Published online Feb 21, 2006. doi: 10.3748/wjg.v12.i7.1049
Revised: July 2, 2005
Accepted: August 26, 2005
Published online: February 21, 2006
AIM: To investigate the in vivo effect of atrial natriuretic peptide (ANP) and its signaling pathway during orthotopic rat liver transplantation.
METHODS: Rats were infused with NaCl, ANP (5 µg/kg), wortmannin (WM, 16 µg/kg), or a combination of both for 20 min. Livers were stored in UW solution (4 °C) for 24 h, transplanted and reperfused. Apoptosis was examined by caspase-3 activity and TUNEL staining. Phosphorylation of Akt and Bad was visualized by Western blotting and phospho-Akt-localization by confocal microscopy.
RESULTS: ANP-pretreatment decreased caspase-3 activity and TUNEL-positive cells after cold ischemia, indicating antiapoptotic effects of ANP in vivo. The antiapoptotic signaling of ANP was most likely caused by phosphorylation of Akt and Bad, since pretreatment with PI 3-kinase inhibitor WM abrogated the ANP-induced reduction of caspase-3 activity. Interestingly, analysis of liver tissue by confocal microscopy showed translocation of phosphorylated Akt to the plasma membrane of hepatocytes evoked by ANP.
CONCLUSION: ANP activates the PI-3-kinase pathway in the liver in vivo leading to phosphorylation of Bad, an event triggering antiapoptotic signaling cascade in ischemic liver.
- Citation: Grutzner U, Keller M, Bach M, Kiemer AK, Meissner H, Bilzer M, Zahler S, Gerbes AL, Vollmar AM. PI 3-kinase pathway is responsible for antiapoptotic effects of atrial natriuretic peptide in rat liver transplantation. World J Gastroenterol 2006; 12(7): 1049-1055
- URL: https://www.wjgnet.com/1007-9327/full/v12/i7/1049.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i7.1049
Ischemia reperfusion injury (IRI) is responsible for primary liver dysfunction and failure after transplantation or liver resection[1,2]. Therefore, reduction of IRI is of high clinical interest. In order to develop preventive strategies, knowledge of the mechanisms leading to cell death after IR is pivotal. The mode of cell death after IR is originally considered to be necrotic, but there is increasing evidence that apoptosis plays a role[3-5]. Apoptosis is a highly regulated process resulting in nuclear fragmentation and condensation as well as disintegration of cells into apoptotic bodies without release of cell contents. Subsequently, tissue macrophages such as Kupffer cells phagocytose the apoptotic bodies. In contrast to apoptosis, necrosis is a passive process characterized by cell lysis. Usually, necrosis of cells causes a local inflammation. However, it has been recognized that apoptotic cells also induce inflammatory response by triggering neutrophil accumulation into the liver[6]. Thus antiapoptotic strategies convey protection against hepatic IRI. Apoptosis occurs during the early phase of reperfusion after liver ischemia and after transplantation[3,5,7]. However, the extent to which it occurs and most importantly its relevance in IRI is still a matter of debate, which might be due to different experimental models for liver IRI[5,7].
Atrial natriuretic peptide (ANP) belongs to the natriuretic peptide family including a number of peptides possessing vasodilating, hypotensive and natriuretic activities[8]. In addition to this cardiovascular profile there is ample evidence that ANP exerts cytoprotective actions in various cells and organs besides the liver[9-12].
In the model of isolated perfused rat liver we observed a rather low amount of apoptotic cells after cold ischemia compared to other reports [3]. We could further show that pretreatment of liver with ANP could lead to a significant reduction of hepatocyte apoptosis and necrosis [3]. ANP-treatment has also been shown to be beneficial in isolated rat hepatocytes exposed to hypoxia [13].
This study was to examine the effect of ANP-preconditioning in an in vivo model of liver IRI rat orthotopic liver transplantation IRI)and the mechanism of cell protection.
Rat ANP, wortmannin and protein kinase A assay kit were purchased from Calbiochem/Novabiochem (Bad Soden, Germany), Complete® was from Roche Diagnostics GmbH (Mannheim, Germany). Rabbit anti-Akt antibody, monoclonal mouse anti-phospho Akt (Ser473) antibody, rabbit anti-phospho Akt (Ser473) antibody, rabbit anti-phospho Bad (Ser136) antibody, rabbit anti-phospho Bad (Ser112) antibody and rabbit anti-Bad antibody were from New England Biolabs GmbH (Frankfurt, Germany). Goat anti-rabbit-IgG was from Dianova (Hamburg, Germany) and goat anti-mouse-IgG1 antibody conjugated to horseradish peroxidase was from BIOZOL (Eching, Germany). The secondary antibody (Alexa Fluor 488 goat anti-mouse) and rhodamine-conjugated phalloidin were from MoBiTec (Göttingen, Germany), [γ32P]-ATP was from Amersham-Pharmacia (Braunschweig, Germany) and the ApopTaq® peroxidase in situ apoptosis detection kit was from Intergen (New York, USA). All other materials were purchased from either Sigma (Taufkirchen, Germany) or VWR International™ (Munich, Germany).
Syngeneic male Lewis rats (donors: 207±12 g; recipients: 276±18 g) were purchased from Charles River Wiga (Sulzfeld, Germany) and housed in a temperature-and humidity-controlled room under a constant 12 h light/dark cycle. Animals had free access to water and rat chow (SSniff, Soest, Germany), but were fasted with free access to water 12 h prior to the operation. All animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” published by the National Institute of Health (NIH publication 86-23 revised 1985). Studies were performed with the permission of the government authorities.
In transplantation experiments donor animals obtained either an intravenous infusion of NaCl or ANP (5 µg/kg). For investigation of signalling pathways additional rats were treated with wortmannin (16 µg/kg), DMSO (0.1%), or a combination of ANP and wortmannin for 20 min prior to hepatectomy. Blood pressure and heart rate were continuously monitored by a catheter in the carotid artery. A jugular venous catheter served to apply substances and substitute plasma volume. Body temperature was kept at 36.5 - 37.5 °C by means of a heating pad. Donor livers were preserved by retrograde aortal flush with 10 mL UW-solution and stored at 4 °C for 24 h. Before implantation, the livers were rinsed with cold Ringer’s solution (10 mL) via the portal vein at a hydrostatic pressure of 10 cm H2O. Orthotopic liver transplantation was performed as previously described [4]. Grafted livers were simultaneously reperfused after completion of the arterial anastomosis. Portal clamping time was less than 20 min in all experiments.
The graft’s common bile duct was cannulated with a PE-tube and bile was collected with its volume determined. Plasma samples (400 µL) were obtained from the recipient before hepatectomy and 60 and 120 min after reperfusion of the transplanted liver. The volume of the blood drawn was replaced by saline. After starting reperfusion, rats received 1.0 mL of albumin (5%) and 0.5-1.0 mL sodium bicarbonate to maintain blood pressure and physiological pH. To avoid fluid loss and drying of the liver, the abdominal cavity was covered with Saran wrap. After 20 min preconditioning, 24 h cold ischemia, or 120 min reperfusion, organs were placed in 4% paraformaldehyde or snap frozen in liquid nitrogen.
Liver samples were fixed in paraformaldehyde, embedded in paraffin, and cut into 6µm sections. TUNEL positive cells [15] were determined by staining of liver sections with the ApopTaq® peroxidase in situ apoptosis detection kit according to the manufacturer’s instructions. The Apop Taq® staining results were evaluated in combination with morphological criteria. For counting of apoptotic cells, an area of 1.96 mm2 (approximately 4000 hepatocytes) was observed by a Leitz Laborlux S microscope.
For analysis of pAkt snap frozen livers were cut into 6 µm sections. Slices were dried overnight at room temperature. Staining of pAkt was performed using the monoclonal anti-pAkt antibody in 0.2% BSA as the primary antibody (1 h, RT) and Alexa Fluor 488 goat anti-mouse antibody (1 h, RT). Sections were observed under confocal laser microscope (LSM 510 Meta, Zeiss, Jena, Germany). All histological evaluations were performed in a blinded fashion.
Fifty mg of liver tissue was homogenized in 1.5 mL of lysis buffer (50 mM Tris-HCl, 5 mM EGTA, 1 mM PMSF, 1 mM Na-vanadate pH 7.0, 40 µL Complete®) containing 1% Triton® X-100 (Roth, Karlsruhe, Germany) with a dounce homogenizer. After centrifugation of samples (14 000 r/min, 15 min, 4 °C), the supernatant was diluted with SDS-containing sample buffer. Samples were stored at -20 °C for Western blotting.
Liver tissue was homogenized as described above, 100 µg of protein in 100 µL lysis buffer was incubated with 2.5 µL of primary antibody (rabbit anti-Bad) shaking overnight at 4 °C. The antibody-antigen complex was precipitated by incubation with 10 µL of washed agarose-A-beads for 2 h, followed by centrifugation. The beads were washed three times with cold lysis buffer, and resuspended in 40 µL of 3× SDS-containing sample buffer. After addition of 40 µL 1× sample buffer samples were boiled at 95 °C for 5 min followed by centrifugation.
Homogenized livers were treated as described before. Proteins in total liver homogenates or immunoprecipitates were separated by SDS-Page and visualized after elec-trophoretical transfer via binding of specific primary and HRP-conjugated secondary antibodies followed by chemoluminescent detection. Detection and quantification were performed with a Kodak image station (NEN, Cologne, Germany).
A commercial PKA assay kit was used. Samples (100 µg) were homogenized in “extraction buffer” and centrifuged as described in the manufacturer’s manual. Supernatants were used for measurement of phosphorylation activity of PKA by in vitro phosphorylation of the specific peptide substrate kemptide with [γ32P]-ATP.
After homogenization of 100 mg liver tissue in 1 mL lysis buffer (25 mM HEPES, 5 mM MgCl2, 1 mM EGTA, pH 7.5, Complete®), samples were centrifuged (14 000 r/min, 10 min, 4 °C). Caspase-3 like activity in the supernatants was determined as previously reported[3]. Generation of free fluorescent 7-amino-4-trifluoro-methylcoumarin was measured with a Fluostar analyser (BMG GmbH, Offenburg, Germany).
All experiments were performed at least three times per treatment group. Results were expressed as mean ± SE. Statistical significance between groups was determined with one sample or Student’s t test using GraphPad Prism® Version 3.02 for Windows (GraphPad Software Inc., San Diego, USA). P < 0.05 was considered statistically significant.
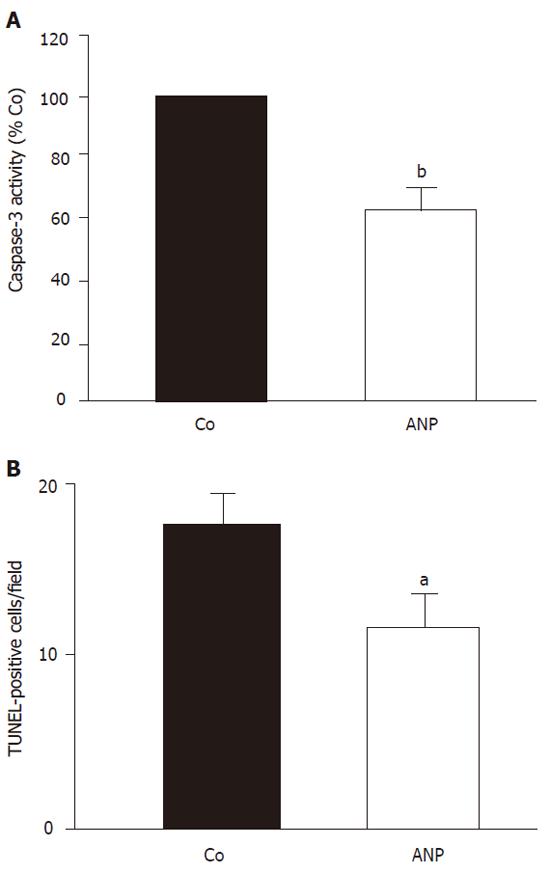
Apoptotic processes were monitored by measuring caspase-3 activity and TUNEL staining of liver sections. After 24 h cold ischemia, an increase of hepatic caspase-3 activity (40.5%) compared to sham operated animals was observed, which sustained after 2 h reperfusion (25%). Figure 1A demonstrates a significant attenuation of caspase-3 activity by ANP-preconditioning, which was also observed in 2 h reperfused organs after 24 h cold storage.
TUNEL-staining of corresponding liver sections confirmed these data. After 24 h cold ischemia apoptotic cells were most prominent. Both hepatocytes and endothelial cells showed positive TUNEL staining combined with characteristic apoptotic morphology.
Figure 1B shows that preconditioning of donor livers with ANP (5 µg/kg) significantly decreased the number of TUNEL-positive cells after 24 h cold ischemia. A similar although less pronounced effect was observed after 2 h reperfusion.
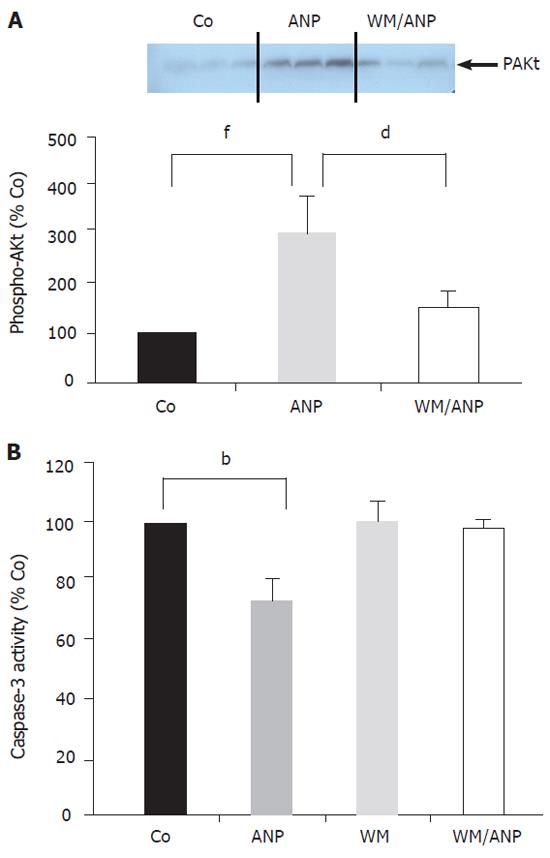
Infusion of ANP (5 µg/kg) for 20 min into the donor animals resulted in a marked increase of phosphorylated Akt (pAkt) in the liver, which was abrogated in the presence of the PI-3-Kinase inhibitor wortmannin (Figure 2A).
Figure 2B demonstrates that application of wortmannin (16 µg/kg) prior to ANP infusion completely blocked the inhibition of caspase-3 activity by ANP seen in ischemic liver tissue (24 h). Thus, the PI-3-Kinase pathway was intimately involved in the antiapoptotic effect of ANP-preconditioning.
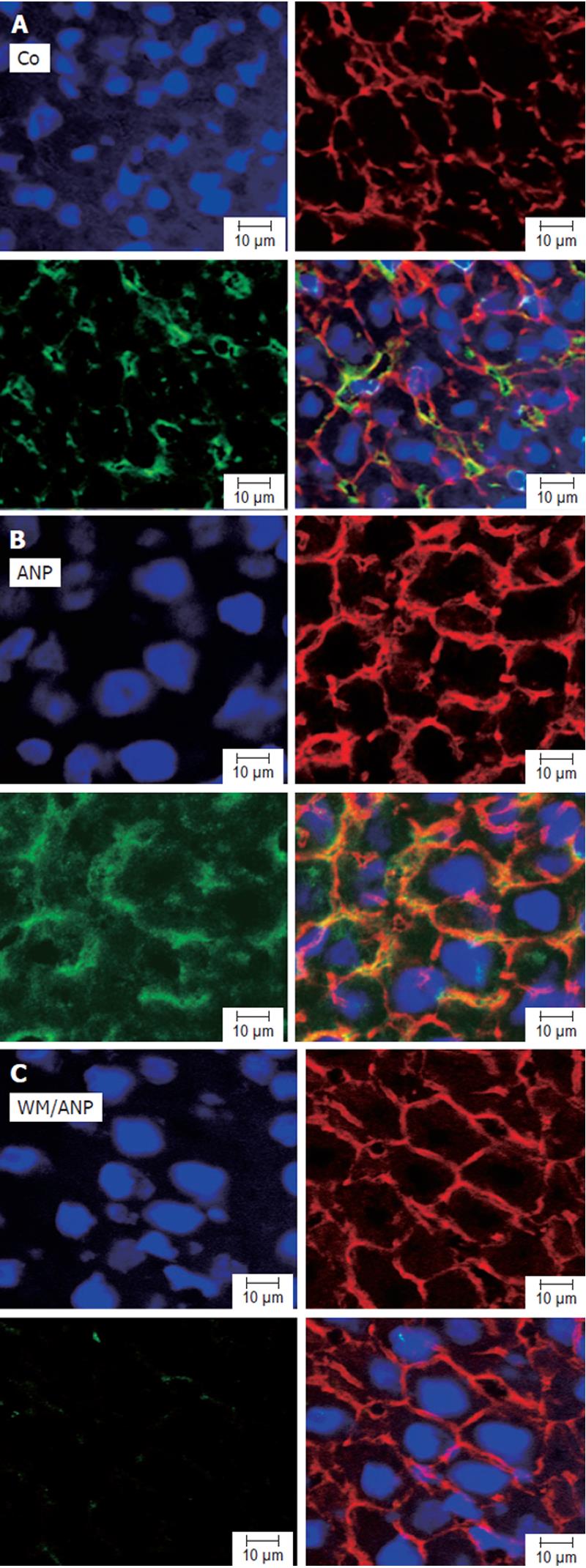
Analysis of pAkt in liver tissue by confocal microscopy confirmed that ANP-pretreatment could lead to a marked increase of pAkt. Interestingly, confocal microscopy revealed a strong localization of pAkt at the plasma membrane of hepatocytes (Figures 3A, 3B). Cotreatment of animals with wortmannin abrogated both the increase as well as the plasma membrane localization of pAkt induced by ANP (Figure 3C).
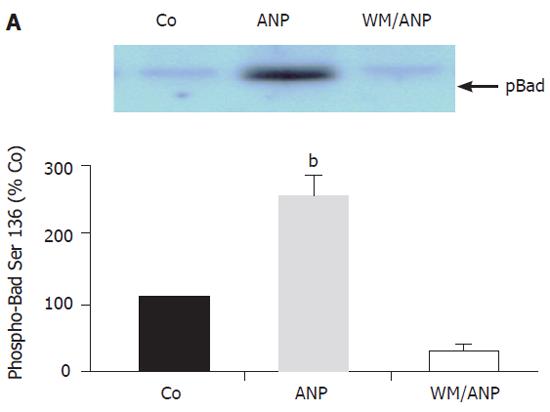
To further examine the antiapoptotic signalling in the liver induced by ANP in vivo, a pivotal downstream target of pAkt (the Bad protein) was investigated. A representative Western blot indicated that Bad was phosphorylated at Ser136 by pAkt induced by ANP (Figure 4). ANP in vivo did not activate PKA and consequently phosphorylated Bad at Ser112 (Figure 5A, Figure 5B).
This study showed that pretreatment of rats with ANP could reduce apoptotic processes mainly occurring after 24 h cold ischemia in orthotopic rat liver transplantation. In this setting ANP activated the PI-3-Kinase/Akt pathway leading to Bad phosphorylation and inhibition of caspase-3 activity, revealing that the PI-3-Kinase/Akt pathway is an important cytoprotective mechanism in IRI in vivo.
PI-3 Kinases are heterodimers composed of a catalytic subunit (p110) and a regulatory subunit (p85) activated by receptors with protein tyrosine kinase activity as well as G-protein-coupled receptors[16,17]. Various growth factors such as IGF-1 activate PI-3-Kinase leading to the generation of phosphoinositides [PI(3,4,5)P3] and activation of Akt, a 57 kDa Ser/Thr kinase. PI-3-kinase regulates Akt kinase activity by generated phospholipids[16,17]. One mechanism is through the direct binding of phosphoinositides to the pH domain of Akt, which seems to be critical for the in vivo activation of Akt. A consequence of Akt binding to phospholipids is the translocation of Akt from cytoplasm to the inner surface of plasma membrane[16,17]. This translocation which we also observed in hepatocytes pretreated with ANP, is important for Akt activation. In fact, protection from apoptotic stimuli in cardiomyocytes and liver has been demonstrated using adenovirally mediated gene transfer of membrane-targeted constitutively activated Akt [18-21]. However, the constitutively activated form of Akt targeted to the cell membrane results in supraphysiological levels of kinase activity having the potential to induce oncogenic transformation. These issues of feasibility and safety concerning an Akt gene therapy are certainly much less relevant in a setting of Akt activation by endogenous stimulators such as ANP or others[22-25].
Up to now, protective effects of ANP against IRI have only been demonstrated in ex vivo and in vitro settings such as isolated perfused rat liver[3], isolated liver cells[13,26], and isolated ischemic heart[27]. The underlying mechanisms seem to be highly dependent on the experimental model used. For instance, as recently described in the ex vivo model, ANP protects hepatocytes from apoptosis after cold ischemia by activation of PKA[28]. ANP treatment of hypoxic isolated rat hepatocytes confers its cytoprotection via PKC σ and activation of p38 MAPK[13]. This difference might be explained by different modes of cell death analyzed. Focusing on apoptotic cell death, our data showed that the in vivo setting revealed a different protective signalling, i.e. activation of the PI-3-kinase/Akt pathway compared to the isolated perfused rat liver subjected to IR. Accordingly, activation of Akt can also protect livers against warm IRI[21]. Importantly, both pathways, PKA activation and Akt activation, lead to phosphorylation and inactivation of Bad protein, suggesting that this protein plays a key role in ANP-induced prevention of ischemia-induced apoptosis.
Bad is a member of the family of Bcl-2 proteins functioning as apoptosis-regulating factors[29,30]. Bad in its unphosphorylated form binds to and inactivates antiapoptotic proteins such as Bcl-2 and Bcl-XL leading to proapoptotic functions. Phosphorylation of Bad at either of the two potential sites (Ser112, Ser136) causes Bad to dissociate from Bcl-2 or Bcl-XL respectively and to associate instead with cytoplasmic 14-3-3 proteins preventing Bad from dephosphorylation[16]. Akt phosphorylates Bad at Ser136 in vivo and mutation of Bad Ser136 to alanine abrogates the blocking effect of Akt in Bad-induced apoptosis. In any case, Bad plays a key role in the Akt survival signalling since Bad phosphorylation leads to prevention of cytochrome C release from mitochondria, a hallmark of apoptosis induction[16].
We would like to point out that besides acting through Bad phosphorylation Akt brings about its function also by a variety of other downstream targets, such as caspase-9, forkhead family members or the eNOS enzyme[16]. Akt-mediated phosphorylation and activation of the eNOS enzyme have been reported to be involved in the antiapoptotic effect of insulin in myocardial IR[25]. In line with this report a blockade of the NO synthase worsens hepatic apoptosis and liver transplant preservation injury[31]. Since we did not observe increased phosphorylation of eNOS in ANP-pretreated livers (data not shown), this mechanism does not contribute to the antiapoptotic action of ANP.
In conclusion, Bad inactivation by phosphorylation should be considered as an important therapeutic target preventing IRI during liver transplantation.
The authors thank Andrea Sendelhofert and Anja Heier (Institute of Pathology) for their excellent support in histological analysis.
S- Editor Wang J L- Editor Wang XL E- Editor Wu M
| 1. | Bilzer M, Gerbes AL. Preservation injury of the liver: mechanisms and novel therapeutic strategies. J Hepatol. 2000;32:508-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 131] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 2. | Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15-G26. [PubMed] |
| 3. | Gerwig T, Meissner H, Bilzer M, Kiemer AK, Arnholdt H, Vollmar AM, Gerbes AL. Atrial natriuretic peptide preconditioning protects against hepatic preservation injury by attenuating necrotic and apoptotic cell death. J Hepatol. 2003;39:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Rüdiger HA, Graf R, Clavien PA. Liver ischemia: apoptosis as a central mechanism of injury. J Invest Surg. 2003;16:149-159. [PubMed] |
| 5. | Gujral JS, Bucci TJ, Farhood A, Jaeschke H. Mechanism of cell death during warm hepatic ischemia-reperfusion in rats: apoptosis or necrosis. Hepatology. 2001;33:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 298] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 6. | Faouzi S, Burckhardt BE, Hanson JC, Campe CB, Schrum LW, Rippe RA, Maher JJ. Anti-Fas induces hepatic chemokines and promotes inflammation by an NF-kappa B-independent, caspase-3-dependent pathway. J Biol Chem. 2001;276:49077-49082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 166] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Clavien PA, Rüdiger HA, Selzner M. Mechanism of hepatocyte death after ischemia: apoptosis versus necrosis. Hepatology. 2001;33:1555-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Vollmar AM, Kiemer AK. Immunomodulatory and cytoprotective function of atrial natriuretic peptide. Crit Rev Immunol. 2001;21:473-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Kiemer AK, Weber NC, Fürst R, Bildner N, Kulhanek-Heinze S, Vollmar AM. Inhibition of p38 MAPK activation via induction of MKP-1: atrial natriuretic peptide reduces TNF-alpha-induced actin polymerization and endothelial permeability. Circ Res. 2002;90:874-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Kiemer AK, Hartung T, Vollmar AM. cGMP-mediated inhibition of TNF-alpha production by the atrial natriuretic peptide in murine macrophages. J Immunol. 2000;165:175-181. [PubMed] |
| 11. | Fürst R, Brueckl C, Kuebler WM, Zahler S, Krötz F, Görlach A, Vollmar AM, Kiemer AK. Atrial natriuretic peptide induces mitogen-activated protein kinase phosphatase-1 in human endothelial cells via Rac1 and NAD(P)H oxidase/Nox2-activation. Circ Res. 2005;96:43-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Kiemer AK, Fürst R, Vollmar AM. Vasoprotective actions of the atrial natriuretic peptide. Curr Med Chem Cardiovasc Hematol Agents. 2005;3:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Carini R, De Cesaris MG, Splendore R, Domenicotti C, Nitti MP, Pronzato MA, Albano E. Mechanisms of hepatocyte protection against hypoxic injury by atrial natriuretic peptide. Hepatology. 2003;37:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Schauer RJ, Kalmuk S, Gerbes AL, Leiderer R, Meissner H, Schildberg FW, Messmer K, Bilzer M. Intravenous administration of glutathione protects parenchymal and non-parenchymal liver cells against reperfusion injury following rat liver transplantation. World J Gastroenterol. 2004;10:864-870. [PubMed] |
| 15. | Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6746] [Cited by in RCA: 7191] [Article Influence: 217.9] [Reference Citation Analysis (0)] |
| 16. | Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905-2927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3190] [Cited by in RCA: 3243] [Article Influence: 124.7] [Reference Citation Analysis (0)] |
| 17. | Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis. 2004;9:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 835] [Cited by in RCA: 955] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 18. | Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 632] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 19. | Matsui T, Li L, del MonteF Y, Franke TF, Hajjar RJ, Rosenzweig A. Adenoviral gene transfer of activated phosphatidylinositol 3'-kinase and Akt inhibits apoptosis of hypoxic cardiomyocytes in vitro. Circulation. 1999;100:2373-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 258] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 20. | Miao W, Luo Z, Kitsis RN, Walsh K. Intracoronary, adenovirus-mediated Akt gene transfer in heart limits infarct size following ischemia-reperfusion injury in vivo. J Mol Cell Cardiol. 2000;32:2397-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 103] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Harada N, Hatano E, Koizumi N, Nitta T, Yoshida M, Yamamoto N, Brenner DA, Yamaoka Y. Akt activation protects rat liver from ischemia/reperfusion injury. J Surg Res. 2004;121:159-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Okumura H, Nagaya N, Itoh T, Okano I, Hino J, Mori K, Tsukamoto Y, Ishibashi-Ueda H, Miwa S, Tambara K. Adrenomedullin infusion attenuates myocardial ischemia/reperfusion injury through the phosphatidylinositol 3-kinase/Akt-dependent pathway. Circulation. 2004;109:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Müller C, Dünschede F, Koch E, Vollmar AM, Kiemer AK. Alpha-lipoic acid preconditioning reduces ischemia-reperfusion injury of the rat liver via the PI3-kinase/Akt pathway. Am J Physiol Gastrointest Liver Physiol. 2003;285:G769-G778. [PubMed] |
| 24. | Schulze-Bergkamen H, Brenner D, Krueger A, Suess D, Fas SC, Frey CR, Dax A, Zink D, Büchler P, Müller M. Hepatocyte growth factor induces Mcl-1 in primary human hepatocytes and inhibits CD95-mediated apoptosis via Akt. Hepatology. 2004;39:645-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Gao F, Gao E, Yue TL, Ohlstein EH, Lopez BL, Christopher TA, Ma XL. Nitric oxide mediates the antiapoptotic effect of insulin in myocardial ischemia-reperfusion: the roles of PI3-kinase, Akt, and endothelial nitric oxide synthase phosphorylation. Circulation. 2002;105:1497-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 365] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 26. | Kiemer AK, Baron A, Gerbes AL, Bilzer M, Vollmar AM. The atrial natriuretic peptide as a regulator of Kupffer cell functions. Shock. 2002;17:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Sangawa K, Nakanishi K, Ishino K, Inoue M, Kawada M, Sano S. Atrial natriuretic peptide protects against ischemia-reperfusion injury in the isolated rat heart. Ann Thorac Surg. 2004;77:233-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Kulhanek-Heinze S, Gerbes AL, Gerwig T, Vollmar AM, Kiemer AK. Protein kinase A dependent signalling mediates anti-apoptotic effects of the atrial natriuretic peptide in ischemic livers. J Hepatol. 2004;41:414-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1249] [Cited by in RCA: 1283] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 30. | Downward J. PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol. 2004;15:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 600] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 31. | Yagnik GP, Takahashi Y, Tsoulfas G, Reid K, Murase N, Geller DA. Blockade of the L-arginine/NO synthase pathway worsens hepatic apoptosis and liver transplant preservation injury. Hepatology. 2002;36:573-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |