Published online Mar 21, 2007. doi: 10.3748/wjg.v13.i11.1672
Revised: January 3, 2007
Accepted: February 3, 2007
Published online: March 21, 2007
AIM: To enhance the differentiation of insulin producing cell (IPC) ability from embryonic stem (ES) cells in vitro.
METHODS: Four-day embryoid body (EB)-formatted ES cells were dissociated as single cells for the followed plasmid DNA delivery. The use of Nucleofector™electroporator (Amaxa biosystems, Germany) in combination with medium-contained G418 provided a high efficiency of gene delivery for advanced selection. Neucleofected cells were plated on the top of fibronectin-coated Petri dishes. Addition of Ly294002 and raised the glucose in medium at 24 h before examination. The differentiation status of these cells was monitored by semi-quantitative PCR (SQ-PCR) detection of the expression of relative genes, such as oct-4, sox-17, foxa2, mixl1, pdx-1, insulin 1, glucagons and somatostatin. The percentage of IPC population on d 18 of the experiment was investigated by immunohistochemistry (IHC), and the content/secretion of insulin was estimated by ELISA assay. The mice with severe combined immunodeficiency disease (SCID) pretreated with streptozotocin (STZ) were used to eliminate plasma glucose restoration after pax4+ ES implantation.
RESULTS: A high efficiency of gene delivery was demonstrated when neucleofection was used in the present study; approximately 70% cells showed DsRed expression 2 d after neucleofection. By selection of medium-contained G418, the percentage of DsRed expressing cells kept high till the end of study. The pancreatic differentiation seemed to be accelerated by pax4 nucleofection. When compared to the group of cells with mock control, foxa2, mixl1, pdx1, higher insulin and somatostatin levels were detected by SQ-PCR 4 d after nucleofection in the group of pax4 expressing plasmid delivery. Approximately 55% of neucleofected cells showed insulin expression 18 d after neucleofection, and only 18% of cells showed insulin expression in mock control. The disturbance was shown by nucleofected pax4 RNAi vector; only 8% of cells expressed insulin 18 d after nucleofection. A higher IPC population was also detected in the insulin content by ELISA assay, and the glucose dependency was demonstrated in insulin secretion level. In the animal model, improvement of average plasma glucose concentration was observed in the group of pax-4 expressed ES of SCID mice pretreated with STZ, but no significant difference was observed in the group of STZ-pretreated SCID mice who were transplanted ES with mock plasmid.
CONCLUSION: Enhancement of IPC differentiation from EB-dissociated ES cells can be revealed by simply using pax4 expressing plasmid delivery. Not only more IPCs but also pancreatic differentiation-related genes can be detected by SQ-PCR. Expression of relative genes, such as foxa 2, mixl 1, pdx-1, insulin 1 and somatostatin after nucleofection, suggests that pax4 accelerates the whole differentiation progress. The higher insulin production with glucose dependent modulation suggests that pax4 expression can drive more mature IPCs. Although further determination of the entire mechanism is required, the potential of pax-4-nucleofected cells in medical treatment is promising.
- Citation: Lin HT, Kao CL, Lee KH, Chang YL, Chiou SH, Tsai FT, Tsai TH, Sheu DC, Ho LL, Ku HH. Enhancement of insulin-producing cell differentiation from embryonic stem cells using pax4-nucleofection method. World J Gastroenterol 2007; 13(11): 1672-1679
- URL: https://www.wjgnet.com/1007-9327/full/v13/i11/1672.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i11.1672
Diabetes mellitus (DM) is a world-wide disease and affects lives of millions of people every year. TypeIdiabetes (insulin dependent), due to developing insulin-resistance, is caused by the progressive deficiency of beta cells in islets of Langerhans. Type II diabetes (non-insulin dependent), on the other hand, results from the destruction of insulin receptors or transporting system. Islet-based transplantation and regimens, such as the “Edmonton protocol” , can restore insulin cells and are taken as a promising approach for the medical treatment of typeIdiabetes[1]. However, the shortage of donor tissues has largely restrained this application in the treatment of diabetic patients[2-5].
Embryonic stem (ES) cells have been considered as the potential substitute generating multi-lineage cells in clinical treatment of DM and characterized by multipotency and the ability of vigorous self-renewal proliferation. Recent studies showed that insulin-producing cells (IPC) generated from ES have become the alternative scheme to replace the cadaver-derived pancreatic islet as the source for transplantation. Strategies to induce the differentiation of IPCs from ES cells in vitro have involved supplementation of differentiation medium with a variety of induction and growth factors, such as nourished ES cells with all-trans retinoic acid[6-9], activin A[8,10-12] in IPC induction. However, the low ratio of insulin producing cells in previous study still limits their medical application[13-16].
Manipulating the relative gene expression to enforce ES cell differentiation, alternatively, has been used to increase the specific lineage cell differentiation efficiency[17,18]. For maintaining the genomic integrity and reducing the possibility of carcinogenesis[19], the non-viral transient gene delivery method is more acceptable. However, the low transfection efficiency of electroporation and lipofection (liposome-mediated) in ES cells often limits its application. The Nucleofector™ technology, a new non-viral electroporation-based gene transfer technique, can improve the disadvantageous results caused by traditional transfection, especially for the hard-to-transfect ES and primary cells.
Considering the influence on pancreatic organogenesis, multiple genes have been chosen as the target to manipulate the expression in an attempt to improve IPC differentiation efficiency. The effectiveness of IPC formation has been revealed in pax4, the important gene for beta islet formation and expression. ES cell clone with external pax4 constitutive expression has been constructed by Blyszczuk et al[20], and the efficiency on ES cell commitment to become IPCs has been demonstrated. No significant variant has been observed in ES cell proliferation and the formation of embryoid body (EB)[21] in pax4 expressed ES cell clones indicates that pax4 can be an optimal candidate to be manipulated.
By combining the nucleofector-based transfection (neuleofection) and fibronectin-coated, medium-based IPC induction in vitro, we attempted to demonstrate that using a simple method of transient pax-4 gene delivery/expression could force ES cells to become IPCs effectively. Through neomycin selection, a higher percentage of pax4-expressed ES cells was observed in the experiment accompanied with a higher IPC differentiation.
The murine embryonic stem cell strain of BALB/c mice (ESC26GJ constructed by Lee et al[22], Animal Technology Institute Taiwan) during passages 25 and 50 was used in the present study. This strain that has been transferred by pCX-EGFP can express green fluorescence constitutively.
Undifferentiated murine stem cells were cultured on mitomycin C-treated STO (mouse embryonic fibroblast cell line, ATCC CRL-1503 passages 15-35 were utilized) and supplied to Dulbecco's modified Eagle's medium (DMEM, 4500 mg glucose/L) containing 15% fetal bovine serum (FBS, HyClone defined and tested batches or ES cell grade), 1% nonessential amino acid, 0.1 mmol/L β-mercaptoethanol, 100 U/mL penicillin, 100 μg/mL streptomycin (all from GIBCO-BRL) and leukemia inhibitory factor (LIF, R&D). Cells were cultured in a humidified chamber containing a 5% CO2/air mixture at 37°C, subcultured every 3 d and medium was changed twice a day. Alkaline phosphatase detection kit (Chemicon) was utilized to examine the quality of undifferentiated ES every 5 passages.
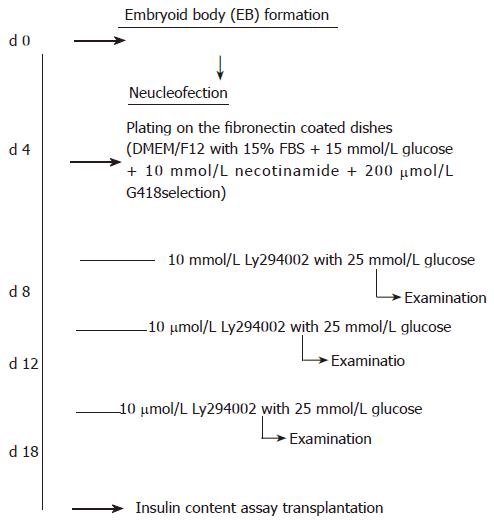
The protocol modified from the study by Blyszczuk et al[23,24] was used in the present study. Briefly, approximately 70% saturated undifferentiated ES cells were harvested and aggregated as hanging drops with the density of 3000 cells/per drop for 4 d (using the medium for undifferentiated ES cells without LIF supplement). EB[25] was harvested by brief centrifugation (800 ×g, 10 min, 28°C), then dissociated to single cells for nucleofection (described in the section below). Nucleofected cells were seeded on a 6 cm-cultivated dish with fibronectin coating, one day after nucleofection, the medium was changed and new medium was added with G418 for selection. The flow chart of induction protocol is shown in Figure 1.
The full-length mouse pax4 gene, a kind gift from Dr. Michael S. German (University of California, San Francisco, USA)[26], was constructed into pIRES2-DsRed plasmid (Clontech, USA) for the following studies. Plasmid DNA was transfected to the RR1 strain of E. coli for scale-up, and plasmid was extracted from E. coli broth by NucleoBondTM PC 2000 EF plasmid DNA purification kit (endotoxin-free, Macherey-Nagel Co., Düren, Germany). The related procedure was referred to in the commodities manuscript. The NucleofectorTM electroporator (Amaxa biosystems, Germany) and the buffer system for mouse ES cells (mouse ES cell NucleofectorTM kit, Amaxa Biosystems, Germany) were utilized for gene delivery[27-29]. Approximately 3 × 105 differentiating cells and 20 μg plasmid DNA were used for a test of electroporation. All A-13, A-23, A-24 and A-30 programs suggested in the manuscript were tested, and no significant difference was shown in both cell mortality rate and delivery efficiency when deliver mock pIRES2-DsRed plasmid DNA was delivered. The program A-30 was utilized for the following examination. After nucleofection, the cells were plated on a fibronectin-coated dish. Neomycin (G418, 200 μg/mL, Sigma, USA) was used to select transfected cells after 24 h of nucleofection.
