Published online Mar 7, 2006. doi: 10.3748/wjg.v12.i9.1403
Revised: May 15, 2005
Accepted: July 20, 2005
Published online: March 7, 2006
AIM: To investigate the effects of long-term albumin administration on survival, recurrence of ascites and onset of other complications.
METHODS: One hundred consecutive patients admitted for first-onset ascites were randomized to receive diuretics plus human albumin 25 g/wk in the first year and 25 g every two wk thereafter (group 1) or diuretics alone (group 2). The primary endpoint was survival without liver transplantation. Secondary endpoints were recurrence of ascites and occurrence of other complications.
RESULTS: Median follow-up was 84 (2-120) mo. Albumin-treated patients had significantly greater cumulative survival rate (Breslow test = 7.05, P = 0.0078) and lower probability of ascites recurrence (51% versus 94%, P < 0.0001). Chronic albumin infusion resulted in a mean increase in survival of 16 mo.
CONCLUSION: Long-term albumin administration after first-onset ascites significantly improves patients’ survival and decreases the risk of ascites recurrence.
- Citation: Romanelli RG, Villa GL, Barletta G, Vizzutti F, Lanini F, Arena U, Boddi V, Tarquini R, Pantaleo P, Gentilini P, Laffi G. Long-term albumin infusion improves survival in patients with cirrhosis and ascites: An unblinded randomized trial. World J Gastroenterol 2006; 12(9): 1403-1407
- URL: https://www.wjgnet.com/1007-9327/full/v12/i9/1403.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i9.1403
Ascites is one of the most frequent complications of cirrhosis, occurring in more than 50% of patients within 10 years of the diagnosis of cirrhosis[1-3]. The development of ascites in cirrhosis severely affects patient’s prognosis and heralds more severe complications, such as spontaneous bacterial peritonitis and the hepatorenal syndrome[1,3]. Two factors are of major importance in the pathogenesis of ascites: sodium retention, leading to extracellular fluid volume expansion; and imbalance in Starling’s equilibrium in liver sinusoids and splanchnic capillaries, which is responsible for translocation of fluid from the intravascular compartment to the abdominal cavity[4,5]. The pathogenesis of sodium retention in cirrhosis is still debated. However, there is no doubt that even if total plasma volume is normal or even increased in cirrhosis, patients with cirrhosis and ascites have a decreased effective arterial blood volume and activation of the renin-angiotensin-aldosterone and sympathetic nervous systems, which stimulate the kidney to retain sodium[4-7]. Starling’s forces in the splanchnic compartment are altered because of the increased hydrostatic pressure due to portal hypertension and the low oncotic pressure due to hypoalbuminemia.
Treatment of grade 2 (moderate) ascites is based on sodium restriction and administration of an aldosterone antagonist, with addition of a loop diuretic in the non-responding patients[8]. The same regimen plus paracentesis is recommended in patients with grade 3 (tense) ascites[8]. At present, the main indications to intravenous albumin in cirrhosis are type I hepatorenal syndrome[8,9] and spontaneous bacterial peritonitis[8-10].
In a previously published, controlled study performed at our institution[11], administration of human albumin to a series of patients with ascites improved the response rate to low-sodium diet and diuretics and reduced the cumulative probability of developing ascites during a 20-mo follow-up, but did not affect survival after a mean follow-up of 20 mo.
We report herein the results of an another randomized study aimed at investigating the effects of long-term human albumin administration in a series of consecutive cirrhotic patients with first-onset ascites. The primary endpoint of this study was survival without liver transplantation. Secondary endpoints were recurrence of ascites and occurrence of other complications.
The study was performed at the Liver Unit, Department of Internal Medicine, University of Florence, in accordance with the Declaration of Helsinki. It was approved by the University of Florence Ethics Committee in October, 1992 and revised periodically thereafter. One hundred consecutive cirrhotic patients admitted from January 1, 1993 to June 30, 2003 because of first-onset, clinically detectable ascites (i.e., grade 2-3 ascites, according to the more recent definition by Moore et al[8]) were included in this randomized, unblinded trial. Exclusion criteria were: age below 35 or over 70 years; active alcohol abuse; previous episode(s) of grade 2-3 ascites; cardiac, respiratory or renal impairment (serum creatinine ≥1.5 mg/dL); diabetes; refractory ascites; hepatocellular carcinoma or other malignancies; present or previous hepatic encephalopathy of any degree; gastrointestinal bleeding at admission (endoscopy); infections; intravascular coagulation; impossibility or unwillingness to return for follow-up; and refusal of informed, written consent. Diagnosis of cirrhosis was based on history, clinical and biochemical data, abdominal ultrasound, gastrointestinal endoscopy and liver biopsy, when not contraindicated.
At admission, eligible patients were randomly assigned to either group 1 (albumin) or group 2 using sealed envelopes containing the treatment assignments. Allocation schedule was generated using a computed random number generation system. Patients from both groups received low-sodium diet and diuretics in increasing dosage, as commonly used at our institution[12] and later recommended by the International Ascites Club[8]. Briefly, they received low (80 mEq/d) sodium diet, spironolactone (100 - 400 mg), frusemide (25-150 mg), as appropriate. Nineteen group 1 and 16 group 2 patients had tense ascites at admission and had 4 L therapeutic paracentesis without plasma volume expansion. After disappearance of ascites, all patients were given low-sodium diet, diuretics and other drugs (e.g. β-blockers), as appropriate, to prevent ascites accumulation and other complications. In addition, group 1 patients intravenously received 25 g albumin per wk in the first year and 25 g every two wk thereafter. Albumin was administered in the outpatient clinic at our institution. All patients were followed in the outpatient clinic every 1-3 mo by members of the clinical staff not involved in this investigation to monitor the development of ascites or other complications of cirrhosis and modify therapy, as needed. Development of grade 2 - 3 ascites during the follow-up was assessed by clinical examination and confirmed by ultrasound and diagnostic paracentesis. Patients with recurrent ascites or other complications received adequate treatment at our institution as inpatients or outpatients, as indicated. In particular, patients with refractory ascites received therapeutic paracenteses of less than 4 L with no intravenous fluid infusion. The following parameters were recorded and analyzed: liver transplant-free survival; causes of death; recurrence of ascites; and onset of other complications.
The data were analyzed using the SPSS 10 (SPSS Inc., Chicago, IL). Being the follow-up loss limited to the first month for withdrawing consent to experimental design, the per protocol analysis was performed as previously described[13]. Student’s t test, Fisher’s exact test and χ2 test were employed for the analysis of data, as appropriate. Dependence of survival from other disease parameters besides albumin treatment was analyzed using univariate analysis. Survival time was analyzed by the Kaplan-Meier method and differences were tested by the Breslow test. Data were expressed as median (range). P < 0.05 was considered statistically significant.
Of the 100 consecutive patients included in this study, 54 were assigned to group 1 (albumin), the remaining 46 patients to group 2 (no albumin). The clinical characteristics of patients at the time of inclusion are shown in Table 1. No significant differences were observed between the two groups with respect to any of the measured biochemical parameters. However, group 1 (albumin) patients had significantly greater Child-Pugh score; as a consequence, significantly more patients in this group were in Child-Pugh class C (Table 1). No patient died during hospital stay. Nine patients in group 1 and eight in group 2 withdrew their consent at discharge or were lost at follow-up immediately afterwards. Median follow-up was 84 (range 2-120) mo.
| Parameters | Group 1 (diuretics plus albumin) | Group 2 (diuretics alone) | P value |
| Age (yr) | 63 (44 - 70) | 63 (47 - 70) | NS |
| Sex (M/F) | 33/21 | 29/17 | NS |
| Etiology | |||
| HBV | 7 | 8 | NS |
| HCV | 36 | 28 | NS |
| Alcohol | 1 | 1 | NS |
| Cryptogenic | 10 | 9 | NS |
| Child-Pugh score | 10 (8 -11) | 9 (8 - 14) | P < 0.0001 |
| Child class (B/C) | 15/39 | 31/15 | P < 0.0001 |
| Hematocrit | 0.33 (0.32 - 0.40) | 0.34(0.25 - 0.41) | NS |
| Serum albumin (g/L) | 30.6 (27.0 - 38.0) | 31.7 (24.0 - 37.0) | NS |
| Serum bilirubin (mg/dL) | 3.15 (1.30 - 4.90) | 2.2 (1.25 - 5.50) | NS |
| Prothrombin activity (%) | 57 (51 - 70) | 62 (30 - 70) | NS |
| MAP (mmHg) | 95 (93 - 96) | 96 (94 - 98) | NS |
| Serum creatinine (mg/dL) | 0.97 (0.93 - 1.00) | 0.98 (0.95 - 0.99) | NS |
| Creatinine clearance (mL/min) | 69 (64 - 85) | 71 (65 - 75) | NS |
Transplant-free survival
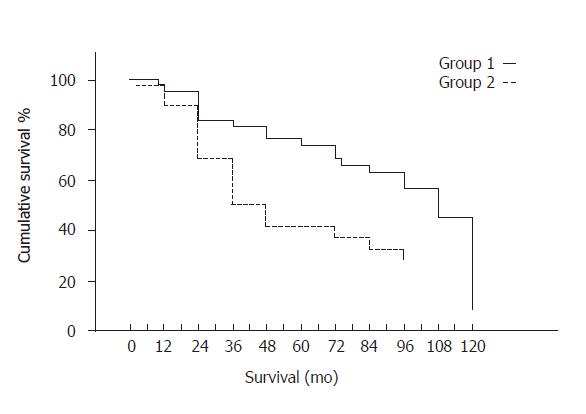
Among the 100 patients recruited, 81 were still alive at 2 years and 57 at 5 years, survival rates being 81% at 2 years and 57% at 5 years. Univariate analysis showed that the most important factor affecting survival was albumin treatment (R-squared = 0.975) (F = 16.55, P = 0.005), followed by Child-Pugh score (F = 6.363, P = 0.021) and age (F = 2.783, P = 0.082). Albumin-treated (group 1) patients had a significantly greater cumulative survival rate than those in group 2 (group 1: median 108, range 99.55-116.45 mo; group 2: median 36, range 22-50 mo, Breslow test = 7.05, P = 0.0079, Figure 1). In particular, 34 out of the 54 patients randomized to receive albumin (75%) were still alive at 24 mo, 31 (69%) at 48 mo, 28 (62%) at 60 and 2 (4%) at 120 mo, while the corresponding figures for group 2 patients were 26 (68%), 11 (29%), 10 (26%) and 3 (8%), respectively.
Fifty-four patients (29 in group 1 and 25 in group 2) died during the follow-up. The causes of death in group 1 and group 2 patients are indicated in Table 2. No significant differences were observed between the two groups with respect to causes of death. Liver transplantation was performed in one patient in the albumin group because of hepatocellular carcinoma, and in three patients in group 2 because of progressive hepatic failure (2 patients) and chronic encephalopathy.
| Causes of death | Group 1 | Group 2 | P value |
| Hepatocellular carcinoma | 9 | 14 | NS |
| Other neoplasms | 2 | 0 | NS |
| Hepatorenal syndrome | 1 | 0 | NS |
| Variceal bleeding | 5 | 4 | NS |
| Liver Failure | 10 | 6 | NS |
| Heart Failure | 0 | 1 | NS |
| Sepsis | 2 | 0 | NS |
Recurrence of grade 2-3 ascites was markedly lower in group 1 patients (38.88%, 21/54) as compared with group 2 patients (84.78%, 39/46, P < 0.0001). In group 1, 4 patients had 3 episodes and 2 patients 2 episodes of ascites, the total number of ascitic episodes being 31; whereas in group 2, ascites recurred 4 times in 1 patient, 3 times in 2 and 2 times in 8 patients, the total number of ascitic episodes being 54 (P < 0.001). Five patients in each group developed refractory ascites (P > 0.05), while 3 patients in group 1 and two in group 2 developed SBP (P > 0.05).
During the follow-up, there were 19 episodes of hepatic encephalopathy in 11 (20%) patients in group 1 and 15 episodes in 11 (24%) patients in group 2. Variceal bleeding occurred in 5 patients in group 1 and 10 patients in group 2 and hepatic failure in 5 patients in group 1 and seven patients in group 2 (P > 0.05). One patient in the latter group received transjugular intrahepatic porto-systemic stent shunt (TIPSS) to stop intractable hemorrhage. Surgical porto-systemic shunt was performed in 1 patient in group 1 and 1 patient in group 2 to control severe chronic anemia due to portal hypertensive gastropathy.
No side effects caused by administration of albumin were observed during the whole study period.
At the beginning of the 1940's, when salt-poor albumin became available, it was widely used in the management of cirrhotic patients with ascites in an attempt to correct the unbalanced Starling’s forces in the splanchnic circulation, so reducing ascites formation, and to improve circulatory and renal function[13,14]. However, few studies investigated the role of albumin in the treatment of ascites and prevention of its recurrence, however, these investigations were uncontrolled and/or included a low number of patients. Albumin infusion usually produced a subjective feeling of “well-being”, as also observed in our previous[11] and current studies, but had variable effects on ascites and edema[14-17].
With the introduction of effective diuretics, the use of albumin for the treatment of ascites in cirrhosis was almost totally abandoned at least in the US. At present, the AASLD recommends albumin only in patients with SBP[18]. Albumin is superior to dextran 70 and polygeline in preventing circulatory dysfunction after paracentesis involving the removal of more than 5 liters of fluid[9] and is therefore widely used outside the US; however, randomized studies have shown no significant difference in survival between patients treated with albumin and those treated with other plasma expanders[19-22].
Despite no evidence from randomized studies support the long-term administration of albumin in patients with cirrhosis and ascites, this practice is widely used at least in Italy, as indicated by a recent study aimed at reaching a consensus among Italian hepatologists as to the use of albumin in patients with cirrhosis and ascites that involved 68 hepatology centers[23]. In the opinion of most experts, long-term use of albumin can help to improve the patient’s general conditions and well being. Seventy-seven percent of the experts involved in this survey agreed that albumin administration can shorten hospital stay or reduce the number of hospital admissions. As a matter of fact, 79% out of the 39 gastroenterological centers participating to a survey organized by the Italian Association of Hospital Gastroenterologists and Endoscopists (AIGO, available on the web site of this association) stated that they usually administer albumin to patients with cirrhosis and ascites.
In a previous randomized study from our department, which included 81 cirrhotic patients with ascites (38 treated with diuretics and 43 with diuretics plus human albumin), and had a follow-up of 20.0 ± 1.9 (range 6-36) mo, patients receiving human albumin had greater rate of ascites disappearance (90.5% versus 74.7%, P < 0.05) and lower cumulative probability of developing ascites, but no improvement in survival[11].
In the current investigation, we extended the follow-up period up to 62.7 ± 4.2 (mean ± SE) mo. The main result of our study was that human albumin administration to patients affected by liver cirrhosis (25 g/wk in the first year and 25 g every two wk thereafter) resulted in a significantly greater cumulative survival rate. In addition, as in the previous investigation, albumin administration markedly reduced ascites recurrence.
The patients in group 1 and group 2 were quite similar with respect to arterial pressure and the measured parameters of liver and renal function. However, despite our attempt to reduce variability by including only cirrhotics with first-onset ascites, patients randomized to receive albumin had a slightly greater Child-Pugh score (Table 1), with more patients in this group belonging to Child class C (39 vs 15). It is conceivable that the effects of any therapeutic intervention, as albumin infusion in the current investigation, will be quite different in patients with different baseline characteristics. This is confirmed by the results of univariate analysis, showing that transplant-free survival was related to treatment, but also to Child-Pugh score. Albumin administration resulted in a gain of 16 mo of mean survival time (88.63 vs 61.39 mo).
Other aspects of this investigation deserve consideration. First, group 2 patients did not receive placebo. This was made to avoid the expenses, discomfort and risks of coming to the outpatient clinic and receive an unnecessary intravenous infusion every 7 - 14 d. On the other hand, patients not receiving albumin are easily identified since they have lower plasma albumin levels. Second, we used the same protocol proved effective in reducing ascites recurrence in our previous investigation[11]. Whether different amounts of albumin would be equally or even more effective in all ascitic patients remains to be established.
The mechanisms determining a better survival in cirrhotic patients with ascites receiving long-term human albumin were not specifically investigated in this study. Albumin is responsible for about 70% of plasma oncotic pressure and therefore plays a major role in modulating the distribution of fluid between compartments. In a hemodynamic study by Brinch et al[24], acute administration of albumin (40 g) expanded plasma and blood volume in cirrhosis, although central blood volume only increased in Child class A patients. In addition, arterial compliance increased and plasma renin activity decreased in Child C patients, indicating that albumin is able to improve the low effective arterial blood volume of cirrhosis, so reducing baroreceptor-induced activation of the renin-aldosterone axis and probably of the other vasoconstriction and sodium-retaining factors. Albumin prevented circulatory dysfunction in patients submitted to large-volume paracentesis[19-21], and circulatory dysfunction was found to be related to a shorter survival[22]. It also proved valuable in the treatment of diuretic-induced hepatic encephalopathy[25] and hyponatremia[26], improved survival in patients with spontaneous bacterial peritonitis and was effective in reversing the hepatorenal syndrome when administered together with vasoconstrictors[8-10,18]. It is tempting to speculate that the favorable effect of human albumin on survival observed in this study could be related to its ability to improve the circulatory dysfunction of liver cirrhosis and ascites, so reducing the degree of activation of the main vasoactive systems and antinatriuretic factors that characterize this particular group of patients and are associated with a poor prognosis[8,27].
The use of albumin has been criticized due to its high costs. In Italy, one vial of albumin (10 g) costs € 43.5; so, expenses for albumin were € 4 524 per patient in the first year and € 2 262 thereafter. However, these charges are comparable or even lower than those of other treatments showed to improve survival, such as antiviral treatment of HBV-HCV-related hepatitis, or immunoprophylaxis of HBV reinfection of liver grafts. The advent of recombinant human serum albumin[28] will probably overcome these economic considerations.
Whether albumin should be administered to all patients with cirrhosis and ascites is a problem related to the overall allocation of available resources. In view of the net gain of 16 mo of adjusted survival time demonstrated in the present investigation, in our opinion albumin can be especially useful in patients on waiting list for liver transplantation.
In conclusion, long-term albumin administration to patients with cirrhosis after the first ascitic episode significantly improves patient survival and decreases the risk of ascites recurrence.
S- Editor Guo SY L- Editor Kumar M E- Editor Ma WH
| 1. | Ginés P, Quintero E, Arroyo V, Terés J, Bruguera M, Rimola A, Caballería J, Rodés J, Rozman C. Compensated cirrhosis: natural history and prognostic factors. Hepatology. 1987;7:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 709] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 2. | D'Amico G, Morabito A, Pagliaro L, Marubini E. Survival and prognostic indicators in compensated and decompensated cirrhosis. Dig Dis Sci. 1986;31:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 368] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 3. | Gentilini P, Laffi G, La Villa G, Romanelli RG, Buzzelli G, Casini-Raggi V, Melani L, Mazzanti R, Riccardi D, Pinzani M. Long course and prognostic factors of virus-induced cirrhosis of the liver. Am J Gastroenterol. 1997;92:66-72. [PubMed] |
| 4. | Arroyo V, Jiménez W. Complications of cirrhosis. II. Renal and circulatory dysfunction. Lights and shadows in an important clinical problem. J Hepatol. 2000;32:157-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 115] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Arroyo V, Colmenero J. Ascites and hepatorenal syndrome in cirrhosis: pathophysiological basis of therapy and current management. J Hepatol. 2003;38 Suppl 1:S69-S89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 169] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 6. | Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodés J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1131] [Cited by in RCA: 1021] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 7. | Levy M. Pathogenesis of sodium retention in early cirrhosis of the liver: evidence for vascular overfilling. Semin Liver Dis. 1994;14:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, Angeli P, Porayko M, Moreau R, Garcia-Tsao G. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003;38:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 611] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 9. | Ginès P, Cárdenas A, Arroyo V, Rodés J. Management of cirrhosis and ascites. N Engl J Med. 2004;350:1646-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 481] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 10. | Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1132] [Cited by in RCA: 1001] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 11. | Gentilini P, Casini-Raggi V, Di Fiore G, Romanelli RG, Buzzelli G, Pinzani M, La Villa G, Laffi G. Albumin improves the response to diuretics in patients with cirrhosis and ascites: results of a randomized, controlled trial. J Hepatol. 1999;30:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 154] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Gentilini P, La Villa G, Romanelli RG, Foschi M, Laffi G. Pathogenesis and treatment of ascites in hepatic cirrhosis. Cardiology. 1994;84 Suppl 2:68-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Runyon BA. Historical aspects of treatment of patients with cirrhosis and ascites. Semin Liver Dis. 1997;17:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Erstad BL, Gales BJ, Rappaport WD. The use of albumin in clinical practice. Arch Intern Med. 1991;151:901-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | LOSOWSKY MS, ATKINSON M. Intravenous albumin in the treatment of diuretic-resistant ascites in portal cirrhosis. Lancet. 1961;2:386-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | WILKINSON P, SHERLOCK S. The effect of repeated albumin infusions in patients with cirrhosis. Lancet. 1962;2:1125-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Runyon BA. Management of adult patients with ascites due to cirrhosis. Hepatology. 2004;39:841-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 239] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 19. | Planas R, Ginès P, Arroyo V, Llach J, Panés J, Vargas V, Salmerón JM, Ginès A, Toledo C, Rimola A. Dextran-70 versus albumin as plasma expanders in cirrhotic patients with tense ascites treated with total paracentesis. Results of a randomized study. Gastroenterology. 1990;99:1736-1744. [PubMed] |
| 20. | Salerno F, Badalamenti S, Lorenzano E, Moser P, Incerti P. Randomized comparative study of hemaccel vs. albumin infusion after total paracentesis in cirrhotic patients with refractory ascites. Hepatology. 1991;13:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 76] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Fassio E, Terg R, Landeira G, Abecasis R, Salemne M, Podesta A, Rodriguez P, Levi D, Kravetz D. Paracentesis with Dextran 70 vs. paracentesis with albumin in cirrhosis with tense ascites. Results of a randomized study. J Hepatol. 1992;14:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Ginès A, Fernández-Esparrach G, Monescillo A, Vila C, Domènech E, Abecasis R, Angeli P, Ruiz-Del-Arbol L, Planas R, Solà R. Randomized trial comparing albumin, dextran 70, and polygeline in cirrhotic patients with ascites treated by paracentesis. Gastroenterology. 1996;111:1002-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 351] [Article Influence: 12.1] [Reference Citation Analysis (1)] |
| 23. | Gentilini P, Bernardi M, Bolondi L, Craxi A, Gasbarrinie G, Ideo G, Laffi G, La Villa G, Salerno F, Ventura E. The rational use of albumin in patients with cirrhosis and ascites. A Delphi study for the attainment of a consensus on prescribing standards. Dig Liver Dis. 2004;36:539-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Brinch K, Møller S, Bendtsen F, Becker U, Henriksen JH. Plasma volume expansion by albumin in cirrhosis. Relation to blood volume distribution, arterial compliance and severity of disease. J Hepatol. 2003;39:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Jalan R, Kapoor D. Reversal of diuretic-induced hepatic encephalopathy with infusion of albumin but not colloid. Clin Sci (Lond). 2004;106:467-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Ginés P, Berl T, Bernardi M, Bichet DG, Hamon G, Jiménez W, Liard JF, Martin PY, Schrier RW. Hyponatremia in cirrhosis: from pathogenesis to treatment. Hepatology. 1998;28:851-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 176] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 27. | Llach J, Ginès P, Arroyo V, Rimola A, Titó L, Badalamenti S, Jiménez W, Gaya J, Rivera F, Rodés J. Prognostic value of arterial pressure, endogenous vasoactive systems, and renal function in cirrhotic patients admitted to the hospital for the treatment of ascites. Gastroenterology. 1988;94:482-487. [PubMed] |
| 28. | Kobayashi K, Nakamura N, Sumi A, Ohmura T, Yokoyama K. The development of recombinant human serum albumin. Ther Apher. 1998;2:257-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |