Published online Apr 14, 2007. doi: 10.3748/wjg.v13.i14.2048
Revised: March 2, 2007
Accepted: March 8, 2007
Published online: April 14, 2007
AIM: To investigate the histopathological and genetic differences between polypoid growth (PG) and non-polypoid growth (NPG) submucosal invasive colorectal carcinoma (CRC).
METHODS: A total of 96 cases of submucosal CRC were divided into two groups according to their growth type; 60 cases of PG and 36 cases of NPG. The size, histological degree of dysplasia, depth of submucosal invasion and lymph node metastasis were compared between the two groups. Furthermore, expression of p53 was detected by immunohistochemical staining, and K-ras gene mutation was examined by polymerase chain reaction based single-strand conformation polymorphism (SSCP).
RESULTS: The average size of the lesions in the NPG group was significantly smaller than those in the PG group (7.5 mm vs 13.8 mm, P < 0.001). The histological degree of dysplasia tended to be more severe in NPG group, while the incidence of submucosal massive invasion and the lymph node metastasis were both significantly higher in the NPG type than in the PG group (64.3% vs 43.3%, P = 0.004; 43% vs 7%, P = 0.008, respectively). In addition, K-ras gene mutations were detected in 67% of lesions in the PG group, but none in the NPG group, while no difference in p53 immunohistochemical expression was found between the two groups.
CONCLUSION: Compared with PG submucosal CRC, NPG type demonstrates more frequent submucosal massive invasion, more lymph node metastasis and a higher degree dysplasia. Genetically, NPG type shows much less frequent K-ras mutation.
- Citation: Hirata I, Wang FY, Murano M, Inoue T, Toshina K, Nishikawa T, Maemura K. Histopathological and genetic differences between polypoid and non-polypoid submucosal colorectal carcinoma. World J Gastroenterol 2007; 13(14): 2048-2052
- URL: https://www.wjgnet.com/1007-9327/full/v13/i14/2048.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i14.2048
Regarding the morphorgenesis of colorectal carcinoma (CRC), the polyp-carcinoma sequences in which a polyp (exophytic) lesion progresses to invasive carcinoma has been stressed[1,2]. This is because most early stage carcinomas have been detected as small lesions with a polypoid appearance until quite recently. Since 1980, following Kudo’s achievements, many early CRC with depressed appearance, which do not resemble a polyp, have been detected in many other countries as well as in Japan[3-5].
This indicates that there are two morphologically different types of early CRC, the exophytic and depressed types. Ikegami and Shimoda classified early CRC into two groups according to their intramucosal growth pattern of the tumor, polypoid growth (PG) and nonpolypoid growth (NPG) types[6]. It has been described that exophytic and depressed lesions develop into PG and NPG types of CRC, respectively. More recently, these two types of CRC have been demonstrated as having biological as well as morphological differences[7,8]. In the present study, we compared PG and NPG submucosal (sm) invasive CRC histopathologically and genetically, and attempted to clarify the biological differences between the two types of submucosal early CRC.
A total of 96 cases of submucosal CRC were resected surgically (63 cases) and endoscopically (33 cases) in The Affiliated Hospital of Osaka Medical College. These lesions were divided into two groups according to their type of growth; 60 cases of PG and 36 cases of NPG. All diagnoses were based on histopathological examinations and none of the patients had hereditary colorectal tumors such as familial adenomatosis polyposis (FAP). Informed consent was obtained from all subjects after a full explanation of the project, while the specimen collecting procedures were approved by the Ethics Committee in Osaka Medical College.
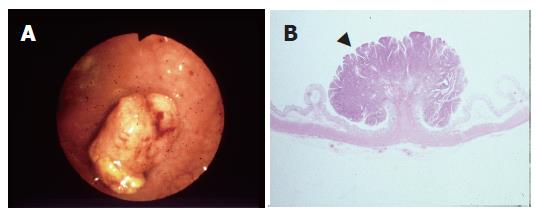
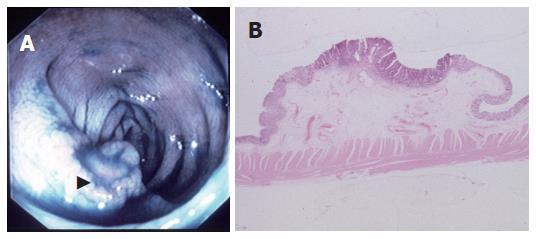
The classification of the lesions was based on the Japanese conventional criteria[9]. Briefly, on the basis of microscopic observation of the cut-surface, a PG lesion is characterized by the polypoid appearance, in which the tumor forms a protrusion due to the intramucosal proliferation (Figure 1A and B), and a NPG lesion is identified as a depressed or flat appearance without prominent intramucosal proliferation (Figure 2 A and B).
All samples were cut into 5 μm thick sections for histologic examination. To determine the extent of submucosal invasion, the submucosal layer was divided into three parts: (1) invasion involving the upper 500 μm of the submucosal layer was regarded as sm1; (2) that involving the middle layer as sm2; and (3) invasion involving the deepest layer as sm3. Sm2 and sm3 were regarded as submucosal massive invasion[11]. The histological grade of differentiation was classified as; well-, moderate-or poor-differentiated adenocarcinoma according to the World Health Organization (WHO) classification for large intestinal tumors[10].
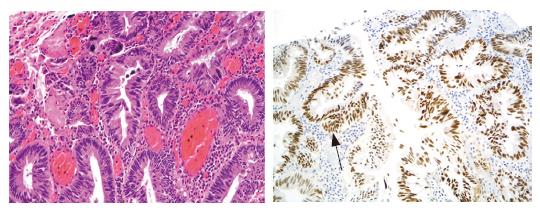
Immunohistochemical staining was performed for the examination of p53 protein[11]. Formalin-fixed paraffin sections were heated in a microwave oven before immunostaining with anti-p53 polyclonal antibody (CM1; Novocastra, Newcastle, UK). Cases showing diffuse overexpression of p53 in almost neoplastic glands were regarded as diffusely positive and cases showing nested or focal overexpression of p53 in neoplastic glands were regarded as focally positive. Cases in which the nuclei of neoplastic glands were stained sporadically or were unstained were regarded as negative. The positive rate was calculated as the percentage of diffuse plus focally positive cases out of the total number of cases.
Formalin-fixed, paraffin-embedded samples were cut serially with a thickness of 10 μm. After identification of a lesion with the highest histological grade of atypia in the entire tumor tissue and after marking the lesion on paraffin sections, the marked lesions were scraped from paraffin sections. The DNA was then extracted by using a DNA isolator PS kit (Wako Pure Chemical, Osaka, Japan) in accordance with the supplied protocol[12].
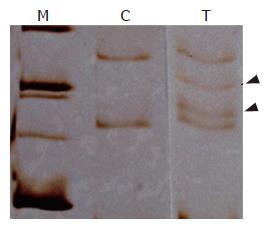
Mutations at codon 12 of K-ras gene were detected by the polymerase chain reaction-single strand conformation polymorphism (PCR-RFLP) method (PCR-SSCP), as previously described elsewhere[13]. Briefly, the extracted DNA was amplified by using PCR with the primers of 5’GCCGGTAGTGTATTAACCTTATGTGTGACAT-3’ and 5’CAAAACAAGATTTACCTCTATTGTTGG-3’. 10 μL of formamide solution was added to 2 μL of PCR products, and heated to 80°C for 5 minutes to dissociated DNA tosingle strand. Then, 10 μL of single strand DNA was applied to a 12% polyacrylic amide gel, and elecphoresis was performed before silver staining. Thus, the presence of point mutation in K-ras codon 12 and the type of base substitution were examined[14].
Data were summarized as mean ± SD. The chi-squared test (or Fisher's exact test when the expected number of any variables was smaller than or equal to five cases) and the student t test were performed to determine the significance of differences in mean values and associations. Findings with a P value of < 0.05 were considered to be statistically significant.
The mean sizes of the lesions in the NPG and PG groups were 7.5 ± 6.2 mm and 13.8 ± 5.4 mm, respectively. The lesions in the NPG group were significantly smaller than those in the PG group (P = 0.001, Table 1, Figure 1A and B, Figure 2A and B). In addition, there was no significant difference in histopathologic differentiation between the two groups (P = 0.238).
The frequency of sm massive invasion was significant higher in the NPG group than in the PG group (P = 0.004), and the incidence of lymph node metastasis was also significantly higher in the NGP group (P = 0.008, Table 2).
The positive rates of p53 overexpression in the NPG and PG groups were 57.1% and 33.3%, respectively, and the difference was not statistically significant (P > 0.05) (Figure 3).
K-ras mutation was detected in 67% of the PG lesions but in none of the NPG lesions (Figure 4).
Colorectal carcinoma has traditionally been considered to develop from adenoma with polypoid appearance, progressing to invasive carcinoma. This concept has been called the adenoma-adenocarcinoma sequence in terms of the histogenesis, and interpreted as the polyp-cancer sequence in terms of morphogenesis[15]. On the other hand, another proposal that some CRC arise from non-polypoid lesions, the so-called de novo sequence, has been reported[16]. Since the 1980s, a large number of tumors with flat and depressed appearance have been detected in Japan, and more recently in many other countries[3-5,17]. It is not well known whether flat/depressed epithelial tumors are distinct from ordinary pedunculated or exophytic polyps and support the de novo theory. In order to understand the possible histogenesis and morphogenesis of CRC, a series of comparative studies of the clinicopathological and biological features of polypoid and flat/depressed lesions has been reported in Japan[18-20]. However, there is a discrepancy in the histopathologic criteria between WHO and Japanese classifications[21], for measuring adenoma and intramucosal adenocarcinoma: intramucosal adenocarcinoma is classified as adenoma with severe dysplasia in the WHO catalog. Therefore, only submucosal early CRC cases were included in the present study.
Of 96 sm invasive early in the study, 60 cases (62.5%) and 36 (37.5) cases were divided into two groups according to their macroscopic growth patterns. Most PG lesions (87%) were morphologically identified as pedunculated or sessile polyp, while some NPG lesions demonstrated gross appearance of central depression with marginal elevation, as labeled IIa + IIc in the Japanese classification of early gastric cancer[22]. Macroscopically, NPG lesions showed a significantly smaller size. Moreover, compared with the PG group, the NPG group demonstrated a worse histologic differentiation, more massive invasiveness, and a higher incidence of lymph node metastasis. These findings indicate that NPG carcinoma might have a different characteristic pattern of development and progression from that of PG lesions. Namely, PG lesions mainly consist of tumors with a low degree of dysplasia and proliferate slowly in the mucosal layer, while NPG lesions mainly consist of tumors with a high degree of dysplasia and infiltrate rapidly to the submucosal layer despite their small size[23]. Therefore, it might be speculated that NPG early CRC is more aggressive that PG type.
Several studies have shown that changes occur in Ki-ras, p53, DCC and Bcl2 gene structure and function during the various stages of human colon carcinogenesis[24-26]. Alterations of these genes are responsible for the establishment of a state of continuous stimulus for cell division and apoptotic inhibition at physiological and pharmacological levels. p53 mutation has been regarded as a late event in the development of advanced CRC. Nuclear immunohistochemical expression of p53 protein has been proved to be related to localization, and of prognostic value for tumor recurrence associated with over-expression of p53 protein[27].
It has been reported that multiple genetic alterations were involved in the development of adenoma-adenocarcinoma sequence[28]. K-ras gene mutation was detected at an early stage of colorectal carcinogenesis[29,30]. However, recent research revealed that K-ras mutation was detected in 47% of exophytic tumor but not in flat type CRC[31]. Our study has also proven that K-ras gene mutation was not found in NPG sm invasive CRC even though it was found in 67% of PG type lesions. This result suggests that NPG type early CRC could have a different genetic background.
In conclusion, our results suggest that NPG early CRC develop from non-polypoid (flat or depressed) lesions, and that there exists a different carcinogenesis pathway from ordinary colorectal adenoma-adenocarcinoma sequence.
Early colorectal cancer (CRC) has been morphologically classified into two growth patterns: polypoid growth (PG) and non-polypoid growth (NPG) types. These two types seem to have different morphogenesis as to their development and progression. The aim of this study was to investigate the histopathological and genetic differences between PG and NPG submucosal invasive CRC.
CRC has traditionally been considered to develop from adenoma with polypoid appearance, progressing to invasive carcinoma. This concept has been called the adenoma-adenocarcinoma sequence in terms of the histogenesis, and interpreted as the polyp-cancer sequence in terms of morphogenesis. On the other hand, another proposal that some CRC arise from non-polypoid lesions, the so-called de novo sequence, has been reported. Since the 1980s, a large number of tumors with flat and depressed appearance have been detected in Japan and more recently, in many other countries. It is not well known whether flat/depressed epithelial tumors are distinct from ordinary pedunculated or exophytic polyps and support the de novo theory. In order to understand the possible histogenesis and morphogenesis of CRC, a series of comparative studies about clinicopathological and biological features of polypoid and flat/depressed lesions have been reported in Japan. However, there is a discrepancy between WHO and Japanese classifications in the histopathologic criteria for adenoma and intramucosal adenocarcinoma. Therefore, only submucosal early CRC cases were included in the present study.
Compared with PG submucosal CRC, NPG type demonstrates more frequent submucosal massive invasion, more lymph node metastasis and higher degree dyspalsia. Genetically, NPG type shows much less frequent K-ras mutation.
These findings suggest that NPG early CRC develop from non-polypoid (flat or depressed) lesions, and that there is a different carcinogenesis pathway from ordinary colorectal adenoma-adenocarcinoma sequence. These results should be useful for the understanding of the carcinogenesis of CRC.
PG and NPG: There are two morphologically different types of early CRC, i.e. exophytic and depressed type. Ikegami and Shimoda classified early CRC into two groups according to their intramucosal growth pattern of the tumor, PG and NPG types. And it has been described that exophytic and depressed lesions develop into PG and NPG types, respectively.
The authors have reviewed extensive series of polypoid and non-polypoid submucosal colorectal carcinoma based on the Japanese literature. In this study, they investigate differences in the histopathology and genetic makeup of these two growth patterns. The study is very interesting and well designed. The title accurately reflects the major topic and contents of the study. The methods used are appropriate for this type of study. The results provide sufficient experimental evidence or data to draw firm scientific conclusions. The discussion is well organized and systematic; the references are appropriate, relevant and up to date. The figures reflect the major findings of the study. However, the genetic evaluation is somewhat limited by the markers they have chosen. Other markers should be studied to further understanding of the differences between these two types of neoplasia.
S- Editor Liu Y L- Editor Roberts SE E- Ediror Chen GJ
| 1. | Day DW, Morson BC. The adenoma-carcinoma sequence. Major Probl Pathol. 1978;10:58-71. [PubMed] |
| 2. | Mitros FA. Polyps: the pathologist's perspective. Semin Surg Oncol. 1995;11:379-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Kudo S, Kashida H, Tamura T, Kogure E, Imai Y, Yamano H, Hart AR. Colonoscopic diagnosis and management of nonpolypoid early colorectal cancer. World J Surg. 2000;24:1081-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 252] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 4. | Noda H, Kato Y, Yoshikawa H, Arai M, Togashi K, Nagai H, Konishi F, Miki Y. Frequent involvement of ras-signalling pathways in both polypoid-type and flat-type early-stage colorectal cancers. J Exp Clin Cancer Res. 2006;25:235-242. [PubMed] |
| 5. | Iwasaki J, Sano Y, Fu KI, Machida A, Okuno T, Kuwamura H, Yoshino T, Mera K, Kato S, Ohtsu A. Depressed-type (0-IIc) colorectal neoplasm in patients with family history of first-degree relatives with colorectal cancer: A cross-sectional study. World J Gastroenterol. 2006;12:3082-3087. [PubMed] |
| 6. | Kanazawa T, Watanabe T, Nagawa H. Does early polypoid colorectal cancer with depression have a pathway other than adenoma-carcinoma sequence? Tumori. 2003;89:408-411. [PubMed] |
| 7. | Vagliasindi A, Sivelli R, Gualtierotti M, Piccolo D, Bertagni A, Sianesi M. Are small-size depressed-type colorectal neoplasias really the expression of a early stage of colorectal cancer. Apropos of a clinical case. Tumori. 2003;89:155-158. [PubMed] |
| 8. | Richter H, Slezak P, Walch A, Werner M, Braselmann H, Jaramillo E, Ost A, Hirata I, Takahama K, Zitzelsberger H. Distinct chromosomal imbalances in nonpolypoid and polypoid colorectal adenomas indicate different genetic pathways in the development of colorectal neoplasms. Am J Pathol. 2003;163:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Schlemper RJ, Kato Y, Stolte M. Diagnostic criteria for gastrointestinal carcinomas in Japan and Western countries: proposal for a new classification system of gastrointestinal epithelial neoplasia. J Gastroenterol Hepatol. 2000;15 Suppl:G49-G57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Egashira Y, Yoshida T, Hirata I, Hamamoto N, Akutagawa H, Takeshita A, Noda N, Kurisu Y, Shibayama Y. Analysis of pathological risk factors for lymph node metastasis of submucosal invasive colon cancer. Mod Pathol. 2004;17:503-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Yao T, Utsunomiya T, Nagai E, Oya M, Tsuneyoshi M. p53 expression patterns in colorectal adenomas and early carcinomas: a special reference to depressed adenoma and non-polypoid carcinoma. Pathol Int. 1996;46:962-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Watanabe T, Muto T. Colorectal carcinogenesis based on molecular biology of early colorectal cancer, with special reference to nonpolypoid (superficial) lesions. World J Surg. 2000;24:1091-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Yuan P, Sun MH, Zhang JS, Zhu XZ, Shi DR. APC and K-ras gene mutation in aberrant crypt foci of human colon. World J Gastroenterol. 2001;7:352-356. [PubMed] |
| 14. | Kurisu Y, Shimoda T, Ochiai A, Nakanishi Y, Hirata I, Katsu KI. Histologic and immunohistochemical analysis of early submucosal invasive carcinoma of the colon and rectum. Pathol Int. 1999;49:608-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Matsui T, Yao T, Iwashita A. Natural history of early colorectal cancer. World J Surg. 2000;24:1022-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Jass JR. Pathogenesis of colorectal cancer. Surg Clin North Am. 2002;82:891-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Ross AS, Waxman I. Flat and depressed neoplasms of the colon in Western populations. Am J Gastroenterol. 2006;101:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Ajioka Y, Watanabe H, Kazama S, Hashidate H, Yokoyama J, Yamada S, Takaku H, Nishikura K. Early colorectal cancer with special reference to the superficial nonpolypoid type from a histopathologic point of view. World J Surg. 2000;24:1075-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | van Wyk R, Slezak P, Hayes VM, Buys CH, Kotze MJ, de Jong G, Rubio C, Dolk A, Jaramillo E, Koizumi K. Somatic mutations of the APC, KRAS, and TP53 genes in nonpolypoid colorectal adenomas. Genes Chromosomes Cancer. 2000;27:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Takahashi Y, Ishii Y, Nagata T, Ikarashi M, Ishikawa K, Asai S. Clinical application of oligonucleotide probe array for full-length gene sequencing of TP53 in colon cancer. Oncology. 2003;64:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Schlemper RJ, Itabashi M, Kato Y, Lewin KJ, Riddell RH, Shimoda T, Sipponen P, Stolte M, Watanabe H. Differences in the diagnostic criteria used by Japanese and Western pathologists to diagnose colorectal carcinoma. Cancer. 1998;82:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | Ishihara S, Watanabe T, Umetani N, Yamagata S, Masaki T, Nagawa H, Muto T. Small advanced colorectal cancers: clinicopathological characteristics and pathogenetic origin. Jpn J Clin Oncol. 2000;30:504-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Miranda E, Destro A, Malesci A, Balladore E, Bianchi P, Baryshnikova E, Franchi G, Morenghi E, Laghi L, Gennari L. Genetic and epigenetic changes in primary metastatic and nonmetastatic colorectal cancer. Br J Cancer. 2006;95:1101-1107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Calistri D, Rengucci C, Seymour I, Leonardi E, Truini M, Malacarne D, Castagnola P, Giaretti W. KRAS, p53 and BRAF gene mutations and aneuploidy in sporadic colorectal cancer progression. Cell Oncol. 2006;28:161-166. [PubMed] |
| 25. | Kojima M, Konishi F, Tsukamoto T, Yamashita K, Kanazawa K. Ki-ras point mutation in different types of colorectal carcinomas in early stages. Dis Colon Rectum. 1997;40:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Kobayashi M, Watanabe H, Ajioka Y, Honma T, Asakura H. Effect of K-ras mutation on morphogenesis of colorectal adenomas and early cancers: relationship to distribution of proliferating cells. Hum Pathol. 1996;27:1042-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Einspahr JG, Martinez ME, Jiang R, Hsu CH, Rashid A, Bhattacharrya AK, Ahnen DJ, Jacobs ET, Houlihan PS, Webb CR. Associations of Ki-ras proto-oncogene mutation and p53 gene overexpression in sporadic colorectal adenomas with demographic and clinicopathologic characteristics. Cancer Epidemiol Biomarkers Prev. 2006;15:1443-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Karakosta A, Golias Ch, Charalabopoulos A, Peschos D, Batistatou A, Charalabopoulos K. Genetic models of human cancer as a multistep process. Paradigm models of colorectal cancer, breast cancer, and chronic myelogenous and acute lymphoblastic leukaemia. J Exp Clin Cancer Res. 2005;24:505-514. [PubMed] |
| 29. | Jass JR, Baker K, Zlobec I, Higuchi T, Barker M, Buchanan D, Young J. Advanced colorectal polyps with the molecular and morphological features of serrated polyps and adenomas: concept of a 'fusion' pathway to colorectal cancer. Histopathology. 2006;49:121-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 187] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 30. | Chiang JM, Chou YH, Chou TB. K-ras codon 12 mutation determines the polypoid growth of colorectral cancer. Cancer Res. 1998;58:3289-3293. [PubMed] |
| 31. | Wark PA, Van der Kuil W, Ploemacher J, Van Muijen GN, Mulder CJ, Weijenberg MP, Kok FJ, Kampman E. Diet, lifestyle and risk of K-ras mutation-positive and -negative colorectal adenomas. Int J Cancer. 2006;119:398-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |