Published online Feb 14, 2006. doi: 10.3748/wjg.v12.i6.961
Revised: May 1, 2005
Accepted: October 20, 2005
Published online: February 14, 2006
AIM: To assess the role of transforming growth factor-β1 (TGF-β1), vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) in the pathogenesis of fibrosis associated with chronic hepatitis C (CHC) and to evaluate the influence of the antiviral therapy on above parameter levels depending on the treatment results (complete response or no response).
METHODS: Study group included 100 patients with CHC, in whom fibrosis in liver specimens was assessed (Scheuer fibrosis score: 1-4 points). Control group included 30 subjects with antibodies anti-HCV present and persistently normal ALT level, without fibrosis (Scheuer fibrosis score: 0 points). Concentration of studied parameters was assayed in the serum by immunoenzymatic method before and after the therapy with interferon alpha-2b and ribavirin.
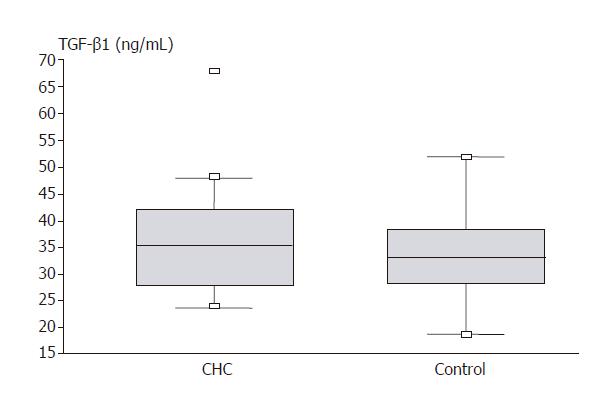
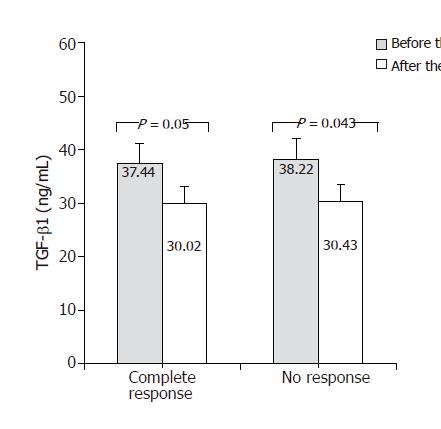
RESULTS: TGF-β1 levels were significantly higher in the study group compared to the control group (35.89 vs 32.37 ng/mL; P = 0.023). Such differences were not found in VEGF and bFGF levels. In patients showing complete response (negative HCV RNA and normal ALT level), significant increase in VEGF (112.8 vs 315.03 pg/mL; P < 0.05) and bFGF (2.51 vs 15.79 pg/mL; P = 0.04) levels were found. Significant decrease in TGF-β1 level was observed both in responders (37.44 vs 30.02 ng/mL; P=0.05), and in non-responders (38.22 vs 30.43 ng/mL; P = 0.043). bFGF levels before the treatment were significantly lower (2.51 vs 5.94 pg/mL; P = 0.04), and after the treatment significantly higher (15.79 vs 4.35 pg/mL; P = 0.01) in patients with complete response than in those with no response.
CONCLUSION: Among the analyzed parameters TGF-β1 seems to play the most important role in the pathogenesis of fibrosis in CHC. Levels of this factor are significantly lower in subjects who do not have fibrosis developed in them. Good therapeutic effect in CHC patients is associated with significant changes in TGF-β1, VEGF, and bFGF levels. bFGF seems to have the highest usefulness in the prognosis of treatment efficacy.
- Citation: Janczewska-Kazek E, Marek B, Kajdaniuk D, Borgiel-Marek H. Effect of interferon alpha and ribavirin treatment on serum levels of transforming growth factor-β1, vascular endothelial growth factor, and basic fibroblast growth factor in patients with chronic hepatitis C. World J Gastroenterol 2006; 12(6): 961-965
- URL: https://www.wjgnet.com/1007-9327/full/v12/i6/961.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i6.961
Hepatitis C virus (HCV) is among the main causes of chronic liver diseases. Consequences of chronic HCV infection and high treatment costs make it a significant worldwide epidemiological, clinical, and social problem.