Primers used in the present study are listed in Table 1. SYBR-green was used for semi-quantitative PCR (SQ-PCR) detection. Briefly, total RNA (approximately 1 μg) of each sample was reversely transcribed in 20 μL using 0.5 μg of oligo-dT and 200 U Superscript II RT (Invitrogen, Carlsbad, CA, USA). Amplification was carried out in a total volume of 20 μL containing 0.5 μmol/L of each primer, 4 mmol/L MgCl2, 2 μL LightCyclerTM-FastStart DNA Master SYBR greenI(Rouche Diagnostics GmbH, Mannheim, Germany) and 2 μL of 1:10 diluted cDNA. PCR was performed in triplicate. The transcript levels of genes were standardized to the corresponding GADPH level, and for each candidate gene, mRNA levels relative to the highest candidate gene level were estimated in percentage[30].
| Marker | Size | Sense | Antisense | Tm |
| Pdx-1 | 451 | ACCATGAACAGTGAGGAGCA | TCCTCTTGTTTTCCTCGGGT | 55 |
| Foxa2 | 199 | GACAAGGGAAATGAGAGGCTG | ACCAAAGGCTCCTTTAAAACAAG | 55 |
| Insulin I | 197 | CCAGCTATAATCAGAGACCA | GTGTAGAAGAAGCCACGCT | 55 |
| Sox 17 | 247 | TCGGACTATGCAGTGTCCGTA | GAAGGTTGCCCGGGGCCGTG | 55 |
| Somatostatin | 315 | ACTAGTCTGCGCTCTGCATCGTCCTG | CCGCGGTCTTCCAGAAGAAGTTCTTG | 55 |
| Glucagon | 253 | ACTAGTGCAGCACGCCCTTCAAGACA | CCGCGGAGCATGTCTCTCAAATTCAT | 60 |
| Mixl1 | 305 | GCACGTCGTTCAGCTCGGAGCAGC | AGTCATGCTGGGATCCGGAACGTGG | 60 |
| Oct-4 | 201 | TGTGGACCTCAGGTTGGTCT | CTTCTGCAGGGCTTTCATGT | 55 |
| GAPDH | 179 | CATCATCCCTGCCTCTACTG | GCCTGCTTCACCACCTTC | 55 |
siRNA was constructed with pSUPER RNAi system (OligoEngine). Hairpin siRNA oligo of pax4 was designed with the following sense and antisense sequences as 5'-AATTGCCCAGCTAAAGGATGA-3' and 5'-TCATCCTTTAGCTGGGCAAT T-3'. The sense and antisense strands of oligos were annealed, and ligated into linearized pSuper vector within the BglII and HindIIIsites as suggested in the manufacturer's instructions. The procedure for the following cloning, scale-up and nucleofection was similar to the description above.
Differentiated ES cells were harvested and re-plated on the cover slide coated with fibronectin for two days before the following immunofluorescence staining, and pretreated with 4% of paraformaldehyde for 5 min for fixation. The original fluorescent signal of eGFP constitutively expressed in cells could not be detected after fixation. Immunoreactive signals of insulin were detected by mouse anti-insulin monoclonal Ab (Sigma), and stained with goat anti-mouse IgG (invitrogen, Cy3-conjugation) subsequently. The GFP in ES cells was detected by rabbit anti-GFP polyclonal Ab (Promega), and followed by goat-anti rabbit IgG antibody (Abcam).
Differentiated ES cells on d 4, 8, 12 and 18 were examined with insulin content in the cells and those secreted into medium as previously described[13]. Briefly, the cells were washed three times with PBS before examination, and the medium was changed with 25 mmol/L glucose for 24 h. The overnight incubated medium was collected, treated by a brief centrifugation (5000 × g, 10 min at 4°C) to remove the insoluble particles, and kept at -80°C till insulin detection. The attached cells were also collected, treated with cold acid-ethanol (0.1 mol/L hydrochloric acid in absolute ethanol) and kept at 4°C overnight for further insulin examination by ELISA (Mercodia, Sweden). The clear supernatants were used to investigate the intracellular insulin content and the values obtained were normalized relative to the total protein content (protein assay reagent, Bio-Rad, USA). The differentiated ES cells on d 18 were used to examine the glucose (5 mmol/L, 15 mmol/L and 30 mmol/L) dependent insulin secretion. The dependency of tolbutamide was used to examine the maturation of IPCs derived from ES cells.
The animal experiment followed “Principles of Laboratory Animal Care” of Taipei Veterans General Hospital and National Yang-Ming University. The 8 to 10 wk old SCID mice were treated with streptozotocin (STZ, 200 mg/kg, Sigma) freshly dissolved in 0.025 mol/L tri-sodium citrate 2 hydrate (pH 4.0). A total of 2 × 105 differentiated ES cells were injected into the subcapsular space of the left kidney of SCID mice following the protocol[10,21]. Blood sample from the retro-orbital plexus was collected every two days and measured by using OneTouch® SureStep plus blood glucose monitoring system (LifeScan Inc. Johnson & Johnson Company).
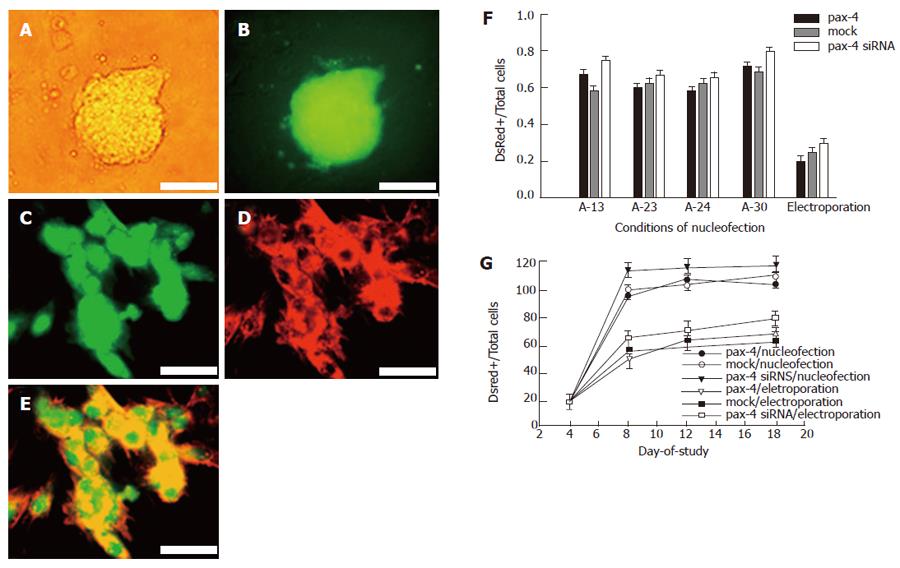
In order to drive ES cells to enter endoderm/mesoderm commitment, the hanging drop method was used for EB formation. The duration of hanging drop was determined by RT-PCR analysis of endoderm-associated gene Gata6, 4 d after hanging-drop with LIF deprivation as previously described[22]. The ES clones of ESC26GJ could express eGFP constitutively (Figure 2A and B). The germline transmission property indicated that the potent integrity was preserved.
The high efficiency of DsRed-N1 expressing vector transfection was shown in EB-dissociated cells. Approximately 70%-80% of cells with red fluorescent were detected 48 h after nucleofection, and the intensity increased 72 h after nucleofection (Figure 2C-E). Four kinds of programs (All A-13, A-23, A-24 and A-30), suggested by the manuscript, were specific for ES cell transfection. However, no significant difference in plasmid delivery efficiency was observed in our experiments (Figure 2F). Compared to another traditional electroporation method (750 V/cm, 100 μs, single pulse), nucleofectorTM had a higher plasmid delivery efficiency (Figure 2F). The fluorescent signal could be detected till the end of the experiment (18 d) under G418 selection, although the intensity was decreased. No significant difference was shown in the gene delivery efficiency when using the DsRed-N1 plasmid incorporated with pax4 or mock control.
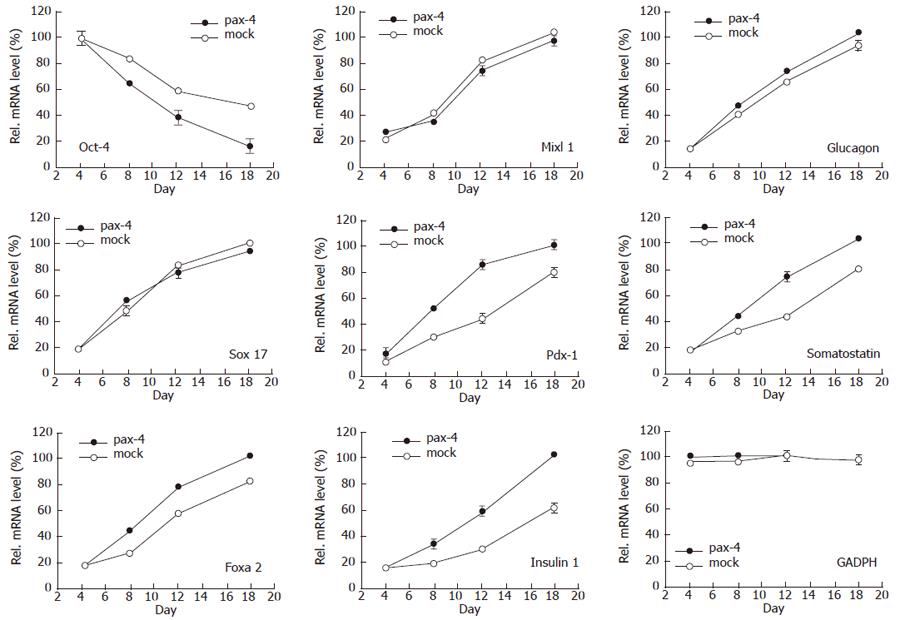
Pax-4 expression and possible immediate effects of differentiation were detected. Not only pax-4, but also oct-4, foxa2 genes were examined by RT-PCR. Differences in oct-4, un-differentiation marker, foxa2, endodermal marker, were revealed. Compared with the undifferentiated ES cells and the ES cells nucleofected with mock plasmid, the lower oct-4 expression was demonstrated when EB derived ES cells nucleofected with pax4 plasmid, and the higher foxa2 gene expression was shown 36 h after nucleofection (data not shown). The gene expression was evaluated by semiquantitative PCR (SQ-PCR). Not only oct-4, but sox-17, foxa2 and mixl1, endodermal markers, pdx-1, insulin 1, glucagons and somatostatin, pancreatic relative genes and GADPH were also examined (Figure 3). Significant influence of pax4 gene expression was shown on oct-4, foxa2, pdx-1, insulin 1 and somatostatin, indicating that pax4 nucleofection could enhance EB-dissociated cells to initiate pancreatic differentiation.
The IPC formation efficiency was evaluated, and the number of insulin producing cells, insulin secretion and insulin content in cell population were all considered.
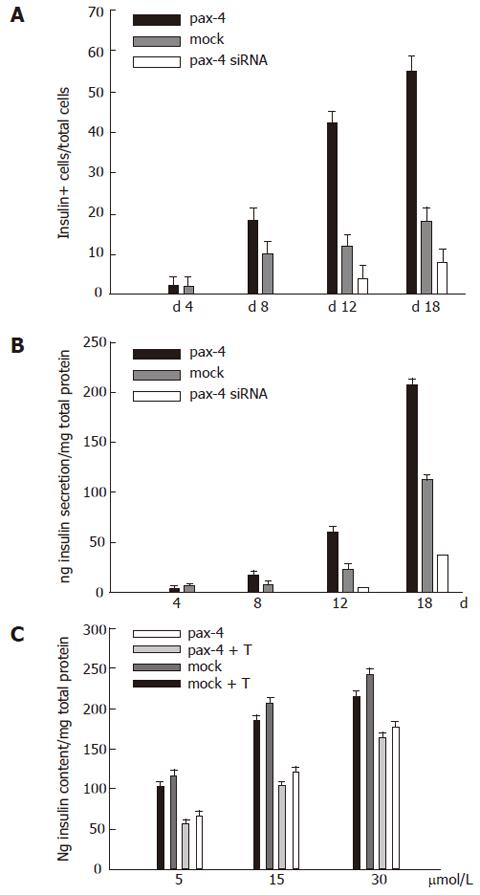
The percentage of insulin-expressed cells in pool was examined in four groups of cells by immunofluoresent staining on d 4, 8, 12 and 18 in the experiment, and the ratio was quantified (Figure 4A). Insulin-expressed cells at onset were observed 4 d after neucleofection. However, the percentage of IPCs increased rapidly on d 12 of the experiment in the group of pax-4 expressed ES cells. On the 18th d of the experiment, approximately 55% of cells with insulin were detected. However, approximately 18% and 8% of cells were revealed in the groups of cells transfected with mock plasmid and pax4 siRNA plasmids, respectively.
Insulin secretion, especially under glucose induction, was a criterion for the maturation of IPC differentiation. Glucose inducible insulin secretion was examined on d 4, 8, 12 and 18 of the experiment. The result is shown in Figure 4B. The significant increase of insulin in the medium was shown in the groups of cells with pax4 on d 12 and 18. The effect of IPC differentiation guidance by medium was demonstrated by the result of the groups of cells with mock plasmid delivery, and no significant insulin raise was detectable in the groups with mock and pax4 siRNA gene expression at the same time. The insulin content in cells of variant groups was demonstrated on 18th d after the examination, and its relationship with glucose concentration in medium was discussed. In the group of ES cells with external pax4 expression, a higher insulin content was observed. The insulin content enhanced when the glucose concentration in the medium was increased. Moreover, the insulin content was susceptible to Tolbutamide (10 μmol/L, Figure 4C).
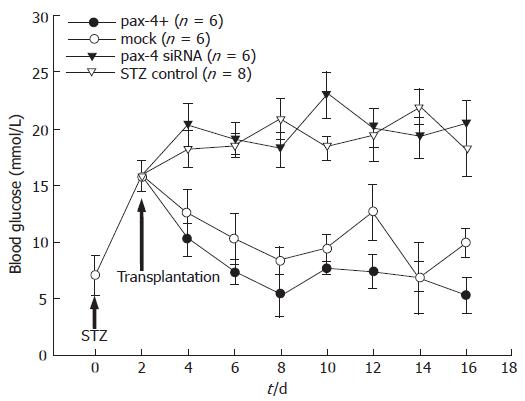
To examine the normoglycemia restoration of IPCs derived from ES cells with pax4 expression in transplantation, SCID mice pretreated with STZ were employed. The space of renal subcapsula in mice could provide the microenvironment suitable for the differentiation of endocrine cells[25]. A total of 1 × 106 pax4+ EB derived ES cells, mock plasmid-nucleofected control and pax4 siRNA plasmid (on d 18 of the experiment) were implanted into the subcapsular space of the left kidney (n = 6, each). Blood glucose was examined every two days before and after the transplantation (Figure 5). Although the blood glucose reduced in both groups of transplanted animals compared to the untreated control group, lower blood glucose was observed in the group of animals implanted pax4+ ES cells. Results showed that IPCs derived from ES cells with pax4+ plasmid nucleofection could restore the blood glucose nearly to its normal level in the STZ- pretreated SCID mice.
A strategy guiding stem cells to commit/differentiate the specific type of cells/tissue effectively is the essential criterion for its further therapeutic application. Meanwhile, the manipulating convenience and autograft transplant possibility should also be considered. Various attempts have been discussed, such as addition of all-trans retinoic acid[6-9] or activin A[8,10-12] in defined medium. However, the efficiency for IPC formation guidance still needs to be proved. Lumelsky et al[31] have developed a five-step protocol by supplement of ITSF in serum-free medium. However, the evidence indicates that insulin detected in the differentiated ES cells might be external and direct toward the medium they used[23,24,32].
During embryogenesis, several growth and transcription factors are involved in β-cell differentiation[21]. Pax4 is a crucial regulator of mammalian pancreas development since the lack of activity prevents the formation of mature pancreatic insulin-producing cells[25,33]. Pax4 has been shown to bind to a cis-acting element of pancreatic islet cell enhancer sequence[34,35], which is present in the promoters of glucagons, insulin and somatostatin, and this interaction is necessary to activate the gene expression. The elegant studies of Pax4-deficient embryonic pancreas, by Dohrman et al[36] and Wang et al[37,38] revealed that the lack of Pax4 activity forces endoderm progenitors to adopt an alternative α-cell fate, and down-regulates the expression of early markers of β-cell differentiation and islet formation, such as Pdx1, Hlxb9, and MafA. Recently, a large-scale screening of molecular epidemiology found that patients carrying missense mutations of Pax4 (R121W, R133W or R37W) show severe defects in the first-phase of insulin secretion in β cells[39], suggesting that the gene mutations of Pax4 are associated with typeIdiabetes.
Raising pax4 expression to enhance IPC differentiation in ES cells has been studied in vitro[21]. ES cells with external pax4 constitutive expression clone have been established, and the manipulation seems intact in ES cells with no significant variant observed in ES cell proliferation and formation of EB[21]. The higher percentage of IPC was observed in the group of pax4 expressed ES cells, indicating that pax4 could be an optimal candidate with safer and more effective in manipulation.
Although external pax4 expression can raise IPC formation, stable clone construction with external pax4 expression is not acceptable in clinical practice if autograft is considered. The attempt to simplify the method was illustrated in the present study, to evaluate the enhancement of IPC formation by using transient pax-4 gene delivery. In order to increase the gene delivery efficiency in ES cells, the nucleofector system was employed, and the refinement was shown. Approximately 70% of nucleofected cells showed the DsRed fluorescence 2 d after nucleofection, higher than the comparative study with traditional electroporation method. The enhancement of IPCs was revealed in ES cell population and insulin secretion and content level were susceptible to the glucose concentration in vitro. From the related gene expression monitored by SQ-PCR, the differentiation was shown. The onset raise of foxa2, pdx-1, insulin and somatostatin gene was shown 8 d after neucleofection, suggesting that pax-4 accelerates the differentiation. Significant differences were observed in genes such as foxa2. The foxa2 gene is specific for endoderm differentiation[40,41]. For pancreatic differentiation, foxa2 has been proved to be an up-stream gene that regulates pdx1 expression[42-44]. Foxa2 is essential for hepatic differentiation[41-45]. The mechanism why raising pax-4 expression level in ES cell population causes IPC differentiation more effectively is not well-understood. Some phenomena are to be solved, for example not all pax-4 neucleofected ES cells (DsRed- expressing cells) become IPCs (insulin secretion). More studies are required to understand the mechanism.
The enhancement of IPC formation derived from endoderm/mesoderm commitment ES cells via nucleofection was demonstrated both in vitro and in vivo. By the transient force of pax4 expression and G418 selection, when the tendency of ES cell differentiation was guided, IPC formation became more effective.
In conclusion, enhancement of IPC differentiation from EB-dissociated ES cells can be revealed by simply using pax4 expressing plasmid delivery. Not only more IPCs can be observed, but also pancreatic differentiation-related genes can be detected by SQ-PCR. Expression of relative genes, such as foxa 2, mixl 1, pdx-1, insulin 1 and somatostatin after nucleofection, suggests that pax4 accelerates the whole differentiation progress. The higher insulin production with glucose dependent modulation suggests that pax4 expression can drive more mature IPCs. Although the entire mechanism is still to be determined, the potential of pax-4-nucleofected cells in medical treatment is promising.
S- Editor Liu Y L- Editor Wang XL E- Editor Chin GJ
| 1. | Korsgren O, Nilsson B, Berne C, Felldin M, Foss A, Kallen R, Lundgren T, Salmela K, Tibell A, Tufveson G. Current status of clinical islet transplantation. Transplantation. 2005;79:1289-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Ben-Yehudah A, Witchel SF, Hyun SH, Chaillet JR, Schatten G. Can diabetes be cured by therapeutic cloning? Pediatr Diabetes. 2004;5 Suppl 2:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Lechner A. Stem cells and regenerative medicine for the treatment of type 1 diabetes: the challenges lying ahead. Pediatr Diabetes. 2004;5 Suppl 2:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Ramiya V, Schatz D. Islet replacement vs. regeneration: hope or hype? Pediatr Diabetes. 2004;5 Suppl 2:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Weir GC. Can we make surrogate beta-cells better than the original? Semin Cell Dev Biol. 2004;15:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Kramer B, Penny C. The quest for factors regulating the development of chick embryonic insulin cells in vitro. Ital J Anat Embryol. 2001;106:451-458. [PubMed] |
| 7. | Micallef SJ, Janes ME, Knezevic K, Davis RP, Elefanty AG, Stanley EG. Retinoic acid induces Pdx1-positive endoderm in differentiating mouse embryonic stem cells. Diabetes. 2005;54:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Shi Y, Hou L, Tang F, Jiang W, Wang P, Ding M, Deng H. Inducing embryonic stem cells to differentiate into pancreatic beta cells by a novel three-step approach with activin A and all-trans retinoic acid. Stem Cells. 2005;23:656-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 149] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Vetere A, Marsich E, Di Piazza M, Koncan R, Micali F, Paoletti S. Neurogenin3 triggers beta-cell differentiation of retinoic acid-derived endoderm cells. Biochem J. 2003;371:831-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Humphrey RK, Bucay N, Beattie GM, Lopez A, Messam CA, Cirulli V, Hayek A. Characterization and isolation of promoter-defined nestin-positive cells from the human fetal pancreas. Diabetes. 2003;52:2519-2525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Ku HT, Zhang N, Kubo A, O'Connor R, Mao M, Keller G, Bromberg JS. Committing embryonic stem cells to early endocrine pancreas in vitro. Stem Cells. 2004;22:1205-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Skoudy A, Rovira M, Savatier P, Martin F, León-Quinto T, Soria B, Real FX. Transforming growth factor (TGF)beta, fibroblast growth factor (FGF) and retinoid signalling pathways promote pancreatic exocrine gene expression in mouse embryonic stem cells. Biochem J. 2004;379:749-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Lin HT, Chiou SH, Kao CL, Shyr YM, Hsu CJ, Tarng YW, Ho LL, Kwok CF, Ku HH. Characterization of pancreatic stem cells derived from adult human pancreas ducts by fluorescence activated cell sorting. World J Gastroenterol. 2006;12:4529-4535. [PubMed] |
| 14. | Zhang L, Hong TP, Hu J, Liu YN, Wu YH, Li LS. Nestin-positive progenitor cells isolated from human fetal pancreas have phenotypic markers identical to mesenchymal stem cells. World J Gastroenterol. 2005;11:2906-2911. [PubMed] |
| 15. | Yao ZX, Qin ML, Liu JJ, Chen XS, Zhou DS. In vitro cultivation of human fetal pancreatic ductal stem cells and their differentiation into insulin-producing cells. World J Gastroenterol. 2004;10:1452-1456. [PubMed] |
| 16. | Cui HF, Bai ZL. Protective effects of transplanted and mobilized bone marrow stem cells on mice with severe acute pancreatitis. World J Gastroenterol. 2003;9:2274-2277. [PubMed] |
| 17. | Kim JH, Do HJ, Choi SJ, Cho HJ, Park KH, Yang HM, Lee SH, Kim DK, Kwack K, Oh SK. Efficient gene delivery in differentiated human embryonic stem cells. Exp Mol Med. 2005;37:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Kobayashi N, Rivas-Carrillo JD, Soto-Gutierrez A, Fukazawa T, Chen Y, Navarro-Alvarez N, Tanaka N. Gene delivery to embryonic stem cells. Birth Defects Res C Embryo Today. 2005;75:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Gardlík R, Pálffy R, Hodosy J, Lukács J, Turna J, Celec P. Vectors and delivery systems in gene therapy. Med Sci Monit. 2005;11:RA110-RA121. [PubMed] |
| 20. | Blyszczuk P, Czyz J, Kania G, Wagner M, Roll U, St-Onge L, Wobus AM. Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells. Proc Natl Acad Sci USA. 2003;100:998-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 299] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 21. | Soria B. In-vitro differentiation of pancreatic beta-cells. Differentiation. 2001;68:205-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 131] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Lee KH, Chuang CK, Wang HW, Stone L, Chen CH, Tu CF. An alternative simple method for mass production of chimeric embryos by coculturing denuded embryos and embryonic stem cells in Eppendorf vials. Theriogenology. 2007;67:228-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Blyszczuk P, Asbrand C, Rozzo A, Kania G, St-Onge L, Rupnik M, Wobus AM. Embryonic stem cells differentiate into insulin-producing cells without selection of nestin-expressing cells. Int J Dev Biol. 2004;48:1095-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Blyszczuk P, Wobus AM. Stem cells and pancreatic differentiation in vitro. J Biotechnol. 2004;113:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Gittes GK, Galante PE, Hanahan D, Rutter WJ, Debase HT. Lineage-specific morphogenesis in the developing pancreas: role of mesenchymal factors. Development. 1996;122:439-447. [PubMed] |
| 26. | Smith SB, Ee HC, Conners JR, German MS. Paired-homeodomain transcription factor PAX4 acts as a transcriptional repressor in early pancreatic development. Mol Cell Biol. 1999;19:8272-8280. [PubMed] |
| 27. | Aluigi M, Fogli M, Curti A, Isidori A, Gruppioni E, Chiodoni C, Colombo MP, Versura P, D'Errico-Grigioni A, Ferri E. Nucleofection is an efficient nonviral transfection technique for human bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:454-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 28. | Iversen N, Birkenes B, Torsdalen K, Djurovic S. Electroporation by nucleofector is the best nonviral transfection technique in human endothelial and smooth muscle cells. Genet Vaccines Ther. 2005;3:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Siemen H, Nix M, Endl E, Koch P, Itskovitz-Eldor J, Brüstle O. Nucleofection of human embryonic stem cells. Stem Cells Dev. 2005;14:378-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Wobus AM, Guan K, Yang HT, Boheler KR. In: Turksen K. Embryonic Stem Cells: Methods and Protocols, Methods in Molecular Biology. Totowa: New Jersey: Humana press 2002; 217-227. |
| 31. | Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1063] [Cited by in RCA: 970] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 32. | Kania G, Blyszczuk P, Wobus AM. The generation of insulin-producing cells from embryonic stem cells--a discussion of controversial findings. Int J Dev Biol. 2004;48:1061-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Habener JF, Stoffers DA. A newly discovered role of transcription factors involved in pancreas development and the pathogenesis of diabetes mellitus. Proc Assoc Am Physicians. 1998;110:12-21. [PubMed] |
| 34. | Brink C, Chowdhury K, Gruss P. Pax4 regulatory elements mediate beta cell specific expression in the pancreas. Mech Dev. 2001;100:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Xu W, Murphy LJ. Cloning of the mouse Pax4 gene promoter and identification of a pancreatic beta cell specific enhancer. Mol Cell Endocrinol. 2000;170:79-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Dohrmann C, Gruss P, Lemaire L. Pax genes and the differentiation of hormone-producing endocrine cells in the pancreas. Mech Dev. 2000;92:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Wang J, Elghazi L, Parker SE, Kizilocak H, Asano M, Sussel L, Sosa-Pineda B. The concerted activities of Pax4 and Nkx2.2 are essential to initiate pancreatic beta-cell differentiation. Dev Biol. 2004;266:178-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 38. | Smith SB, Gasa R, Watada H, Wang J, Griffen SC, German MS. Neurogenin3 and hepatic nuclear factor 1 cooperate in activating pancreatic expression of Pax4. J Biol Chem. 2003;278:38254-38259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Shimajiri Y, Sanke T, Furuta H, Hanabusa T, Nakagawa T, Fujitani Y, Kajimoto Y, Takasu N, Nanjo K. A missense mutation of Pax4 gene (R121W) is associated with type 2 diabetes in Japanese. Diabetes. 2001;50:2864-2869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Besnard V, Wert SE, Hull WM, Whitsett JA. Immunohistochemical localization of Foxa1 and Foxa2 in mouse embryos and adult tissues. Gene Expr Patterns. 2004;5:193-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 41. | Khoo ML, McQuade LR, Smith MS, Lees JG, Sidhu KS, Tuch BE. Growth and differentiation of embryoid bodies derived from human embryonic stem cells: effect of glucose and basic fibroblast growth factor. Biol Reprod. 2005;73:1147-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Gerrish K, Van Velkinburgh JC, Stein R. Conserved transcriptional regulatory domains of the pdx-1 gene. Mol Endocrinol. 2004;18:533-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 43. | Lantz KA, Vatamaniuk MZ, Brestelli JE, Friedman JR, Matschinsky FM, Kaestner KH. Foxa2 regulates multiple pathways of insulin secretion. J Clin Invest. 2004;114:512-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 44. | Lee CS, Sund NJ, Behr R, Herrera PL, Kaestner KH. Foxa2 is required for the differentiation of pancreatic alpha-cells. Dev Biol. 2005;278:484-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 157] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 45. | Ishii T, Yasuchika K, Fujii H, Hoppo T, Baba S, Naito M, Machimoto T, Kamo N, Suemori H, Nakatsuji N. In vitro differentiation and maturation of mouse embryonic stem cells into hepatocytes. Exp Cell Res. 2005;309:68-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |