INTRODUCTION
The term dyspepsia defies definition, although it is widely used in clinical practice. It comes from the Greek word, dys meaning bad and peptein meaning digestion. It causes much confusion among both patients and clinicians. Dyspepsia itself is not a diagnosis but stands for a constellation of symptoms referable to the upper gastrointestinal tract. A recent working party has recommended that dyspepsia refers to pain or discomfort centered in the upper abdomen (ROME II) [1]. Pain is the unpleasant sensation mainly in or around the midline. Discomfort may be characterized by early satiety, fullness, abdominal bloating, belching, nausea, retching and vomiting. When dyspeptic patients have no underlying identifiable disease process to account for their symptoms, then they are considered to be suffering from functional dyspepsia (FD). FD runs a chronic course and the Rome II criteria states that symptoms have to be present for at least 12 wk, which need not be consecutive, in the preceding 12 mo. The symptoms are persistent or recurrent and not associated with a change in bowel pattern or stool form.
FD is a heterogeneous condition and not all patients present with the same symptoms. The patients may be divided into subgroups based on their symptoms cluster. 3 major subgroups are recognized: Ulcer-like dyspepsia, dysmotility-like dyspepsia and nonspecific dyspepsia. Dysmotility-like dyspepsia describes a subgroup of FD patients whose symptom complex seems to suggest a relation to feeding and involvement of an underlying gastric sensory or motor disorder. The pathogenesis of this common disorder remains unclear but probably involves multiple pathophysiological mechanisms with complex interactions involving the enteric nervous system, the afferent sensory pathways and the brain, the so called brain-gut axis. There appears to be disturbed motor functions with altered visceral sensations and a strong association to psychosocial factors. Present opinion is that FD is a biopsychosocial disorder where dyspeptic symptoms may arise from these interactions[2].
The brain-gut peptide CCK and brain-gut indolamine 5-HT share some physiological effects. 5-HT is involved in gut motility, visceral sensation and other aspects of gut function while CCK is involved in mediation of pain in the gut and nociception in the central nervous system (CNS). CCK and fenfluramine (increases neuronal release of 5-HT) both slow gastric emptying and block stress induced hyperphagia[3-7]. Both neurotransmitters have been independently implicated as factors affecting food intake [8,9]. Peripheral and central administration of CCK and fenfluramine produces anorexia in both humans and animals. The CCK induced anorexia depended on serotonergic function, probably at central sites[10]. Furthermore intravenous CCK administration stimulates the release of 5-HT and noradrenaline in the paraventricular and supraoptic nuclei, both important in central modulation of feeding and gastrointestinal motility [11].
Stressful events in life are known to alter ingestive behaviors and associated physiological events such as gastric acid secretion and gastrointestinal motility. Evidence also implicated corticotrophin releasing factor (CRF) in the mediation of stress-induced inhibition of upper gastrointestinal (GI) tract and stimulation of lower GI motor function. Endogenous 5-HT, peripherally released in response to stress, seems to be involved in the central CRF-induced effect on the GI tract[12,13]. Acute psychological stress, if produces significant emotional change may lead to an increase in sensitivity to experimental visceral stimuli. Whether chronic stress has the same effects remain to be seen.
The symptom complex described in dysmotility-like FD is usually related to feeding and also suggests an underlying abnormal GI sensory and motor function. There is an indirect correlation between severity of early satiety and gastric emptying rate, as well as an association between bloating and delayed gastric emptying[58]. Severe postprandial fullness and vomiting are independently associated with delayed gastric emptying of solids[19]. Since the neurotransmitter 5-HT and the neuropeptide CCK have been implicated in the regulation of feeding and the control of GI function, they may play an important role in the pathophysiology of functional dyspepsia.
ROLE OF SEROTONIN IN FUNCTIONAL DYSPEPSIA
Serotonin is a monoamine that acts as both a peripheral transmitter in the gut and a neurotransmitter in the br-ain[14,15]. Within the enteric nervous system (ENS), 5-HT is stored in myenteric neurons and acts as a neurotransmitter[16,17]. It plays an important role in regulating peristalsis and intestinal tone and thought to be one of the most important gut neurotransmitters. Sumatriptan, a 5-HT1 receptor agonist, inhibits antral motor activity, delays gastric emptying and relaxes the gastric fundus[18]. Intravenous injection of 5-HT in dogs have been observed to modulate gastric emptying, and furthermore gastric emptying can be inhibited by injecting fenfluramine into the cerebral ventricles of rats[20,21]. The primary neuronal effect of fenfluramine includes release of 5-HT from nerve terminals and inhibition of reuptake. Some serotonergic drugs induce anorexia through central pathways[22]. Warner suggested that some cases of functional abdominal pain are due to hyperserotoninemia[23]. Furthermore the hyperserotoninaemia of the carcinoid syndrome causes nausea, vomiting, colicky abdominal pain and diarrhea[24]. The majority of patients with FD are however not hyperserotoninemic. Nonetheless, altered sensitivity of 5-HT receptors might have similar consequences as high levels of serotonin. Interestingly, the functional activity of central serotonergic receptors can be studied using a neuroendocrine challenge test.
Neuroendocrine challenge test
The neuroendocrine axis provides an acceptable means of assessing brain 5-HT receptor function. The development of neuroendocrine challenge tests rest on the demonstration that the release of certain anterior pituitary hormones is controlled by brain monoamine pathways. Thus , monoamine function can be assessed by measurement of the hormonal response in plasma which follows stimulation of a particular brain monoamine pathway by a specific drug [25]. The size of the hormonal response is taken as an index of the functional activity of the monoamine synapses with which the drug interacts. It is apparent that for a neuroendocrine challenge test to provide a valid measure of brain 5-HT function, it must demonstrate that the hormone measured is indeed under the control of brain 5-HT pathways and that the drug employed to produce the hormonal response is acting specifically through 5-HT synapses. The release of prolactin (PRL) from the anterior pituitary is under the inhibitory control of dopamine and stimulatory control of 5-HT[25]. When hypothalamic receptors are stimulated by an appropriate 5-HT agonist, an increase in serum PRL takes place, via stimulation of a PRL releasing factor by 5-HT neurons originating in the medial and dorsal raphe nuclei.
Buspirone, an azaspirodecanedione, stimulates central 5-HT1A receptors at the hypothalamic level and brings about PRL release in a dose dependent manner[26,27]. Its effects can be blocked by the antagonist methysergide and pindolol, a specific 5-HT1A receptor blocker. It crosses the blood brain barrier easily and has a rapid onset of action. The extent of prolactin release can thus be used reliably as a measure of central 5-HT1A receptor sensitivity.
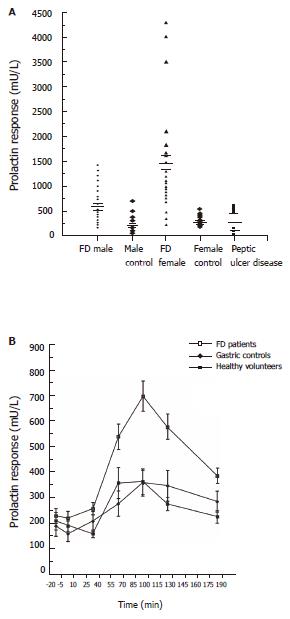
The response to the buspirone challenge test in FD patients was compared to normal healthy subjects and patients with peptic ulcer disease[63,64]. Considerable greater prolactin responses were found in FD patients than in healthy controls or PUD patients (Figure 1A). The mean (+/- SEM) increase in plasma PRL after buspirone in male FD patients was 672.2 +/- 65.0 Mu/L, in contrast to male healthy controls with 222.9 +/- 59.9 Mu/L. The corresponding results for female FD and controls are 1428.8 +/- 232.5 Mu/L and 352.5 +/- 33.8 Mu/L respectively. The mean PRL response for the PUD group was 325+/- 91.1 Mu/L. A highly significant difference was obtained when the FD patients was compared to the healthy controls (males; P < 0.05, females; P < 0.001). Differences between the responses became apparent around 60 minutes (Figure 1B). There is a significant difference in the prolactin response to Buspirone challenge, between males and females. Why is there such a difference is still not very clear. There is also evidence to suggest that, the prolactin response in females, varies at different times in the female menstrual cycle. All the neuroendocrine challenges were carried out at the same period of the cycle. Interestingly, gastric emptying rates have also being observed to be influenced by the menstrual cycle[74]. This may explain the fact why FD, is more common in women and generally their symptoms are more severe compared to man. Our results indicated that central serotonergic receptors are considerably more sensitive in FD patients and provide evidence that FD is a disorder characterized by a neurochemical dysfunction in the brain.
Figure 1 A :Prolactin response to buspirone challenge in healthy controls and patients with functional dyspepsia and peptic ulcer disease B :Prolactin response to buspirone 60 mg in male functional dyspeptics, peptic ulcer disease patients and healthy controls.
Serotonin neuronal system of the brain have been postulated to modulate many basic physiological tasks, including gating of pain perception, control of eating and GI sensorimotor functions. An abnormal central 5-HT receptor function may interfere with any one or all of the above functions. Interference with the pain gating system may affect patients perception to pain or give rise to an abnormal response to nociception from visceral receptors. Fenfluramine, a 5-HT releasing agent and reuptake inhibitor, reduces hunger ratings, delays onset of feeding and provokes termination of a bout of eating. The presence of hypersensitive central serotonergic receptors would function in the same manner as an increase release of 5-HT. Interestingly, FD patients are known to have abnormal feeding behavior with symptoms that are related to meals. Furthermore, gastric motility has been shown to be affected by injection of 5-HT into the cerebral ventricles of animals[4]. Thus a hypersensitive central 5-HT receptor function could easily affect gastric motility.
ROLE OF CHOLECYSTOKININ (CCK) IN FUNCTIONAL DYSPEPSIA
CCK is an established brain-gut peptide that plays an important regulatory role in gastrointestinal function[28]. CCK is involved in the control of food intake and satiety in both man and animals[29-31]. It inhibits gastric motility and emptying via capsaicin-sensitive vagal pathways[32,33]. In humans, CCK regulates gastric emptying under physiological conditions[34,72]. An altered response to CCK may be responsible for the dyspeptic symptoms in FD and may be accountable for the frequently observed abnormal gastric motility. The sensitivity of FD patients to CCK has been tested using a CCK challenge test.
CCK challenge test
Subjects for the provocation test undergo an overnight fast. The test was performed using a synthetic CCK-octapeptide (CCK-8), sincalide. Response to CCK was assessed by an intravenous CCK-8 infusion (6 nanograms/kg/min) over 10 min in a double-blind, cross-over design using normal saline as placebo. A break of 15 min was given between infusion. The CCK test was deemed positive when the infusion reproduced the patients’ symptoms (epigastric pain or discomfort, nausea, abdominal distension, bloating, belching and vomiting). Patients’ response to the infusion was assessed by a third independent observer and patients scored their response (reproduction of the symptoms) on a visual analogue scale.
When FD patients were subjected to the CCK-8 challenge test, majority (90%) of them responded positively to the challenge test[75]. Commonly reported symptoms included abdominal pain, abdominal bloating and fullness, belching, nausea and occasional vomiting (dysmotility type symptoms). Most of the healthy subjects complained only of very minor symptoms including mild nausea and minor abdominal discomfort (Figure 2A). No subject reported any symptoms on saline (placebo) infusion. Interestingly it was also shown that, intravenous atropine was able to abolish the response to the CCK-8 provocation test in a dose-dependent fashion (Figure 2B). Similarly, oral loxiglumide (CCK-A antagonist) 800 mg, consumed 1 h before the CCK challenge, was successful in controlling the symptoms (Figure 2C). Solid phase gastric emptying were also measured in the FD patients using scintigraphic assessment of a standard breakfast with Tc-99M tin colloid and compared with healthy controls. Solid-phase gastric emptying (analyzed in terms of half-emptying times) differed significantly between the two groups (Figure 2D).
Figure 2 A: Response to CCK-8 challenge in healthy controls, patients with functional dyspcpsia and peptic ulcer disease; B: Effects of atropine on CCK-8 infusion in functional dyspeptic patients.
C: Effects of loxiglumide 800mg on CCK-8 infusion in functional dyspeptic patients; D: Solid phase emptying rates in functional dyspeptic patients and healthy controls.
These results indicated that a high proportion of the patients with dysmotility-type FD have an abnormal response to CCK-8 infusion. This abnormal hyperresponsiveness to CCK may account for the genesis of dyspeptic symptoms in FD. It is interesting to note that gastric emptying is also delayed in the FD group. CCK-8 contribution to the slowing of gastric emptying may be due to the stimulation of pyloric contractions and suppression of antral and proximal duodenal motility[34]. The response to CCK-8 challenge may be mediated via a vagovagal reflex arc, resulting in the perturbation of gastric motility. It is possible that CCK-A receptors are involved with the sensory afferent limb, while the cholinergic receptors are responsible for the motor efferent limb of this reflex arc. Ingestion of a meal (especially a fatty meal) leads to the release of CCK that acts locally in a paracrine fashion, and the information generated conveyed to the CNS via vagal afferent fibers. This pathway is also responsible for the feedback inhibition of gastric tone and motility. The gastric motor changes can lead to dyspeptic symptoms in FD patients.
CHOLECYCSTOKININ AND SEROTONIN INTERACTIONS IN FUNCTIONAL DYSPEPSIA
Stallone first provided evidence for the interactions between 5-HT and CCK in the control of feeding behavior[10].
It was demonstrated that while the 5-HT receptor antagonist, metergoline, acted to attenuate the CCK-induced anorexia, the peripheral 5-HT antagonist xylamidine, had no effect. Therefore the inhibitory effect of CCK-8 on food intake depended upon central serotonergic activity[10,35,37]. Studies have shown that dl-fenfluramine can reduce the rate of gastric emptying[21,36]. The reduction in gastric emptying produced by dl-fenfluramine could be blocked by the CCK-A receptor antagonist, devazepide[38]. This suggested that the serotonergic effects on gastric emptying depended upon CCK mechanism. It has also been shown that administration of CCK-8 can excite 5-HT neurons[39], and result in 5-HT being released in the hypothalamic paraventricular nuclei (PVN) and supraoptic nuclei[11,41] both important in central modulation of feeding and gastrointestinal motility. It is likely that endogenous CCK, released post prandially, activates the central 5-HT system, leading to increased release in the PVN. There is evidence that 5-HT acts within the PVN to suppress carbohydrate intake[42]. Activation of central 5-HT mechanisms by CCK-8 may then be involved in the control of satiety. Likewise efferent fibers descending from the CNS to the ENS are important in the control of gastrointestinal functions. Abnormalities involving either the CCK or the 5-HT pathways can result in perturbation of feeding and disruption of gastrointestinal motility. Thus the symptoms encountered in FD may result from a dysfunction of either of the above pathways or interaction between the two.
We examined a group of FD patients (early satiety, upper abdominal pain or discomfort, post-prandial bloating, fullness, nausea and vomiting) who has an abnormal response to the CCK challenge test, a delayed solid-phase gastric emptying and determined their central 5-HT receptor activity using the buspirone stimulation test. Solid phase gastric emptying (half emptying time) were significantly prolonged in the dyspeptic group compared to the controls (mean +/- SE; 90.3 +/- 3.9 min vs 54.6 +/- 5.9 min ). Dyspeptic patients (mean +/- SE; Females: 1450+/- 132.5 Mu/L, Males: 672.5 +/- 84.9 Mu/L) were found to have a significantly higher (P < 0.001) PRL response when compared to the healthy volunteers ( mean +/- SE ; Females: 352 +/- 33.5 Mu/L, Males: 187 +/- 44.3 Mu/L).
These results indicated that FD patients with an abnormal response to CCK-8 challenge also have hypersensitive central 5-HT receptor functioning. However, no correlation can be obtained between PRL response and severity of response to CCK challenge (R= 0.24, NS ). Patient’s response was rated on a Visual Analogue Scale, which is very subjective and patient dependent. A weighted scoring system may be better in assessing patient’s response and may thus show a significant correlation then.
DISCUSSION
Dysfunctional motility[19,43,44], visceral hypersensitivity[45,46], impaired accommodation[47,48] and disordered feeding behaviour are pathophysiological abnormalities that have been described in FD. Each of these disorders may result from primary pathologies arising from the ENS, aberrant signal transmission in the afferent and efferent nerves and abnormal integration within the CNS, which can lead to disruption in peripheral GI sensorimotor functions. Psychological factors, either acute or chronic[12,49,50-54] (stress, negative life events, personality traits or illness seeking behaviors) and presence of certain nutrients in the intestine[55-57] (abnormal exaggerated response) can influence pathways of the brain-gut axis resulting in the observed pathophysiological abnormalities and the resultant symptoms.
It has been reported that agents that modulate 5-HT function may be useful in the treatment of visceral hypersensitivity, either directly on perception or through alteration in visceral tone or motility[59-61]. Visceral sensation can be modified at various levels of the brain-gut axis. It is believed that FD patients perceive visceral stimuli in an abnormal manner[62]. It is still uncertain at what level the dysfunction originates. Altered threshold of visceral mechanoreceptor sensitivity, abnormalities in sensory input transmission and a decrease pain threshold at the CNS may all be responsible. Evidence for central dysfunction exists in that central serotonergic receptors have been shown to be hypersensitive in FD patients[63,64]. This was demonstrated by an exaggerated prolactin response to the buspirone challenge test. Furthermore gastric emptying was also delayed in this group of patients. More importantly the gastric emptying rates and the prolactin response were very highly correlated[63]. This observation suggests that in FD, hypersensitive central 5-HT receptors may be involved in mechanisms giving rise to abnormalities of gastric emptying.
Central 5-HT pathways are also implicated in the mechanisms of nociception. Descending 5-HT systems in the supraspinal and spinal pathways are involved in the control of nociception and thus can modify visceral perception and awareness of pain. Pathways arising from the brainstem and projecting to the dorsal horn of the spinal cord can alter the sensitivity of the dorsal horn neurons and thus centrally control the intensity of perception of pain [65,66]. Similar mechanisms have been implicated in the increase perception to visceral sensation[67].
The neuropeptide CCK act on vagal gastric afferents to inhibit gastric emptying and decrease food intake[29,34,70]. CCK receptors have been demonstrated on gastric afferent nerves[71]. Studies have compared various gastrointestinal responses to exogenous and endogenous CCK at plasma levels measured after a meal or intestinal nutrients. Similar plasma CCK levels after endogenous release and exogenous administration, appear to cause equal degrees of gallbladder contraction[73] and inhibition of gastric emptying in man[72,74]. Animal studies suggest that CCK acts via a vagal afferent pathway to decrease gastric motility and this pathway is important in mediating CCK induced delay in gastric emptying[32]. By acting on the vagal afferents, CCK is seen as part of the mechanism by which information from the peripheral GI tract is conveyed centrally to the CNS to modulate feeding behaviour and the autonomic control of the digestive tract. The abnormal response to CCK octapeptide infusion observed in FD patients may be due to sensitized gastric mechanoreceptors or modification in the transmission of sensory impulse. The satiety effect of CCK depends on intact central 5-HT function[10]. It may
be possible that hypersensitive central 5-HT receptors may up-regulate the sensory receptors in the gut wall that then respond abnormally to the CCK infusion. Interestingly postprandial CCK levels in a normal healthy person are not significantly different from FD patients. It is conceivable that the gut in a sensitized subject may react more vigorously to external stimuli (stress or presence of nutrients).
SUMMARY
An interaction between the CCK and serotonergic pathways may explain the constellation of symptoms observed in FD. An altered central 5-HT receptors functioning can reset the threshold for sensitivity to CCK (more sensitive to stimuli) in the gut receptors. Consequently, a normal peripheral stimulus (presence of nutrients in the small intestine or food in stomach) will result in an abnormal afferent input that leads to a distortion of GI perception and function[68,69], either through intrinsic ENS reflexes or through the autonomic or central nervous systems. This will also help to explain how stress and psychological factors may precipitate dyspeptic symptoms in FD patients. Depending on the presence and degree of the stressor at the time, different dyspeptic symptoms will predominate.
S- Editor Guo SY L- Editor Zhang JZ E- Editor Wu M