No pathological fibrosis is found during the initial stages of the disease, however in course of its development, the process increases, and the final stage is cirrhosis[1]. Fibrosis as an after-effect of chronic liver inflammation is compared to wound healing[2,3]. It is supposed that growth factors taking part in classical reaction of wound healing may play an important role in similar repair process occurring in the liver. Nature of fibrogenesis is excessive deposition of collagen and other extracellular matrix (ECM) components in the organ parenchyma. ECM protein synthesis takes place mainly in hepatic stellate cells[2-4]. Inhibition and regression of fibrosis is connected with the limitation of excessive ECM protein production and degradation of components already produced[5,6]. Growth factors may be detected in blood that is circulated. There are premises that they could serve as markers of fibrosis and inflammatory activity, and could be useful in non-invasive hepatological diagnostics, especially in those situations when there are contraindications for liver biopsy[2].
Among several known isomeric forms of transforming growth factor-β (TGF-β), the most significant role in pathogenesis of liver fibrosis is played by TGF-β1. Main source of TGF-β1 in the liver are activated stellate cells and Kupffer cells. The role of this factor in fibrosis development has been studied for several years[5-10]. Results of those studies confirm the existence of a connection between the factor’s concentration in the blood and progression of liver lesions[8-10], as well as reduction of those concentrations following treatment leading to the decrease of fibrosis stage[10].
Vascular endothelial growth factor (VEGF) stimulates angiogenesis both in regular conditions and in neoplastic processes[11-15]. It has been verified to be closely involved in the development of hepatocellular carcinoma and hepatic metastases[15].
Basic fibroblast growth factor (bFGF, FGF-2) is found in almost all tissues of mesoderm and endoderm origins. Studies conducted on animal models show participation of the cytokine in regeneration of tissues, angiogenesis and in wound healing process[16,17]. A role played by bFGF in the processes of liver regeneration and fibrosis is not known. However, mentioned actions of the factor towards other tissues suggest possibility of its influence on fibrosis[18].
The only way of liver fibrosis prevention known so far is effective causal treatment of hepatitis[19]. Preferred treatment for patients with CHC is a combined therapy with interferon alpha and ribavirin. In antiviral treatment recombined interferons alpha (alpha 2a and alpha 2b) are used, recently usage of pegylated interferons has become a standard. Ribavirin is an analog of guanosine. Apart from producing a virostatic effect, the drug shows probably also immunomodulative activity. Aim of CHC treatment is inhibition of HCV replication shown by the disappearance of HCV RNA in peripheral blood, which may lead to the decrease of inflammatory activity and stage of fibrosis found in histopathological examination.
We undertook this study to assess the role of TGF-β1, VEGF, and bFGF in the pathogenesis of liver fibrosis, evaluate effect of antiviral treatment on concentration of these factors depending on the effect of the treatment (full response or lack of response), and define a biochemical marker useful for prognosis of antiviral treatment efficacy.
Ethical approval for the study was obtained from the Bioethical Committee of Medical University of Silesia. All serum and tissue samples were taken with patients’ informed consent.
Study group included 100 patients (48 females and 52 males), aged between 19 and 64 (average 42 ± 3 years) with chronic hepatitis C (CHC).
The diagnosis of CHC was confirmed by the presence of serum HCV-RNA assayed with reverse transcription polymerase chain reaction (RT-PCR) method (Amplicor v.2 Roche Diagnostic test) and persistently elevated alanine aminotransferase (ALT) level for at least 6 mo. All studied patients had liver biopsy performed with Hepafix kit (B. Braun, Melsungen AG, Germany). Grade of inflammatory activity and stage of fibrosis were assessed according to Scheuer numeric scale[20]. Patients with pathologically confirmed cirrhosis met the criteria for the A class in Child-Pugh scale.
Control group included 30 patients with present anti-HCV antibodies and normal ALT activity, with no or minimal necroinflammatory activity and no pathological fibrosis found in liver specimen pathological examination.
All patients starting the therapy and those belonging to the control group had blood collected in the beginning of the study. Blood samples were also taken from the study group subjects after the completion of therapy. Obtained serum was frozen at -70 °C until assay.
TGF-β1, VEGF, and bFGF concentrations were measured by immunoenzymatic method (Quantikine Immunoassay, R&D Systems Inc, Minneapolis, USA).
In therapy of CHC patients interferon alpha 2b and ribavirin were used (RebetronTM, Schering-Plough). Interferon alpha was administered at 3 MU dose thrice a week, ribavirin at a dose of 1 200 mg/d for patients ≥75 kg of body weight, or at a dose of 1 000 mg/day for patients with body weight lower than 75 kg. Treatment was continued for 48 wk.
Serum levels of TGF-β1, VEGF, and bFGF were measured before and after the treatment.
In this study, comparison was made between the two groups of patients - those with complete sustained response to applied antiviral treatment (undetectable HCV RNA and normalization of ALT activity 6 mo after the end of therapy) and lack of this response (continuous elevated aminotransferase activity and presence of HCV RNA after conclusion of therapy).
Values were expressed as the mean ± SE. Hypothesis of studied parameters, mean values equality between the compared groups were assessed using Student’s t test for related and unrelated variables preceded with Fisher’s test for variance homogeneity and Mann-Whitney U test. P < 0.05 was considered statistically significant.
General characteristics of patients with CHC and subjects belonging to the control group are presented in Table 1.
| n | Male/female | Age (yr) | ALT (U/L) | AST (U/L) | Alkaline phosphatase (U/L) | Bilirubin (µmol/L) | |
| CHC | 100 | 52/48 | 42.08 ± 3 | 108.7 ± 10.12 | 75.6 ± 8.76 | 141.7 ± 6.78 | 18.9 ± 2.42 |
| Control | 30 | 16/14 | 40.79 ± 2 | 21.3 ± 1.92 | 18.5 ± 1.73 | 136.4 ± 6.56 | 13.8 ± 1.76 |
Figure 1 shows the results of TGF-β1 assays in compared groups. In group of patients with CHC, there was significantly higher serum concentration of the factor compared to control group (35.89 ± 1.28 vs 32.37 ± 1.41 ng/mL; P = 0.023).
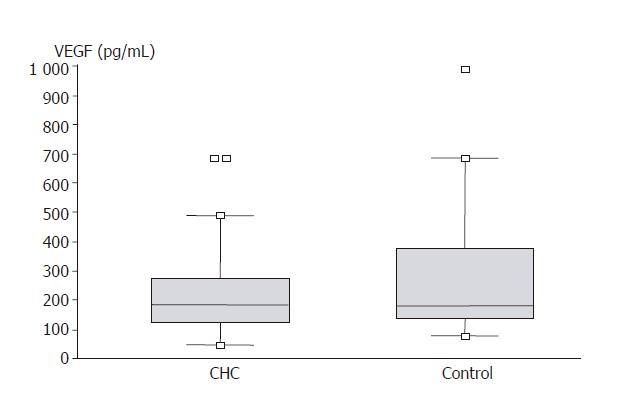
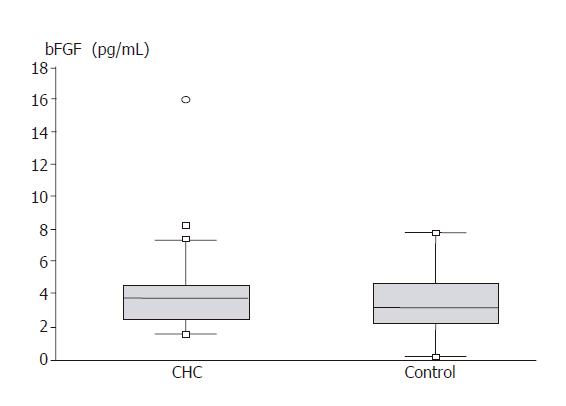
VEGF serum levels in compared groups are demonstrated in Figure 2. No significant differences of this growth factor concentration were found between the hepatitis C group and control (231.84 ± 24.55 vs 262.89 ± 33.99 pg/mL). Figure 3 presents bFGF concentration. No significant differences were found between CHC group and control (4.35 ± 0.58 vs 3.30 ± 0.39 pg/mL).
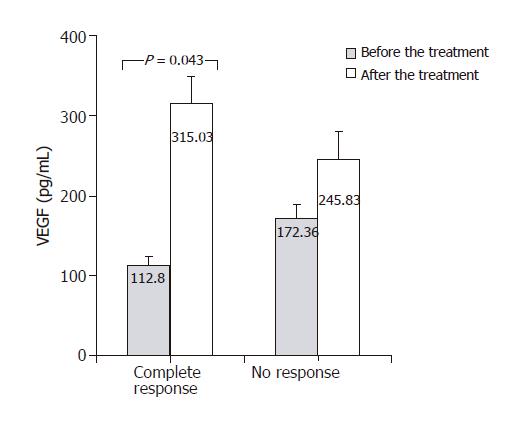
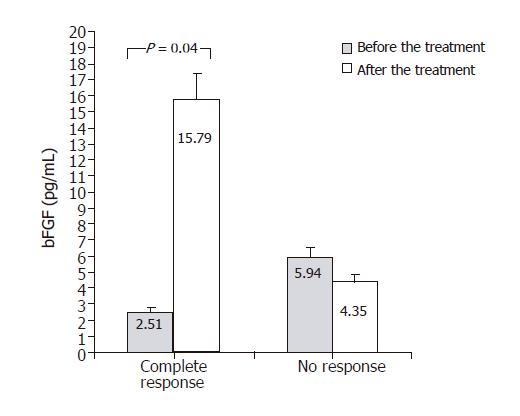
In Figure 4, TGF-β1 concentrations measured before and after antiviral treatment are compared. In both groups – responders and non-responders – significant decrease in serum TGF-β1 concentration was found following antiviral treatment. TGF-β1 level in responders decreases from 37.44 ± 4.31 to 30.02 ± 4.16 ng/mL (P = 0.05) and in non-responders from 38.22 ± 3.93 to 30.34 ± 3.7 ng/mL (P = 0.043). Figure 5 shows changes of VEGF concentration following treatment with interferon and ribavirin. In group of responders, significant increase of this factor concentration in blood was found (112.8 ± 10.2 vs 315.03 ± 29.3 pg/mL; P < 0.05), unlike the group of non-responders. In Figure 6, concentrations of bFGF in treated patients are shown. Significant increase of the concentration was demonstrated in patients with complete response to therapy (2.51 ± 0.29 vs 15.79 ± 1.8 pg/mL; P = 0.04). The concentration did not change significantly in the group of non-responders.
bFGF levels before the treatment were significantly lower (2.51 vs 5.94 pg/mL; P = 0.04), and after the treatment significantly higher (15.79 vs 4.35 pg/mL; P = 0.01) in patients with complete response than in those with no response.
Process of liver fibrosis has been recently a subject of numerous studies. Attempts are made to recognize accompanying pathogenetic phenomena and define factors influencing its progression. Reports were published concerning the possibility of fibrosis stage decrease following effective causal treatment, for example, following treatment of CHC with interferon and ribavirin[6,10,21,22]. Some authors have reported even cases of regression of cirrhosis that has been considered irreversible so far[2,6,10,19,23-26]. This study made an attempt to define the role that may be played by chosen growth factors in processes occurring in course of chronic hepatitis.
TGF-β1 is the growth factor of the best known and confirmed pro-fibrogenic activity. In our study it was demonstrated that TGF-β1 concentration in patients with fibrosis in course of CHC was indeed higher compared to the patients in whom there was no fibrosis development. Tsushima et al noted significant decrease of TGF-β1 concentration in serum, and inhibited expression of TGF-β1 mRNA in hepatic tissue following antiviral treatment, regardless of the clinical result (both for responders and non-responders)[10]. Similar observations have been made during our study. For both groups of patients significant decrease of the factor concentration was noted, regardless of the final result of treatment. This could suggest existence of direct, inhibitory influence of interferon alpha and/or ribavirin on the expression of TGF-β1 that is independent of the influence of the drug on HCV replication.
VEGF, as a factor specifically inducing proliferation of vascular endothelium cells, plays an important role in angiogenesis, and seems to play on indirect role in regeneration processes. One of the repair processes in which VEGF plays its role is probably “wound healing” process taking place in liver, and being a consequence of chronic inflammatory condition. Studies including analyses of VEGF participation in the development of hepatocellular carcinoma were performed[11-15], but only single attempts to assess its blood concentration and hepatic expression in hepatitis have been reported. Akiyoshi et al[27] found significant increase of VEGF concentration in the serum of patients with acute hepatitis of typical course, but in cases of fulminant hepatitis those concentrations were lower than in control group and increased in patients who recovered. Low VEGF concentrations were noted by the authors in cases of cirrhosis, but they did not show significant differences compared to control group in cases of chronic hepatitis. Jinno et al obtained different results. In their study VEGF concentrations appeared to be significantly higher in cases of chronic hepatitis and cirrhosis compared to healthy individuals[28]. In our study we did not find statistically significant differences of VEGF concentrations in blood between patients with hepatitis C and control group. No papers estimating possible changes in the cytokine concentration following antiviral therapy have been published yet. Our study showed that in patients meeting the criteria of response to this type of treatment there was a significant increase of VEGF serum concentration. The phenomenon was not observed in non-responsive patients. VEGF concentration increase following effective therapy, after which one could expect inhibition of fibrosis, justifies the assumption that stimulation of angiogenesis by this factor may be a key element in regeneration processes accompanying regression of liver fibrosis.
bFGF, a growth factor with well documented participation in classic process of “wound healing”, may also play a role in repair processes taking place in liver damaged by inflammatory condition, however, number of published reports on this topic is very low. Jinno et al found that bFGF blood concentrations increase with the liver disease progression[18]. In our study bFGF levels were not statistically different in case and control groups. Following treatment with interferon and ribavirin, significant increase of the factor concentration was found in patients, for whom the therapy proved to be effective. Moreover, it was noted that complete therapeutic response occurred in patients whose initial concentration of this cytokine was significantly lower, and significantly higher following the treatment than in non-responders group. As one of the probable bFGF activity courses is stimulation of endothelium cells proliferation[16,17], it may be assumed that increase of the cytokine concentration following effective treatment may be linked with intensification of angiogenesis, just like in the case of VEGF.
Studies showed that antiviral treatment, especially effective one, causes significant changes in concentrations of some growth factors. Studies documenting decrease of fibrosis stage following the therapy and concluded with persistent inhibition of virus replication are increasing. Combination of those facts justifies assumption that those cytokines may play an important role in pathogenesis of changes taking place in liver during chronic inflammatory conditions. Studies documenting participation of the growth factors other than TGF-β1 in those processes are, as for now, very sparse. It seems, however, that the topic deserves interest. Precise knowledge concerning processes leading to the development of fibrosis could contribute to elaboration of methods aimed at prevention of the disease progression and complications.
Among the factors analyzed in our study, TGF-β1 plays the most significant role in pathogenesis of fibrosis in course of CHC. It supports results of other studies.
We have demonstrated that good therapeutic effect in patients with CHC is connected with influence of drugs on TGF-β1, VEGF, and bFGF serum concentration. These findings suggest possible usefulness of these factors in assessment of antiviral treatment efficacy and indication of medication failure. It seems that bFGF shows the best prognostic value regarding final antiviral treatment results.
S- Editor Guo SY L- Editor Elsevier HK E- Editor Kong LH
| 1. | Poynard T, Ratziu V, Benhamou Y, Opolon P, Cacoub P, Bedossa P. Natural history of HCV infection. Baillieres Best Pract Res Clin Gastroenterol. 2000;14:211-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38 Suppl 1:S38-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1291] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 3. | Albanis E, Safadi R, Friedman SL. Treatment of hepatic fibrosis: almost there. Curr Gastroenterol Rep. 2003;5:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Reeves HL, Friedman SL. Activation of hepatic stellate cells--a key issue in liver fibrosis. Front Biosci. 2002;7:d808-d826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 338] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 5. | Arthur MJ. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G245-G249. [PubMed] |
| 6. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [PubMed] |
| 7. | Taniguchi H, Kato N, Otsuka M, Goto T, Yoshida H, Shiratori Y, Omata M. Hepatitis C virus core protein upregulates transforming growth factor-beta 1 transcription. J Med Virol. 2004;72:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Nelson DR, Gonzalez-Peralta RP, Qian K, Xu Y, Marousis CG, Davis GL, Lau JY. Transforming growth factor-beta 1 in chronic hepatitis C. J Viral Hepat. 1997;4:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 107] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fibrosis. Front Biosci. 2002;7:d793-d807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 326] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 10. | Tsushima H, Kawata S, Tamura S, Ito N, Shirai Y, Kiso S, Doi Y, Yamada A, Oshikawa O, Matsuzawa Y. Reduced plasma transforming growth factor-beta1 levels in patients with chronic hepatitis C after interferon-alpha therapy: association with regression of hepatic fibrosis. J Hepatol. 1999;30:1-7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Deli G, Jin CH, Mu R, Yang S, Liang Y, Chen D, Makuuchi M. Immunohistochemical assessment of angiogenesis in hepatocellular carcinoma and surrounding cirrhotic liver tissues. World J Gastroenterol. 2005;11:960-963. [PubMed] |
| 12. | Sun XY, Wu ZD, Liao XF, Yuan JY. Tumor angiogenesis and its clinical significance in pediatric malignant liver tumor. World J Gastroenterol. 2005;11:741-743. [PubMed] |
| 13. | Imura S, Miyake H, Izumi K, Tashiro S, Uehara H. Correlation of vascular endothelial cell proliferation with microvessel density and expression of vascular endothelial growth factor and basic fibroblast growth factor in hepatocellular carcinoma. J Med Invest. 2004;51:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Li X, Feng GS, Zheng CS, Zhuo CK, Liu X. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol. 2004;10:2878-2882. [PubMed] |
| 15. | Shi B, Wang X, Yang Z. Vascular endothelial growth factors and liver diseases. Hepatogastroenterology. 2001;48:1145-1148. [PubMed] |
| 16. | Wang JK, Gao G, Goldfarb M. Fibroblast growth factor receptors have different signaling and mitogenic potentials. Mol Cell Biol. 1994;14:181-188. [PubMed] |
| 17. | Gospodarowicz D. Fibroblast growth factor. Chemical structure and biologic function. Clin Orthop Relat Res. 1990;257:231-248. [PubMed] |
| 18. | Jin-no K, Tanimizu M, Hyodo I, Kurimoto F, Yamashita T. Plasma level of basic fibroblast growth factor increases with progression of chronic liver disease. J Gastroenterol. 1997;32:119-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Arthur MJ. Reversibility of liver fibrosis and cirrhosis following treatment for hepatitis C. Gastroenterology. 2002;122:1525-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 183] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1130] [Cited by in RCA: 1190] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 21. | Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, Ling MH, Albrecht J. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 802] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 22. | Poynard T, Ratziu V, Benmanov Y, Di Martino V, Bedossa P, Opolon P. Fibrosis in patients with chronic hepatitis C: detection and significance. Semin Liver Dis. 2000;20:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 113] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Bonis PA, Friedman SL, Kaplan MM. Is liver fibrosis reversible. N Engl J Med. 2001;344:452-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 129] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Rockey DC. Antifibrotic therapy in chronic liver disease. Clin. Gastroenterol Hepatol. 2005;3:95-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Markham CH, Diamond SG. Further evidence to support disconjugate eye torsion as a predictor of space motion sickness. Aviat Space Environ Med. 1992;63:118-121. [PubMed] |
| 26. | Abergel A, Darcha C, Chevallier M, Ughetto S, Henquell C, Pol S, de Ledinghen V, Canva V, Bronowicki JP, Tran A. Histological response in patients treated by interferon plus ribavirin for hepatitis C virus-related severe fibrosis. Eur J Gastroenterol Hepatol. 2004;16:1219-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Akiyoshi F, Sata M, Suzuki H, Uchimura Y, Mitsuyama K, Matsuo K, Tanikawa K. Serum vascular endothelial growth factor levels in various liver diseases. Dig Dis Sci. 1998;43:41-45. [PubMed] [DOI] [Full Text] |
| 28. | Jinno K, Tanimizu M, Hyodo I, Nishikawa Y, Hosokawa Y, Doi T, Endo H, Yamashita T, Okada Y. Circulating vascular endothelial growth factor (VEGF) is a possible tumor marker for metastasis in human hepatocellular carcinoma. J Gastroenterol. 1998;33:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 102] [Article Influence: 3.8] [Reference Citation Analysis (0)] |