Published online Jan 7, 2007. doi: 10.3748/wjg.v13.i1.141
Revised: May 15, 2006
Accepted: July 10, 2006
Published online: January 7, 2007
AIM: To demonstrate the change and effect of nociceptin/orphanin FQ in the colon of rats with cathartic colon.
METHODS: The cathartic colon model was established by feeding rats rhubarb for 3 mo, the changes of colonic electromyography were investigated by both suspension muscle strips test and serosal recordings of colonic myoelectrical activity. Immunohistochemical staining (S-P methods) and image analysis were used to determine the changes of nociceptin/orphanin FQ in the proximal colon and distal colon of rats with cathartic colon.
RESULTS: Suspension muscle strips test in vitro showed OFQ (10-9-10-6 mol/L) concentration dependently caused an immediate tonic contraction in the isolated colon. But the increase of tension in cathartic colon was less than control groups (P < 0.01). Intravenous administration of OFQ (1 μg/kg) caused phasic contractions in the proximal colon, while the amplitude of phasic contractions caused by OFQ in cathartic colon was much lower than that in the control groups (2.58 ± 0.41 vs 4.16 ± 0.53, t = -2.6, P = 0.012). OFQ was highly expressed in the myenteric plexus of the rat colon but not in the muscle cells. The immunoreactivity of OFQ in the proximal colon in cathartic colon rats decreased significantly compared with the control group (P = 0.001).
CONCLUSION: Colonic smooth muscle of cathartic colon showed low sensitivity to the stimulation of OFQ, suggesting that it might be caused by the abnormal distribution of OFQ or the abnormalities of receptors, leading to the disorganization of dynamic and incoordinated contractions.
- Citation: Li HY, Yan X, Xue QL, Zhou YN, Gao Y, Wang R, Liu YM, Ran JT. Effects of nociceptin/orphanin FQ on rats with cathartic colon. World J Gastroenterol 2007; 13(1): 141-145
- URL: https://www.wjgnet.com/1007-9327/full/v13/i1/141.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i1.141
Slow transit constipation (STC) is a common syndrome in idiopathic constipation. It is a severe disorder of colonic motility. Because of lack of understanding of the etiology, current medical treatments for STC are often ineffective[1]. It has been shown that predominant symptoms in STC correlate with the enteric nervous system (ENS) abnormalities[2]. Animal experiments have shown that stimulant laxatives can damage the ENS and cause changes of some enteric neurotransmitters and thus slow gastrointestinal tract transit occurs[3].
The peptide nociceptin, also called orphanin-FQ (N/OFQ), is a 17-amino-acid peptide and was identified in 1995[4]. Because it binds an opioid receptor like 1 (ORL1)receptor with a high affinity, it has been reported to be an endogeneous agonist for ORL1 receptor. In situ hybridization studies have revealed the wide distribution of the ORL1 receptor mRNA in the central nervous system of rats[5], especially in the coli involved in pain control, so most of the researches focused on the role of N/OFQ in analgesics. However, by the activation of the ORL1 receptor, N/OFQ can also influence reward, anxiety, feeding and memory processes[6,7], cardiovascular and renal functions[8,9], gastric and intestinal motility[10,11] and secretions[12,13].
The gastrointestinal tract is an important model system for the research of pharmocological characterization of opioid receptors. Therefore, if N/OFQ can also act as a brain-bowel peptide like classical opioid, it may play a role in the regulation of gastrointestinal tract functions. In this study, with a rat model of cathartic colon, we determined the effects of OFQ on mechanical activity of the rat colon and conducted immunohistochemical studies on rat colon to assess the changes of OFQ.
Fifty Wistar rats of either sex, obtained from the Experimental Animal Center, Lanzhou University (Lanzhou, China), weighing 290 ± 50 g, were divided randomly into control group (n = 25) and cathartic colon group treated with rhubarb (n = 25). Rats were housed in cage, one per cage under standard laboratory conditions (room temperature, 18-28°C, relative humility, 40%-80%). Control rats were given soft chows, while the rats in rhubarb group were given chows premixed with rhubarb powder. The initial rhubarb dosage was 200 mg/kg.d, and another 200 mg/kg was added every day until it reached 1000 mg/kg.d for several days until loose stool disappeared. Then, rhubarb was added at 200 mg/kg.d again to 3600 mg/kg.d for 3 mo.
Tetrodotoxin (TTX) and naloxone were obtained from Sigma Co. USA. OFQ (1-17) was obtained from Physical Laboratories, School of Life Science, Lanzhou University (Lanzhou, China). Rabbit antinociceptin antiserum (Chemicon Pharmaceuticals Inc, 1:500. Goat anti-rabbit biotinylated secondary antibody (Rocland Laboratories, USA, 1:15 000).
Wistar rats (body weight, 290 ± 50 g) were starved overnight and killed by head-strike, longitudinal muscle strips were isolated from proximal colon, muscle strips (10 mm in length and 3 mm in width) were suspended between 2 platinum electrodes in a 30 mL organ bath filled with Krebs-Henseleit buffer containing 118 mmol/L NaCl, 4.8 mmol/L KCl, 2.5 mmol/L CaCl2, 25 mmol/L NaHCO3, 1.2 mmol/L KH2PO4, 1.2 mmol/L MgSO4, 11 mmol/L glucose and 0.1% bovine serum albumin. Krebs-Henseleit buffer solution was continuously gassed with 95%O2-5%CO2 and maintained at 37°C and pH 7.4. Mechanical activity was recorded on a polygraph through isometric transducers. Muscle strips were stretched in 1-mm increments and repeatedly exposed to 10-6 mmol/L carbachol to determine L0, the length at which the maximal active tension response developed. The resting of tension was kept constant during the equilibration period, dose-response curves were constructed after applying OFQ (10-9-10-6 mol/L) to longitudinal muscle stripes from proximal colon. Doses of OFQ were administered at 20-min intervals. To investigate the neural pathways responsible for the contractile action of OFQ, we examined the effects of TTX and naloxone on OFQ-induced contractions. Muscle strips were preincubated with each antagonist for 10 min followed by incubation with OFQ (n = 8).
Colonic motility studies in anesthetized rats were carried out according to the procedures described previously (Nagasaki et al, 1989.) In brief, rats were fasted for 20 h and anesthetized with urethane (1.2 g/kg s.c.). After midline laparotomy, a strain gauge force transducer (F-081S, Star Medical, Japan) was sutured on the serosal surface of the proximal colon to record the circular muscle contraction. For intravenous administration of drugs, the right femoral vein was cannulated. After the contractile response to bethanechol (30 mg/kg i.v) became stable, OFQ (1 μg/kg) was administered intravenously (n = 7).
The animals were deeply anaesthetized with 5% isoflurane and perfused transcardially with aerated calcium-free Tyrode’s solution, followed by a fixative containing 4% paraformaldehyde and 0.2% picric acid in 0.1 mol/L phosphate buffer (pH 7.2-7.4). The proximal and distal colons were removed immediately after perfusion, post-fixed in the same fixative for 4h and then cryoprotected in 10% sucrose in phosphate-buffered saline (PBS, pH 7.4) overnight.
A series of sections were cut and mounted on gelatin-subbed slides. Elimination of endogeneous peroxidase activity was performed with 0.3% hydrogen peroxide in PBS at room temperature for 30 min. The sections were pre-blocked with 3% normal goat serum, 0.5% triton X-100, and 1% bovine serum albumin in PBS for 30 min. Then incubated in rabbit antinociceptin antiserum (Chemicon Pharmaceuticals Inc, 1:500) at 4°C overnight. The sections were rinsed with PBS and incubated for 1h with goat anti-rabbit biotinylated secondary antibody (Rocland Laboratories, USA, 1:15 000). Then they were incubated in an avidin-biotin complex coupled to horseradish peroxidase for 10 min at room temperature. Finally, after rinsing with PBS, the nociceptin-positive neurons and nerve fibers were visualized using 3, 3’-diaminobenzidine (DAB, Maixin Chemical Co. Fuzhou, China). The slides were then dried, dehydrated in ethanol (70%-100% gradually), cleared in xylene, and coverslipped with mounting solution. Immunohistochemical image analysis was made to assess the changes of OFQ both in proximal and distal colons in two group rats (n = 10).
All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Results were expressed as mean ± SE. Data were analyzed by SPSS10.0. Differences were analyzed by one-way analysis of variance ANOVA and independent t test. Probabilities of P < 0.05 were considered significant.
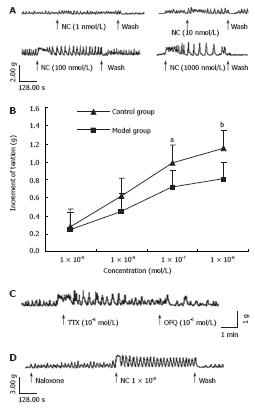
In vitro studies using longitudinal muscle revealed that OFQ (10-9-10-6 mol/L) induced contractions in the colon. The threshold concentration of OFQ to induce contractions was 10-9 mol/L. In the proximal colon of two group rats, OFQ (10-9 to 10-6 mol/L) induced contractions in a dose-dependent manner (Figure1A), but the increase of tension in cathartic colon was lower than in the control groups (Figure1B). To determine if OFQ activity was mediated by neural pathways or if it was a direct myogenic effect, OFQ-induced contractions were examined in the presence of TTX. In the presence of TTX, OFQ failed to elicit additional contractions (Figure 1C). The contractions induced by OFQ (1 × 10-6 mol/L) were not affected by classical opioid antagonist, naloxone (1 × 10-6 mol/L) (Figure1D).
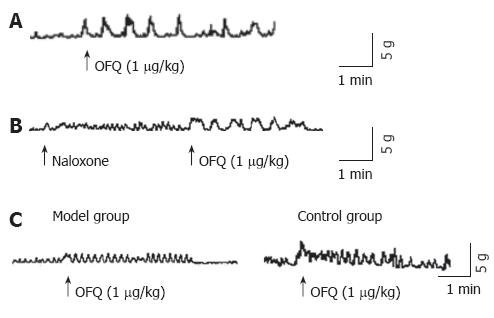
Intravenous administration of OFQ caused phasic contractions in the proximal colon (Figure 2A), it was not affected by classical opioid antagonist, naloxone (1 × 10-6 mol/L) (Figure 2B), but the amplitude of phasic contractions caused by OFQ in cathartic colon was much lower than control groups (Figure 2C, 2.58 ± 0.41 vs 4.16 ± 0.53, t = -2.6, P = 0.012).
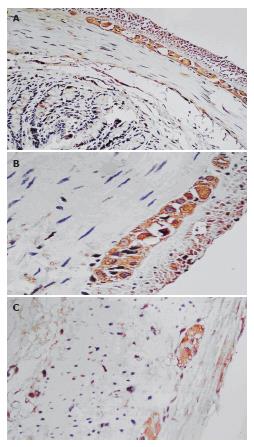
Immunohistochemistry of OFQ in the rat gastrointestinal tract showed that Orphain FQ immunoreactive (OFQ-IR) neurons and nerve fibers were visualized in the myenteric plexus of the rat colon (Figure 3A). The immunoreactivity of OFQ in the myenteric plexus in the proximal cathartic colon of rats decreased significantly as compared with the control group. (176.42 ± 5.792 vs 137.367 ± 25.508. P = 0.001) (Figure 3B, 3C). The negative control showed no staining of OFQ-IR in the myenteric plexus of the gastrointestinal tract.
Due to the diet and social-psychological changes, constipation has become an important factor affecting the quality of human life in modern society, therefore the morbidity is getting higher and higher. STC is a common syndrome in idiopathic constipation. It is a severe disorder of colonic motility characterized by a reduction in the frequency, amplitude and duration of propulsive contractions in the large bowel. Because of lack of understanding of the etiology, current medical treatments on STC are often ineffective. Animal experiments have shown that stimulant laxatives can damage ENS and cause the changes of some enteric neurotransmitters and thus the slow gastrointestinal tract transit occurs.
The innervation of the gastrointestinal tract is unique among the visceral organs. Neurons originating in the enteric ganglionated plexuses within the gut wall coordinate intrinsic reflexes associated with intestinal motility, epithelial secretion, and mucosal blood flow[14]. The myenteric plexus lies between the longitudinal and circular smooth muscle layers and regulates intestinal motor function. Bowel motility is controlled by a local network of intramural nerves[15]. Effects of opioid peptides on GI motility have been studied. Morphine can cause constipation due in part to slowing of GI transit and inhibition of active intestinal secretion, which was the normal side effect in clinical practice[16].
Despite its being homologous to classical opioids, dynorphin A, the OFQ/ORL1 system represents a new peptide-based signaling pathway, which is pharmacologically distinct from the opioid system[17]. Probing into the distribution and function of N/OFQ and ORL1 receptor in the gastrointestinal tract of the animal model will contribute to the overall understanding of the characteristics of this neuronic system, accelerating related researches of the gastrointestinal motility regulated by OFQ/ORL1 receptor system and promote the general comprehensivness of the opioid system. Thus, the model of rats with cathartic colon was established by feeding the rats with rhubarb for 3 months so as to illustrate the etiology of STC.
Our in vitro studies showed that OFQ induced contractions of colonic longitudinal muscle strips. In the proximal colon, OFQ (10-9-10-6 mol/L) induced contractions in a dose-dependent manner (Figure 1). But the increase of tension in cathartic colon was lower than the control groups (Figure 2). The mechanisms underlying OFQ-induced contractions were further investigated in the proximal colon. To determine if OFQ activity was mediated by neural pathways or if it was a direct myogenic action, OFQ induced contractions were examined in the presence of TTX, it has been supposed that TTX can abolish neuronal transmitter transition[18]. In the presence of TTX, OFQ failed to evoke additional contractions, suggesting that action of OFQ is mediated by neural pathways. It was coincidental with the present study that OFQ was highly expressed in the myenteric plexus of the rat colon, but not in the muscle cells. The in vitro, inhibitory effects of OFQ on EFS-evoked muscle contractions has been reported for mouse vas deferens and guinea pig ileum[19]. EFS-evoked muscular contractions were significantly reduced by 50% in guinea pig ileum by OFQ (10-7 mol/L) and was significantly reduced in mouse vas deferens by OFQ. Thus, the mechanisms and sites of action of OFQ may differ among different regions of gastrointestinal tract and different species.
Our study in vivo showed that intravenous admini-stration of OFQ (1 μg/kg) significantly increased muscle contractions in the proximal colon. These observations indicate that OFQ also stimulates colonic motility in vivo. The effects cannot be affected by classical antagonist, naloxone, but can be abolished in the presence of TTX, suggesting that the action of OFQ is nerve mediated. Currently, the physiological role of OFQ in colonic motility is unknown. However, Takahashi et al[20] in an in vivo study showed that intravenous administration of OFQ (3 pmol/kg to 3 nmol/L) significantly increased muscle contractions in the proximal colon. Furthermore, OFQ (1 nmol/L, subcutaneously) accelerated the colonic transit by promoting migrating colonic contractions in rats[21]. These observations also indicate that OFQ can stimulate colonic motility in vivo.
The immunoreactivity of OFQ in the myenteric plexus in the proximal region of the cathartic colon of rats decreased significantly compared with the control group. It has been suggested that OFQ prefered to be located in the stimulating motor neuron[22]. Colonic smooth muscle of cathartic colon showed low sensitivity to the stimulation of OFQ and this suggested it might becaused by the abnormal distribution of OFQ or the abnormalities of receptors, leading to the disorganization of dynamic and uncoordinated contractions.
In conclusion, OFQ seems to modulate the colonic transit independently from the classical opioid peptides[23]. We confirmed that OFQ is present in the gastrointestinal tract and has an effect on colonic motility. The physiological role of the nociceptin-QRL1 system in the colon is not clear but it may be significant to pathophysiological processes that underlie motor dysfunction of the bowel[24]. These findings indicate that OFQ is a brain-gut peptide and plays a role in the control of gastrointestinal functions[25]. The abnormalities of enteric nervous system are responsible for slow transit constipation. It also indicates that chronic application of stimulant laxatives can induce disorganization and damage the enteric nervous system[26,27] and accelerate the pathological changes of STC. But the pathophysiology of STC is complex and not easily approached through the data from animals alone. Further studies according to the clinical characteristics of STC patients could provide additional insight into this issue. It is necessary to investigate the mechanism underlying the biological actions of N/OFQ on GI. Because OFQ seems to exert its stimulatory action in the colon[28,29], in the future, synthesized analogues of OFQ may be used to treat constipation secondary to colonic inertia[30].
S- Editor Liu Y L- Editor Ma JY E- Editor Ma WH
| 1. | Krammer H, Schlieger F, Singer MV. [Therapeutic options of chronic constipation]. Internist (Berl). 2005;46:1331-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Bassotti G, Villanacci V. Slow transit constipation: a functional disorder becomes an enteric neuropathy. World J Gastroenterol. 2006;12:4609-4613. [PubMed] |
| 3. | Liu BH, Mo P, Zhang SB. Effects of mu and kappa opioid receptor agonists and antagonists on contraction of isolated colon strips of rats with cathartic colon. World J Gastroenterol. 2004;10:1672-1674. [PubMed] |
| 4. | Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1538] [Cited by in RCA: 1522] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 5. | Mollereau C, Mouledous L. Tissue distribution of the opioid receptor-like (ORL1) receptor. Peptides. 2000;21:907-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 194] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Ciccocioppo R, Economidou D, Fedeli A, Massi M. The nociceptin/orphanin FQ/NOP receptor system as a target for treatment of alcohol abuse: a review of recent work in alcohol-preferring rats. Physiol Behav. 2003;79:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Mamiya T, Yamada K, Miyamoto Y, König N, Watanabe Y, Noda Y, Nabeshima T. Neuronal mechanism of nociceptin-induced modulation of learning and memory: involvement of N-methyl-D-aspartate receptors. Mol Psychiatry. 2003;8:752-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Mao L, Wang JQ. Cardiovascular responses to microinjection of nociceptin and endomorphin-1 into the nucleus tractus solitarii in conscious rats. Neuroscience. 2005;132:1009-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Mogil JS, Pasternak GW. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol Rev. 2001;53:381-415. [PubMed] |
| 10. | Krowicki ZK, Kapusta DR, Hornby PJ. Orphanin FQ/nociceptin and [Phe(1)Psi(CH(2)-NH)Gly(2)] nociceptin(1-13)-NH(2) stimulate gastric motor function in anaesthetized rats. Br J Pharmacol. 2000;130:1639-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Broccardo M, Guerrini R, Petrella C, Improta G. Gastrointestinal effects of intracerebroventricularly injected nociceptin/orphaninFQ in rats. Peptides. 2004;25:1013-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Ishihara S, Minowa S, Tsuchiya S, Horie S, Watanabe K, Murayama T. Gastric acid secretion stimulated by centrally injected nociceptin in urethane-anesthetized rats. Eur J Pharmacol. 2002;441:105-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Lippl F, Schusdziarra V, Huepgens K, Allescher HD. Inhibitory effect of nociceptin on somatostatin secretion of the isolated perfused rat stomach. Regul Pept. 2002;107:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | D'Agostino B, Marrocco G, De Nardo M, Calò G, Guerrini R, Gallelli L, Advenier C, Rossi F. Activation of the nociceptin/orphanin FQ receptor reduces bronchoconstriction and microvascular leakage in a rabbit model of gastroesophageal reflux. Br J Pharmacol. 2005;144:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Bassotti G, Villanacci V, Maurer CA, Fisogni S, Di Fabio F, Cadei M, Morelli A, Panagiotis T, Cathomas G, Salerni B. The role of glial cells and apoptosis of enteric neurones in the neuropathology of intractable slow transit constipation. Gut. 2006;55:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 147] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 16. | Mehendale SR, Yuan CS. Opioid-induced gastrointestinal dysfunction. Dig Dis. 2006;24:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Grond S, Meuser T, Pietruck C, Sablotzki A. [Nociceptin and the ORL1 receptor: pharmacology of a new opioid receptor]. Anaesthesist. 2002;51:996-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Sakaba T. Roles of the fast-releasing and the slowly releasing vesicles in synaptic transmission at the calyx of held. J Neurosci. 2006;26:5863-5871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Zhang G, Murray TF, Grandy DK. Orphanin FQ has an inhibitory effect on the guinea pig ileum and the mouse vas deferens. Brain Res. 1997;772:102-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Takahashi T, Bagnol D, Schneider D, Mizuta Y, Ishiguchi T, LePard K, Galligan JJ, Watson SJ, Owyang C. Orphanin FQ causes contractions via inhibiting purinergic pathway in the rat colon. Gastroenterology. 2000;119:1054-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Takahashi T, Mizuta Y, Owyang C. Orphanin FQ, but not dynorphin A, accelerates colonic transit in rats. Gastroenterology. 2000;119:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | O'Donnell AM, Ellis LM, Riedl MS, Elde RP, Mawe GM. Distribution and chemical coding of orphanin FQ/nociceptin-immunoreactive neurons in the myenteric plexus of guinea pig intestines and sphincter of Oddi. J Comp Neurol. 2001;430:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 23. | Calo' G, Guerrini R, Rizzi A, Salvadori S, Regoli D. Pharmacology of nociceptin and its receptor: a novel therapeutic target. Br J Pharmacol. 2000;129:1261-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 351] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 24. | Osinski MA, Brown DR. Orphanin FQ/nociceptin: a novel neuromodulator of gastrointestinal function? Peptides. 2000;21:999-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Yazdani A, Takahashi T, Bagnol D, Watson SJ, Owyang C. Functional significance of a newly discovered neuropeptide, orphanin FQ, in rat gastrointestinal motility. Gastroenterology. 1999;116:108-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Nadal SR, Calore EE, Manzione CR, Puga FR, Perez NM. Effects of long-term administration of Senna occidentalis seeds in the large bowel of rats. Pathol Res Pract. 2003;199:733-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Kapur RP. Neuropathology of paediatric chronic intestinal pseudo-obstruction and related animal models. J Pathol. 2001;194:277-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Abdelrahman AM, Pang CC. Regional hemodynamic effects of nociceptin/orphanin FQ in the anesthetized rat. Eur J Pharmacol. 2002;450:263-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Tada H, Nakagawa K, Yamamura T, Takahashi T. Antagonistic effects of CompB on orphanin FQ-induced colonic contractions in rats. Eur J Pharmacol. 2002;454:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Broccardo M, Linari G, Guerrini R, Agostini S, Petrella C, Improta G. The effects of [Arg14, Lys15] nociceptin/orphanin FQ, a highly potent agonist of the NOP receptor, on in vitro and in vivo gastrointestinal functions. Peptides. 2005;26:1590-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |