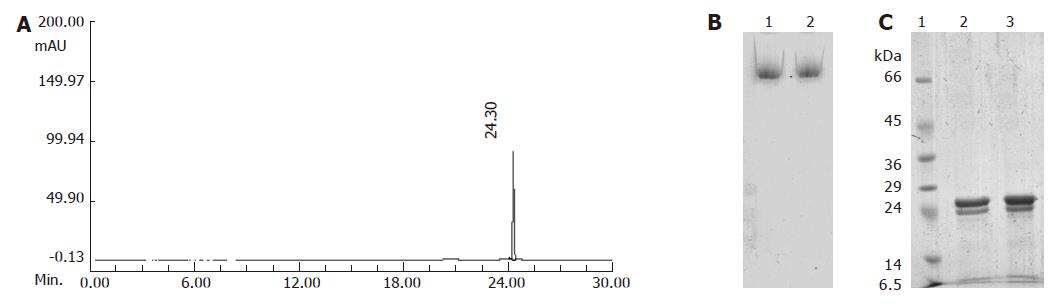
Dried pea (Pisum sativum) seeds were procured from the local market. Ferrous sulfate (FeSO4·7H2O) was obtained from Sisco Research Laboratories, Mumbai, India. Unless otherwise stated, all chemicals, enzymes were purchased from Sigma Chemical Co. (St. Louis, MO). Purification of ferritin from pea seeds was performed as described by Laulhere et al[25]. Briefly, 150 g powdered pea seed was suspended and homogenized in 3 volumes of 10 mmol/L phosphate buffer at pH 7.5, containing 1 mmol/L EDTA. The suspension was filtered through three layers of muslin cloth and further clarified with centrifugation at 10 000 r/min for 20 min at 4°C (Sorval RC 5B). The supernatant was subjected to MgCl2 precipitation and centrifuged at 12 000 r/min for 35 min and collected the reddish-brown pellet. The pellet was dissolved in water and subjected to Sepharose-6B gel filtration chromatography. The ferritin rich fractions, identified by high iron (420 nm) to protein ratio (280 nm), pooled and concentrated using Amicon 5K filters. The protein was estimated by BCA protein assay kit (Sigma Chemical Co.). Purified pea ferritin was characterized using capillary electrophoresis, PAGE and SDS-PAGE (Figure 1).
The simulated in vitro digestion was performed as described previously by Glahn et al[23] with minor modifications. Briefly, reagents required for the digestion such as saline, 40 g/L pepsin (0.1 mol/L HCl), pancreatin-bile salt mixture (0.05 g pancreatin and 0.3 g bile extract/25 mL 0.1 mol/L NaHCO3) were prepared freshly and mixed with 5% Chelex-100 and incubated for 30 min at room temperature to remove the endogenous iron present in these solutions. The Chelex-100 was removed from these solutions by passing it through a sintered glass funnel.
Peptic digestion was carried out in 50 mL screw-cap culture tubes. One hundred microlitre of stock purified pea ferritin (780 μg of iron/2.5 mg protein/mL) was diluted to 9 mL with saline in the presence and absence of 1:5 and 1:1 molar ratios of ascorbic acid and phytic acid (inositol hexa phosphate dodeca sodium salt isolated from rice, Sigma Cat#P3168), respectively. The pH of the samples was adjusted to 2.0 with 6 mol/L HCl and 0.5 mL of the pepsin solution was added and the volume adjusted to 10 mL. The tubes were immersed in a shaking water-bath at 37°C for 60 min. For intestinal digestion, the pH of the sample (also referred to as the “digesta”) was increased to 6 with 1 mol/L NaHCO3, 2.5 mL of pancreatin-bile extract mixture added and further adjusted to pH 7 with 1 mol/L NaOH. The final volume made up to 15 mL with saline and the digestion was carried out in the upper chamber of the transwell plate for 2 h as described below. Saline and ferrous sulfate treated similarly were fed to cells and run simultaneously as control and reference, respectively.
Caco-2 cells were obtained from the National Center for Cell Sciences (Pune, India) and used in experiments at passage 15-20. Cells were seeded at a density of 50 000 cells/cm2 in 6-well plates (Corning, India). The cells were grown in Dulbecco’s modified Eagle’s medium with 20% fetal bovine serum (FBS), 10% non-essential amino acids and 0.4 mmol/L glutamine and 1% antibiotic-antimycotic solution. The cells were maintained at 37°C in an incubator with a 5% CO2/95% air atmosphere at constant humidity. Once the cells reached confluence, the concentration of FBS in the medium was reduced to 10% and it was changed every 2 d. These cells were used for iron bioavailability experiments at 12-14 d after seeding.
Immediately prior to the intestinal digestion, the growth medium was removed from culture wells and the monolayer washed thrice with MEM (MEM was used because it contains less amount of endogenous iron compared to the DMEM). A fresh 2 mL aliquot of MEM (5% chelex-100 treated) covered the cells during the experiment. A sterilized insert ring fitted with a dialysis membrane (12 000 MW cut-off, Spectrapore) using an adaptor was introduced into the wells, thus creating a two-chamber system. An aliquot of 2 mL intestinal digest was pipetted into the upper chamber. The plate was covered and incubated in humidified CO2 incubator at 37°C for a period of 2 h with regular mixing at 15 min intervals. After the intestinal digestion, the upper chamber was removed and the plates were further incubated for a period of 22 h. At the end of this the cells were washed thrice with ice-cold saline and collected by scraping into 400 μL saline. The contents were sonicated for 20 s using a probe sonicator and centrifuged at 5000 r/min for 5 min. Protein concentration in the cell lysates was estimated using BCA protein assay kit.
A human ferritin sandwich ELISA method developed in-house and validated against recombinant ferritin (94/572) obtained from WHO International Laboratory for Biological Standards (NIBSC, UK) was used to estimate the caco-2 cell ferritin content[26]. Ferritin content was estimated either in neat or diluted caco-2 cell lysate using human liver ferritin IgG-HRP and the substrate system orthophenylene diamine-H2O2. The color intensity was measured using ELISA plate reader (Multiskan Accent, Lab systems, USA). Iron and inorganic phosphate content of purified pea ferritin was determined by bathophenonthroline method[27] and micro-method of Chen et al[28], respectively. Purified pea ferritin (1 mg/mL) was electrophoresed (Biofocus 3000, Bio-Rad) in an uncoated silica capillary column (50 μm ID, 375 μm OD, 50 cm length) using 10 mmol/L phosphate buffer pH 2.3 at 20 kV and detected at 215 nm.
To study the digestive stability, purified pea ferritin (1 mg/mL) at pH 7.2 (10 mmol/L phosphate buffer) and at gastric pH 2 (saline-HCl) was incubated in the presence and absence of 1.6 mg/mL of pepsin at 37°C for 1 h. At the end of the incubation, the pH of the solution was adjusted to pH 7.2 with 1 mol/L sodium bicarbonate. Aliquots of the reaction mixtures were immediately mixed with sample buffer with or without SDS. The samples were analyzed on 6% native PAGE and 12.5% SDS-PAGE. The gels were stained for iron by Prussian blue staining method[29] or for protein by the coomassie brilliant blue method.
Gel filtration chromatography of purified pea ferritin was performed using TSK-2000 SW (Altex) column connected to HPLC (Agilent, Model: 1100). Briefly, 200 μg of purified pea ferritin was incubated at pH 7.2 or pH 2 for 20 min and then subjected to size fractionation using 10 mmol/L phosphate buffered saline pH 7.5, at a flow rate of 1 mL/min and the elution was monitored at 280 nm for protein and at 420 nm for protein bound iron.
Purified pea ferritin (1 mg/mL) was incubated for 20 min at room temperature either in 10 mmol/L phosphate buffer saline pH 7.2 or in saline HCl pH 2. Circular dichroism (CD) was measured with a spectropolarimeter (JASCO-810) using 1 mm cell at 0.2 nm intervals and 1 nm bandwidth. Spectra were signal averaged by adding five accumulations. The baseline was corrected by subtracting the spectra of respective buffer blanks obtained under identical conditions. Percentage of secondary structure was calculated using the web-based program K2D (http://www.emblheidelberg.de/~andrade/k2d).
Statistical analysis was performed using the software package SPSS-7. Each experiment was conducted in triplicate and analyzed in duplicate. The ferritin data were log transformed and the means were compared using one-way ANOVA followed by least significant difference (LSD) post-hock test. The results were considered significant if P value was < 0.05.
Purification of pea ferritin using MgCl2 precipitation followed by gel filtration on Sepharose-6B column resulted in reddish-brown colored protein fractions. Capillary zone electrophoresis of pooled fractions showed a single peak suggesting high purity of the protein (Figure 1A). Native PAGE of this protein followed by staining for protein (Figure 1B) and iron (data not shown) revealed a single band and intense reaction with iron confirming the identity of ferritin. Upon SDS-PAGE, the purified ferritin showed two bands at 28 and 26 kDa regions (Figure 1C). The iron and phosphate concentrations of pea ferritin were 325 and 375 μg/mg protein, respectively. The yield of purified protein was 13-16 mg protein/kg of seeds using the above purification procedure.
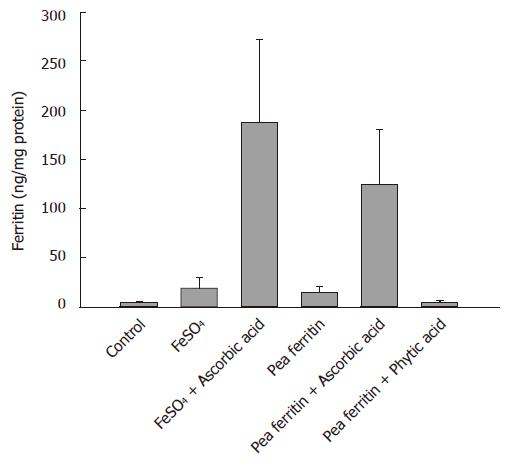
The bioavailability of iron from both FeSO4 and pea ferritin was assessed using simulated in vitro digestion/Caco-2 cell model either in the presence or absence of ascorbic acid (1:5 molar ratio) and ferritin in the presence of phytic acid (1:1 molar ratio) (Figure 2). The caco-2 cell ferritin formation was significantly increased (P < 0.001) with iron from FeSO4 (19.3 ± 9.8 ng/mg protein) or pea ferritin (13.9 ± 6.19 ng/mg protein) compared to the blank digest (3.7 ± 1.8 ng/mg protein). Addition of ascorbic acid along with either FeSO4 (188.8 ± 82.8 ng/mg protein) or pea ferritin (125.1 ± 55.49 ng/mg protein) resulted in marked increase in the ferritin formation compared to controls without ascorbic acid (P < 0.001). Addition of phytic acid along with pea ferritin reduced the caco-2 cell ferritin formation compared to pea ferritin alone (4.45 ± 2.2 ng/mg protein) (P < 0.001). However, the ferritin content of caco-2 cells exposed to FeSO4 was significantly higher than pea ferritin either in the presence or absence of ascorbic acid (P < 0.004).
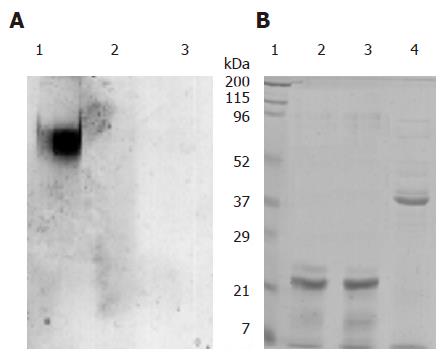
The digestive stability of purified pea ferritin was studied in the presence and absence of pepsin at gastric pH (pH 2). At pH 7.2, pea ferritin predominantly migrated as a single high molecular weight protein band on native PAGE (Figure 3A 1). However, no band was observed either in the absence or presence of pepsin at gastric pH (Figure 3A 2 and 3). Subjecting the protein to SDS-PAGE revealed 28 and 26 kDa subunits at pH 7.2 and 2 (Figure 3B 2 and 3), no other band except a protein band at 36 kDa region (pepsin) in the presence of pepsin (Figure 3B 4).
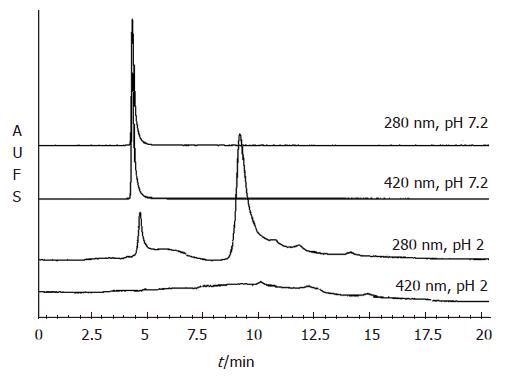
The quaternary structural changes of pea ferritin as a function of pH were studied using gel filtration chromato-graphy on TSK-2000 column (Figure 4). At pH 7.2, pea ferritin eluted at 4.8 min as a single peak having absorption at 280 (protein) and 420 nm (protein bound iron). Pea ferritin incubated at pH 2 eluted at 9.5 min predominantly as low molecular weight protein when monitored at 280 nm and only minor fraction of protein eluted at 4.8 min. However, the peak due to absorption of protein bound iron at 420 nm was completely absent at pH 2 (Figure 4).
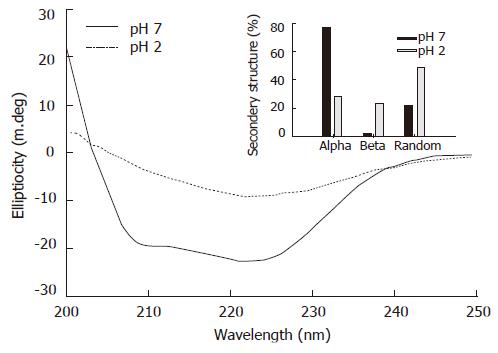
The secondary structural changes of pea ferritin as a function of pH were studied using far UV CD spectral analysis. At native pH, purified pea ferritin showed a typical α-helical spectrum, and acidification led to loss of secondary structure (Figure 5). Analysis of the CD spectrum indicated high content of α-helix (76%) in the protein followed by β-sheet (2%) and random coil (22%) conformation at pH 7.2 (Figure 5, inset). However, on acidification α-helical content of pea ferritin decreased (28%) while β-sheet (23%) and random coil (49%) conformations increased.
On a chemical basis, dietary iron is classified as non-heme iron of plant origin (inorganic ferric or ferrous iron) and heme iron of animal origin. Ferritins are ubiquitous iron storing proteins present in animals and plants and each ferritin molecule is known to store about 4500 atoms of iron[14]. Since ferritin iron is masked by a protein coat, it is likely that ferritin-bound iron is less sensitive to chelators such as phytates present in the diet and, therefore, forms a bioavailable source of iron. This is further supported by observations that ferritin iron bioavailability is comparable to that of FeSO4 in human subjects[16,30]. The bioavailability of iron from soybeans, which contain large amounts of ferritin iron, has been shown to be high despite relatively high concentrations of phytates[13]. Based on these observations, on the complex chemistry of ferritin iron and on the unusual stability of ferritin protein, it was proposed that ferritin might escape the digestion process and be directly absorbed by the enterocyte[13-14]. However, survival of a protein harsh gastric conditions is unlikely. Moreover, dietary factors such as phytates chelate ferric iron and make it unavailable for absorption in the gut while ascorbic acid and other reducing compounds convert ferric iron into ferrous iron, which is absorbed in the gut by a specific membrane receptor, divalent metal ion transporter-1[31].
Considering the importance of plant ferritin as a target molecule for biofortification of iron and its success in enhancing the iron density of staple foods[8], systematic investigations on the bioavailability of ferritin iron and its associated mechanisms are needed. Therefore, we have purified the pea seed ferritin and used this as a source of iron. The purified protein was found to contain high amounts of iron and phosphate, characteristic of plant ferritins. The subunit composition and secondary structure of the protein are in agreement with those reported by others[14,25,32].
The bioavailability and the miscibility of pea ferritin iron was assessed using the well-established system of simulated in vitro digestion/Caco-2 cell model. Ascorbic and phytic acids are known to promote and inhibit iron bioavailability from non-heme iron sources, such as FeSO4, respectively. In this study, we found that bioavailability of iron from FeSO4 was significantly enhanced by addition of ascorbic acid to the digest. Interestingly, pea ferritin iron bioavailability was also found to be enhanced by ascorbic acid and inhibited by phytic acid. These results are in agreement with the known effects of phytate on non-heme iron absorption[3,4]. The observation that reduction of phytate concentration by over-expression of Aspergillus phytase in transgenic maize over expressing soybean ferritin gene enhances the bioavailability of iron[18], also supports the above findings. However, the bioavailability of pea ferritin iron was relatively less than that of FeSO4 possibly due to a high phosphate content of purified pea ferritin or incomplete dissolution of the released iron-phosphate core in the chyme. These results suggest that the gastric and duodenal milieu dissociates iron from pea ferritin core and the iron thus released is accessible for either reduction or chelation by ascorbic and phytic acids, respectively.
In an attempt to understand the stability of pea ferritin at gastric pH, we incubated the protein at pH 2 in the presence and absence of gastric enzyme pepsin followed by native or SDS-PAGE analysis. The results revealed dissociation of protein even in the absence of pepsin with the appearance of only a weak smear of protein stain on native-PAGE, possibly due to dissociation of protein at gastric pH. The appearance of low molecular weight peaks during gel filtration analysis of acidified ferritin further confirms the above findings. Interestingly, absence of absorption at 420 nm (due to protein-bound iron) implies release of iron core from ferritin at gastric pH. Taken together, these observations suggest that pea ferritin is dissociated at gastric pH and releases its iron core into the digestive medium. The released iron core is then accessible for reduction by ascorbic acid or chelation by phytic acid, leading to modulation of iron induced ferritin formation in caco-2 cells.
In general, acidification is known to denature proteins by altering the quaternary, tertiary and secondary structural elements leading to loss of function such as enzyme activity or ligand binding properties. At native pH (pH 7.2), purified pea ferritin showed a typical α-helical spectrum similar to the observations reported with horse spleen ferritin[32,33]. However, on acidification (pH 2) there was a significant loss of α-helical structure and increase in the β-sheet and random coil conformation, implying severe secondary structural alternations in the protein at gastric pH. Collectively, the findings from PAGE, gel filtration and CD spectroscopic studies on pea ferritin strongly suggest loss of quaternary and secondary structures of the protein at gastric pH. Thus, under these conditions it is unlikely that the denatured pea ferritin protein retains its iron binding ability.
Interestingly, these observations contrast with the human studies using either purified reconstituted soybean or horse spleen ferritin[16,30]. These studies have concluded that the relative bioavailability of ferritin iron is close to 1.0 when compared with FeSO4. Based on studies in rats, it was proposed that ferritin iron is bioavailable in the presence of dietary inhibitors[15]. However, studies in women with low iron stores suggest a lower bioavailability (~ 0.46) of iron from intrinsically labelled soybean when compared to FeSO4, which is comparable to the results of the present study[13]. Other possible reasons for the relatively high bioavailability of ferritin iron in human subjects (30%-35%) could be the variations in gastric conditions and iron status prevailing in the study subjects[13,16,30]. It is also noteworthy that unlike the in vitro digestion model, gastric digestion and emptying in humans is a dynamic process. Therefore, the released ferritin iron has equal chances of interaction with either enterocyte or chelators/reducers in the food, and thereby the observed high bioavailability of ferritin iron in humans. The other likely reason may be subtle differences between pea and soy ferritins.
In conclusion, purified pea ferritin under gastric con-ditions losses its quaternary and secondary structural elements and undergoes peptic digestion leading to release of protein bound iron. The released iron is accessible to ascorbic and phytic acids to bring about reduction or chelation reflecting as increased or reduced bioavailability of pea ferritin iron, the characteristic properties of non-heme iron pool. Therefore, apart from increasing the iron density through plant ferritin, ensuring substantial reduction in phytate concentrations appears to be nece-ssary for biotechnological approaches to use plant ferritin as a tool in crop-biofortification.
We thank Dr. B. Sesikeran, Director and Dr. B. Sivakumar former Director for their support. We appreciate the technical help of Mr. T.G. Thippeswamy for HPLC analysis and Dr. N. Balakrishna for statistical analysis.
S- Editor Wang J L- Editor Ma JY E- Editor Liu Y
| 1. | Stoltzfus RJ, Dreyfuss , ML . Guidelines for the use of iron supplements to prevent and treat iron deficiency anemia. Washington (DC): ILSI Press 1998; 1-39. |
| 2. | World Health Organization. Iron Deficiency Anemia Assessment, Prevention and Control. Geneva: WHO 2001; 15-16. |
| 3. | Narasinga Rao BS, Vijayasarathy C, Prabhavathi T. Iron absorption from habitual diets of Indians studied by the extrinsic tag technique. Indian J Med Res. 1983;77:648-657. [PubMed] |
| 4. | Hallberg L, Rossander L, Skånberg AB. Phytates and the inhibitory effect of bran on iron absorption in man. Am J Clin Nutr. 1987;45:988-996. [PubMed] |
| 5. | Madhavan Nair K. Alternate strategies for improving iron nutrition: lessons from recent research. Br J Nutr. 2001;85 Suppl 2:S187-S191. [PubMed] |
| 6. | Hurrell RF. Prospects for improving the iron fortification of foods. Nutritional Anemias. New York: Raven 1992; 193–208. |
| 7. | Briat JF, Lobréaux S. Iron storage and ferritin in plants. Met Ions Biol Syst. 1998;35:563-584. [PubMed] |
| 8. | Lucca P, Hurrell R, Potrykus I. Fighting iron deficiency anemia with iron-rich rice. J Am Coll Nutr. 2002;21:184S-190S. [PubMed] |
| 9. | Layrisse M, Martínez-Torres C, Renzy M, Leets I. Ferritin iron absorption in man. Blood. 1975;45:689-698. [PubMed] |
| 10. | Ambe S, Ambe F, Nozuki T. Mossbauer study of iron in soybean seeds. J Agric Food Chem. 1987;35:292-296. [DOI] [Full Text] |
| 11. | Beard JL, Burton JW, Theil EC. Purified ferritin and soybean meal can be sources of iron for treating iron deficiency in rats. J Nutr. 1996;126:154-160. [PubMed] |
| 12. | Skikne B, Fonzo D, Lynch SR, Cook JD. Bovine ferritin iron bioavailability in man. Eur J Clin Invest. 1997;27:228-233. [PubMed] |
| 13. | Murray-Kolb LE, Welch R, Theil EC, Beard JL. Women with low iron stores absorb iron from soybeans. Am J Clin Nutr. 2003;77:180-184. [PubMed] |
| 14. | Theil EC. Iron, ferritin, and nutrition. Annu Rev Nutr. 2004;24:327-343. [PubMed] |
| 15. | Theil EC, Briat JF. Plant ferritin and non-heme iron nutrition in humans. Harvest Plus Technical Monograph 1. Washington, DC and Cali: International Food Policy Research Institute and International Center for Tropical Agriculture (CIAT) 2004; . |
| 16. | Lönnerdal B, Bryant A, Liu X, Theil EC. Iron absorption from soybean ferritin in nonanemic women. Am J Clin Nutr. 2006;83:103-107. [PubMed] |
| 17. | Lynch SR, Beard JL, Dassenko SA, Cook JD. Iron absorption from legumes in humans. Am J Clin Nutr. 1984;40:42-47. [PubMed] |
| 18. | Drakakaki G, Marcel S, Glahn RP, Lund EK, Pariagh S, Fischer R, Christou P, Stoger E. Endosperm-specific co-expression of recombinant soybean ferritin and Aspergillus phytase in maize results in significant increases in the levels of bioavailable iron. Plant Mol Biol. 2005;59:869-880. [PubMed] |
| 19. | Hunt JR. Absorption of iron from ferritin. Am J Clin Nutr. 2005;81:1178-1179; author reply 1178-1179;. [PubMed] |
| 20. | Glahn RP, Wien EM, Van Campen DR, Miller DD. Caco-2 cell iron uptake from meat and casein digests parallels in vivo studies: use of a novel in vitro method for rapid estimation of iron bioavailability. J Nutr. 1996;126:332-339. [PubMed] |
| 21. | Glahn RP, Rassier M, Goldman MI, Lee OA, Cha J. A comparison of iron availability from commercial iron preparations using an in vitro digestion/Caco-2 cell culture model. J Nutr Biochem. 2000;11:62-68. [PubMed] |
| 22. | Au AP, Reddy MB. Caco-2 cells can be used to assess human iron bioavailability from a semipurified meal. J Nutr. 2000;130:1329-1334. [PubMed] |
| 23. | Glahn RP, Lee OA, Yeung A, Goldman MI, Miller DD. Caco-2 cell ferritin formation predicts nonradiolabeled food iron availability in an in vitro digestion/Caco-2 cell culture model. J Nutr. 1998;128:1555-1561. [PubMed] |
| 24. | Chitchumroonchokchai C, Schwartz SJ, Failla ML. Assessment of lutein bioavailability from meals and a supplement using simulated digestion and caco-2 human intestinal cells. J Nutr. 2004;134:2280-2286. [PubMed] |
| 25. | Laulhere JP, Laboure AM, Briat JF. Mechanism of the transition from plant ferritin to phytosiderin. J Biol Chem. 1989;264:3629-3635. [PubMed] |
| 26. | Madhavan Nair K, Bhaskaram P, Balakrishna N, Ravinder P, Sesikeran B. Response of hemoglobin, serum ferritin, and serum transferrin receptor during iron supplementation in pregnancy: a prospective study. Nutrition. 2004;20:896-899. [PubMed] |
| 27. | Hurrell RF, Lynch SR, Trinidad TP, Dassenko SA, Cook JD. Iron absorption in humans: bovine serum albumin compared with beef muscle and egg white. Am J Clin Nutr. 1988;47:102-107. [PubMed] |
| 28. | Chen PS, Toribara TY, Warner H. Micro determination of phosphorus. Anal Chem. 1956;28:1756-1758. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5935] [Cited by in RCA: 5305] [Article Influence: 230.7] [Reference Citation Analysis (0)] |
| 29. | Gaal O, Medgyesi GA, Vereczkey A. Electrophoresis in the separation of biological molecules. New York: John Wiley & Sons 1980; 251-259. |
| 30. | Davila-Hicks P, Theil EC, Lönnerdal B. Iron in ferritin or in salts (ferrous sulfate) is equally bioavailable in nonanemic women. Am J Clin Nutr. 2004;80:936-940. [PubMed] |
| 31. | Fleming MD, Trenor CC, Su MA, Foernzler D, Beier DR, Dietrich WF, Andrews NC. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet. 1997;16:383-386. [PubMed] |
| 32. | Listowsky I, Blauer G, Enlard S, Betheil JJ. Denaturation of horse spleen ferritin in aqueous guanidinium chloride solutions. Biochemistry. 1972;11:2176-2182. [PubMed] |
| 33. | Ishitani K, Niitsu Y, Listowsky I. Characterization of the different polypeptide components and analysis of subunit assembly in ferritin. J Biol Chem. 1975;250:3124-3128. [PubMed] |