Published online Mar 21, 2007. doi: 10.3748/wjg.v13.i11.1747
Revised: February 10, 2007
Accepted: March 6, 2007
Published online: March 21, 2007
AIM: To explore the effect of intratumoral expressions of interleukin-12 (IL-12) and interleukin-18 (IL-18) on clinical features, angiogenesis and prognosis of gastric carcinoma.
METHODS: The expressions of IL-12 and IL-18 from 50 samples of gastric cancer tissue were analyzed by immunohistochemistry, and microvessel density (MVD) was determined with microscopic imaging analysis system.
RESULTS: The positive expression rates of IL-12 and IL-18 were 44% (22/50) and 26% (13/50), respectively. IL-12 was significantly associated with pathologic differentiation, depth of invasion, lymph node metastasis, distant metastasis, and TNM stage, and IL-18 was closely related to distant metastasis. Intratumoral IL-12 and IL-18 expressions were not statistically related to MVD scoring. IL-12-positive patients survived significantly longer than those with IL-12-negative tumors, but there was no significant difference between IL-18-positive patients and IL-18-negative ones. The multivariate analysis with Cox proportional hazard model revealed IL-12, MVD and T stage were independent prognostic factors.
CONCLUSION: The positive expressions of IL-12 and IL-18 can play an important role in progression and metastasis of gastric cancer, and IL-12 might be an independent factor of poor prognosis in gastric carcinoma.
- Citation: Ye ZB, Ma T, Li H, Jin XL, Xu HM. Expression and significance of intratumoral interleukin-12 and interleukin-18 in human gastric carcinoma. World J Gastroenterol 2007; 13(11): 1747-1751
- URL: https://www.wjgnet.com/1007-9327/full/v13/i11/1747.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i11.1747
The immune response to cancer is predominantly affected by cell-mediated immunity[1], involving antigen-presenting cells, CD4+ and CD8+ T-lymphocytes, and the production of interleukins (IL), such as IL-2, IL-12, IL-18.
IL-12, mainly secreted by antigen-presenting cells and B lymphocytes, is a disulfide-linked heterodimer composed of two subunits, p35 and p40. IL-12 stimulates T-lymphocytes and natural killer (NK) cells proliferation and cytotoxic activity, and induces the production of several cytokines [e.g., interferon-γ (IFN-γ)], therefore plays an important role in promoting Th1-type response which favor cell-mediated immunity. Recently IL-12 was reported to inhibit angiogenesis and upregulate E-cadherin, thus contributing to antitumor and antimetastatic effects[2].
IL-18 is initially described as IFN-γ-inducing factor in 1989, which up-regulates several cytokines, such as IFN-γ, TNF-α, IL-1β, and promotes Th1 cell differentiation. IL-18, mainly produced by dendritic cells and macrophages, has structural homology with IL-1, and provides a synergistic antitumor immunity in combination with IL-12. Most of the biological activities of IL-18 overlap with those of IL-12, but these two interleukins seemed to act in separate mechanisms[3,4].
This study was designed to investigate the status of IL-12 and IL-18 in gastric cancer, and probe into the possibility of the cytokines in determining clinicopathologic features, angiogenesis and prognosis of gastric cancer.
Paraffin-embedded tumor specimens from 50 randomly selected patients with gastric cancers who underwent surgery at our institute from June 1997 to December 1998 were examined. All experiments were performed after obtaining informed consent from patients according to institutional rules. The patients ranged in age from 20 to 77 years (median, 60 years). Thirty-four patients were male and 16 were female. The median follow-up period was 68 mo (range, 1-79 mo). Haematoxylin and eosin-stained sections of each primary tumor were reviewed by a gastrointestinal pathologist to ensure uniformity in the assessment of Tumor Node Metastasis (TNM) stage and differentiation. We attributed highly or moderately differentiated adenocarcinoma to “well differentiated”, and attributed poorly differentiated adenocarcinoma, mucinous carcinoma and signet cell carcinoma to “poorly differentiated”. The detailed profiles of patients and tumor are summarized in Table 1.
| Variables | n | IL-12 | IL-18 | |||
| Positive (%) | P | Positive (%) | P | |||
| Gender | M | 34 | 14 (41.2) | 0.558 | 10 (29.4) | 0.5081 |
| F | 16 | 8 (50.0) | 3 (18.8) | |||
| Age (yr) | < 60 | 25 | 14 (56.0) | 0.087 | 7 (28.0) | 0.747 |
| ≥ 60 | 25 | 8 (32.0) | 6 (24.0) | |||
| Differentiated | Well | 12 | 2 (16.7) | 0.029 | 3 (25.0) | 1.0001 |
| Poorly | 38 | 20 (52.6) | 10 (26.3) | |||
| pT category | T1-2 | 24 | 5 (20.8) | 0.002 | 4 (16.7) | 0.148 |
| T3-4 | 26 | 17 (65.4) | 9 (34.6) | |||
| pN category | N0 | 26 | 6 (30.8) | 0.002 | 6 (23.1) | 0.624 |
| N1-3 | 24 | 16 (66.7) | 7 (29.2) | |||
| pM category | M0 | 40 | 13 (32.5) | 0.0031 | 6 (15.0) | 0.0011 |
| M1 | 10 | 9 (90.0) | 7 (70.0) | |||
| TNM stage | I-II | 28 | 7 (25.0) | 0.005 | 6 (21.4) | 0.406 |
| III-IV | 22 | 15 (68.2) | 7 (31.8) | |||
Consecutive 4-μm sections were recut from each paraffin block, and were immunostained for IL-12, IL-18 and CD31.
Four-micrometer-thick sections were dewaxed and subjected to antigen heat retrieval. Endogenous peroxidase activity and nonspecific binding were blocked by incubation with 3% H2O2 and nonimmune serum, respectively. After being washed with phosphate-buffered saline (PBS), they were incubated overnight with primary antiobodies (anti-human IL-12 monoclonal antibody, R&D Systems, USA; anti-human IL-18 monoclonal antibody, Santa Cruz, UK; anti-human CD31 monoclonal antibody, DAKO, Denmark) at a 1:500 dilution. Next, they were incubated with Envision+ reagent (DAKO, Denmark) for 30 min at room temperature, then the reaction complex was visualized with DAB chromogen and nuclei were counterstained with haematoxylin. Diluted goat serum was used as negative control for the primary antibody.
The specimens immunostained for IL-12 and IL-18 were assessed without knowledge of the clinicopathologic features. At least 500 carcinoma cells were examined from 5 different fields randomly selected (× 200) within the same section under light microscopy to determine the staining status of IL-12 and IL-18, and samples were considered positive when the unequivocal staining of cytoplasm and/or nuclear compartment was seen in more than 10% of the tumor cells.
Tumor sections were scanned under low-power magnification (× 200) to select most intense vascularization areas, and the microvessel density (MVD) were determined by Axioplan 2 imaging system and KS 400 image analysis software (ZEISS, Germany). Briefly, the signal collected from the sections by the camera were intensified and analyzed automatically, and MVD was represented by the value of CD31-positive staining area over the chosen field.
The association between IL-12 or IL-18 and clinicopathologic features of patients was analyzed by the Chi-square test or Fisher’s exact probability test. The relationship between IL-12 or IL-18 expressions and MVD was calculated by Mann-Whitney test. The concordance of IL-12 and IL-18 expressions was determined by Kappa test. The significance of IL-12 or IL-18 to patient survival was examined using Kaplan-Meier method and log rank test. The influence of each variable on survival was assessed by Cox proportional hazard model. All statistical analyses were performed using SPSS for Windows (Version 13.0). The accepted level of significance was P < 0.05.
Of the 50 specimens examined, 22 (44%) and 13 (26%) demonstrated positive IL-12 and IL-18 expressions, respectively. IL-12 was mainly located in the cytoplasm of carcinoma cells but not in nuclei (Figure 1A), and the strongly positive expression was also presented in the germinal center of lymphoid follicles. IL-18 was distributed in both the cytoplasm and nuclear compartment of the carcinoma cells (Figure 1B), and the germinal center of lymphoid follicle. Adjacent normal gastric mucosa was not immunoreactive with anti-human IL-12 or IL-18 antibody.
Table 1 summarizes the relationship between clinicopathologic features and IL-12 or IL-18 expressions. IL-12 expression was directly associated with pathologic differentiation (P = 0.029), depth of invasion (P = 0.002), lymph node metastasis (P = 0.002), distant metastasis (P = 0.003) and TNM stage (P = 0.002). Although there was no significant association with gender, age, pathologic differentiation, depth of invasion, lymph node metastasis and TNM stage, IL-18 tended to be positively stained in the gastric carcinoma with distant metastasis (P = 0.001).
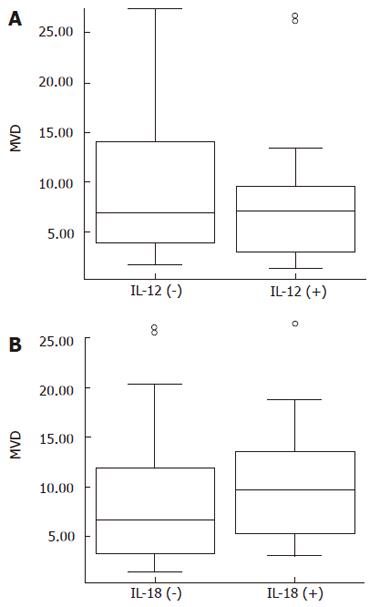
The median MVD in positive IL-12 gastric cancer specimens (7.13, 1.40-26.49) was slightly higher than in negative IL-12 specimens (6.87, 1.76-27.45) (Figure 2A, P = 0.379). The median MVD in positive IL-18 group (9.61, 2.99-27.45) was 1.46-fold higher than in negative IL-18 group (6.58, 1.40-26.49), but the difference was not statistically significant (Figure 2B, P = 0.215).
The number of gastric cancer specimen immunostained by IL-12 or IL-18 is listed in Table 2. The Kappa value representing the relationship between IL-12 and IL-18 expression was 0.194 (P = 0.139).
| Variables | IL-18 (+) | IL-18 (-) | Patients (n) | P |
| IL-12 (+) | 8 | 14 | 22 | 0.139 |
| IL-12 (-) | 5 | 23 | 28 | |
| No. of patients | 13 | 37 | 50 |
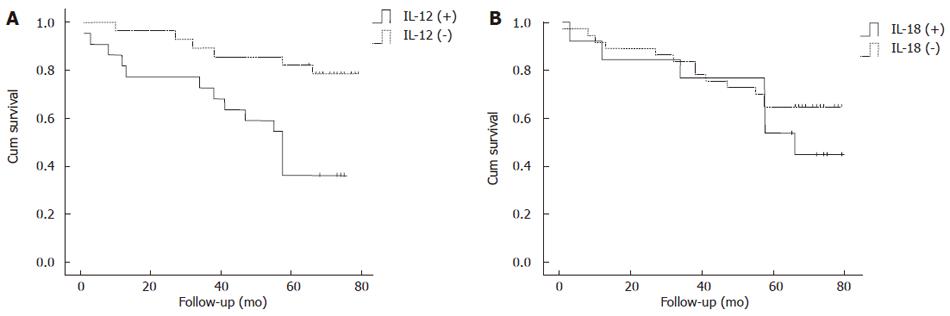
Kaplan-Meier product limit estimates of overall survival are plotted in Figure 3. Patients with positive IL-12 expression had a significantly lower survival rate than those with negative expressions (P = 0.002), and the 5-year survival rates for IL-12 negative and positive gastric carcinoma were 82.1% and 37.4%, respectively. No significant difference was found between IL-18 expressions and the overall survival (P = 0.342), and the 5-year survival rates for IL-18 negative and positive gastric carcinoma were 64.9% and 53.8%, respectively. Cox proportional hazard model demonstrated that IL-12, MVD and depth of invasion (T stage) were independent prognostic factors (Table 3).
| Variables | B | SE | Wald | df | P | Exp (B) | 95% CI |
| IL-12 | 0.955 | 0.237 | 16.290 | 1 | < 0.001 | 2.599 | 1.634-4.132 |
| MVD | 0.897 | 0.390 | 5.287 | 1 | 0.021 | 2.452 | 1.142-5.268 |
| T stage | 0.786 | 0.349 | 5.076 | 1 | 0.024 | 2.194 | 1.108-4.347 |
This study has found immunohistochemically that IL-12 and IL-18 were expressed in normal lymphoid cells and some tumor cells within gastric cancer tissues, but not in adjacent normal gastric mucosa. To our knowledge, no studies to date have demonstrated the intratumoral expressions of IL-12 or IL-18 in gastric cancer, or have associated those to patient characteristics and prognosis.
As a cytokine, IL-12 has multiple functions in tumor-related immunity, it could direct immune reaction from a deleterious Th2 to a protective Th1 response. Overexpression of interleukin-12 enables dendritic cells to activate NK cells and confer systemic antitumor immunity[5], and the injured NK activity of peripheral blood mononuclear cell (PBMC) from patients with metastatic cancer could be restored by the addition of IL-12[6]. IL-12 administered in combination with pulse IL-2 displayed prominent antitumor activity, and induced rapid and complete regression of primary and metastatic Renca tumors[7], and IL-12 gene therapy is believed to be more effective than any other cytokines for induction of solid tumor regression[2]. The positive rate of IL-12 in this panel of gastric cancer was 44%, and IL-12 expression was associated with poorly differentiated type, the depth of invasion, lymph node or distant metastasis, and TNM stage. Multivariate survival analysis even suggested that IL-12 should be a negative independent prognostic factor besides T stage and MVD, which was contrary to our initial expectation. Paleri and his colleagues assessed intratumoral expression of IL-12 and IL-7 in head and neck squamous cell cancers (HNSCC), and proved a trend towards better survival associated with high expression of IL-12[8]. There could be variable antitumor immune mechanisms in tumors with different origin and different pathologic type, and IL-12 seemed not to take participate in antitumor immunity of gastric cancer. Cell-mediated immunity might be suppressed in advanced gastric cancer, the polarization of Th2-type T-cell prevails, and the overexpression of IL-4 would downregulate β2 subunit of IL-12 receptor, resulting in the malfunction of IL-12 signal conducting pathway[9]. Furthermore, deficient antigen presentation by the downregulation of major histocompatibility complex (MHC) classIexpression, and reduced or lost expression of T cell epitopes on tumor cells, could interfere in the antigen presenting pathway[10]. In the absence of antigenic stimulation, IL-12 would induce FasL mediated and caspase-3 dependent T cell apoptosis, and Janus kinase (JAK) was involved in this process[11], thus impairing the antitumor immunity. These findings might in part explain in this study that IL-12 tended to be expressed in poorly differentiated, more aggressive gastric cancers, and related directly to poor prognosis.
IL-18 was only expressed in 13 (26%) of 50 cases, and was not associated with pathologic differentiation, depth of invasion, lymph node metastasis, TNM stage and prognosis. But IL-18 was presented in 70% of gastric cancer with distant metastasis (M1), significantly higher than M0-stage group (15%) (P = 0.001). Merendino et al[12] reported that serum IL-18 level was significantly higher in breast cancer patients with liver or bone metastases than in patients without metastasis or healthy individuals, implying that IL-18 could be regard as a metastatic marker for breast cancer, irrespective of its biological activities. The preoperative serum IL-18 level may represent a significant postoperative prognostic determinant in patients with gastric carcinoma, and patients with IL-18 levels over 310 pg/mL experienced a significantly lower survival rate after surgery than under 310 pg/mL[13]. IL-18 could significantly increase MMP-9 production at both mRNA and protein levels[14], thus enhancing the aggressiveness of malignant tumors, facilitating cancer metastasis through the induction of vascular cell adhesion molecule-1 (VCAM-1), and promoting cancer cell adhesion and liver metastasis in vivo[15]. Live metastasis frequently appeared in late stage gastric cancer, and most of the M1-stage patients in this study presented liver involvement, which accounted for the phenomenon of high expression of IL-18 in M1-stage gastric cancer.
There were several reports suggesting the synergistic antitumor activity of IL-12 and IL-18[4,16], and one of mechanisms was the inhibition of angiogenesis through the secretion of IFN-γ. But we did not find any accordance of intratumor IL-12 and IL-18 expression in gastric cancer, and microvessel density was not associated with either of these two cytokines. IL-12 and IL-18 might have different roles in the regulation of gene expression in NK and T cells, and IL-18 may act as a strong coinducer of Th1 or Th2 cytokines depending upon the cell type, and IL-18 could induce the production of IL-13 in NK and T cells, thus promoting Th2 polarization[17].
In summary, our findings have shown that IL-12 and IL-18 were expressed in certain subtypes of gastric cancer, but these two cytokines seemed not to be associated with antitumor immunity, but with progression and metastasis of gastric cancer, and IL-12 was even one of the independent indicators of unfavorable prognosis. Although the sample size was relatively small, some general patterns had clearly emerged, and warranted a further more detailed study to illuminate the effects of intratumoral cytokines.
Gastric carcinoma is one of the most frequent and lethal malignancies in China. The biological behavior and the mechanism of antitumor immunity of gastric carcinoma remain to be illuminated. The immune response to gastric carcinoma is predominantly affected by cell-mediated immunity, involving antigen-presenting cells, CD4+ and CD8+ T-lymphocytes, and the production of interleukins (ILs), and the research of cytokines is the hotspot in this area.
IL-12 and IL-18 are the main cytokines included in cell-mediated immunity. These two cytokines play important roles in promoting Th1-type response which favor cell-mediated immunity, and could inhibit angiogenesis, thus contributing to the antitumor and antimetastatic effects. Most of the biological activities of IL-12 overlap with those of IL-18, but the two interleukins seem to act in separate mechanisms.
This study has demonstrated the intratumoral expressions of IL-12 or IL-18 in gastric cancinoma, IL-12 was significantly associated with pathologic differentiation, depth of invasion, lymph node metastasis, distant metastasis, and TNM stage, and IL-18 was closely related to distant metastasis. These two cytokines seemed not to be associated with antitumor immunity, but with progression and metastasis of gastric cancer, and IL-12 was even one of the independent indicators of unfavorable prognosis. This work has provided us more knowledge about the distinct aspects of gastric cancer behavior.
The authors have investigated the expression and clinical significance of IL-12 and IL-18 in gastric carcinoma. Although the number of sample was small, this study is well designed and deserves to be read.
S- Editor Liu Y L- Editor Ma JY E- Editor Lu W
| 1. | Terabe M, Berzofsky JA. Immunoregulatory T cells in tumor immunity. Curr Opin Immunol. 2004;16:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 190] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 2. | Imagawa Y, Satake K, Kato Y, Tahara H, Tsukuda M. Antitumor and antiangiogenic effects of interleukin 12 gene therapy in murine head and neck carcinoma model. Auris Nasus Larynx. 2004;31:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Cao R, Farnebo J, Kurimoto M, Cao Y. Interleukin-18 acts as an angiogenesis and tumor suppressor. FASEB J. 1999;13:2195-2202. [PubMed] |
| 4. | Hikosaka S, Hara I, Miyake H, Hara S, Kamidono S. Antitumor effect of simultaneous transfer of interleukin-12 and interleukin-18 genes and its mechanism in a mouse bladder cancer model. Int J Urol. 2004;11:647-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Miller G, Lahrs S, Dematteo RP. Overexpression of interleukin-12 enables dendritic cells to activate NK cells and confer systemic antitumor immunity. FASEB J. 2003;17:728-730. [PubMed] |
| 6. | Egan MA, Chong SY, Megati S, Montefiori DC, Rose NF, Boyer JD, Sidhu MK, Quiroz J, Rosati M, Schadeck EB. Priming with plasmid DNAs expressing interleukin-12 and simian immunodeficiency virus gag enhances the immunogenicity and efficacy of an experimental AIDS vaccine based on recombinant vesicular stomatitis virus. AIDS Res Hum Retroviruses. 2005;21:629-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Wigginton JM, Komschlies KL, Back TC, Franco JL, Brunda MJ, Wiltrout RH. Administration of interleukin 12 with pulse interleukin 2 and the rapid and complete eradication of murine renal carcinoma. J Natl Cancer Inst. 1996;88:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 114] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Paleri V, Pulimood A, Davies GR, Birchall MA. Interleukins 7 and 12 are expressed in head and neck squamous cancer. Clin Otolaryngol Allied Sci. 2001;26:302-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817-824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 790] [Cited by in RCA: 797] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 10. | Grabbe S, Granstein RD. Modulation of antigen-presenting cell function as a potential regulatory mechanism in tumor-host immune reactions. In Vivo. 1993;7:265-269. [PubMed] |
| 11. | Fan H, Walters CS, Dunston GM, Tackey R. IL-12 plays a significant role in the apoptosis of human T cells in the absence of antigenic stimulation. Cytokine. 2002;19:126-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Merendino RA, Gangemi S, Ruello A, Bene A, Losi E, Lonbardo G, Purello-Dambrosio F. Serum levels of interleukin-18 and sICAM-1 in patients affected by breast cancer: preliminary considerations. Int J Biol Markers. 2001;16:126-129. [PubMed] |
| 13. | Kawabata T, Ichikura T, Majima T, Seki S, Chochi K, Takayama E, Hiraide H, Mochizuki H. Preoperative serum interleukin-18 level as a postoperative prognostic marker in patients with gastric carcinoma. Cancer. 2001;92:2050-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Zhang B, Wu KF, Cao ZY, Rao Q, Ma XT, Zheng GG, Li G. IL-18 increases invasiveness of HL-60 myeloid leukemia cells: up-regulation of matrix metalloproteinases-9 (MMP-9) expression. Leuk Res. 2004;28:91-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Vidal-Vanaclocha F, Fantuzzi G, Mendoza L, Fuentes AM, Anasagasti MJ, Martín J, Carrascal T, Walsh P, Reznikov LL, Kim SH. IL-18 regulates IL-1beta-dependent hepatic melanoma metastasis via vascular cell adhesion molecule-1. Proc Natl Acad Sci USA. 2000;97:734-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 252] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 16. | Coughlin CM, Salhany KE, Wysocka M, Aruga E, Kurzawa H, Chang AE, Hunter CA, Fox JC, Trinchieri G, Lee WM. Interleukin-12 and interleukin-18 synergistically induce murine tumor regression which involves inhibition of angiogenesis. J Clin Invest. 1998;101:1441-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 310] [Article Influence: 11.5] [Reference Citation Analysis (0)] |